Introduction
Light, the most powerful environmental cue, or
zeitgeber, is indispensable for synchronizing the brain's
endogenous biological rhythms with the 24-h terrestrial day. This
synchronization underpins temporal organization across all levels
of physiology, profoundly influencing sleep architecture, affective
state and metabolic homeostasis (1). While the human retina has been
traditionally conceived as the exclusive domain of image-forming
vision, a paradigm shift has illuminated its central role in
mediating a suite of non-image-forming (NIF) responses to light.
These vital functions, including circadian photoentrainment,
neuroendocrine modulation and pupillary control, are primarily
orchestrated by a unique population of intrinsically photosensitive
retinal ganglion cells (ipRGCs) (2,3).
These neurons, expressing the blue-light-sensitive photopigment
melanopsin, form a direct conduit to key subcortical brain regions,
most notably the suprachiasmatic nucleus (SCN) in the hypothalamus,
which serves as the master circadian pacemaker (4).
From an advanced ophthalmic and neuroscience
perspective, the retina is a highly sophisticated interface between
environmental photic information and the central nervous system's
timing machinery (5). Operating in
concert with classical photoreceptors (rods and cones), ipRGCs
detect ambient illuminance and transduce this information into
neural signals that govern the sleep-wake cycle, regulate the
secretion of the chronobiotic hormone melatonin and directly
modulate brain circuits involved in mood (6). Pathological disruption of this
pathway, arising from degenerative ophthalmic diseases, the
physiological process of aging, or the pervasive alteration of our
light environment by modern technology, can precipitate a state of
circadian desynchronization, leading to impaired sleep quality,
affective disorders and a host of other systemic health problems
(7).
A growing body of evidence from complex animal
models and human clinical studies highlights the profound
vulnerability of the NIF system in the context of retinal pathology
(8–10). Diseases such as glaucoma, which
leads to the progressive loss of retinal ganglion cells, and
retinal degenerations such as retinitis pigmentosa, can selectively
or disproportionately compromise ipRGC function (11). This damage results in a cascade of
downstream consequences, including circadian arrhythmia, severely
disrupted sleep architecture and mood instability that transcends
the psychological effect of vision loss itself. Conversely, the
precise understanding of these pathways has unlocked novel
therapeutic possibilities. Targeted manipulation of light exposure,
through spectrally-tuned light therapy or advanced spectral
filtering technologies, offers the potential to strategically
modulate retinal input to circadian centers, heralding new
frontiers in both ophthalmic and behavioral medicine (12), The present review synthesized
current, in-depth knowledge of retinal light perception and its
complex implications for circadian biology, sleep and mood, with a
specific focus on the intricate mechanisms and clinical
significance in ophthalmic disorders.
The molecular and cellular mechanisms of
retinal light perception
The retina's capacity to inform the brain about the
presence and quality of environmental light is mediated by a
sophisticated and heterogeneous assembly of photoreceptive and
neuronal cells (13). While the
roles of rods and cones in image formation are well-established, a
deeper understanding of the retina's non-visual functions requires
a detailed examination of the intrinsic photosensitivity of ipRGCs,
their unique molecular machinery and their intricate integration
within the retinal network (14).
The cellular architecture for photoreception
includes not only the classical photoreceptors but also the 1–3% of
retinal ganglion cells that are ipRGCs (15). These cells express the G-protein
coupled receptor photopigment melanopsin and can function as
autonomous light detectors. This intrinsic photosensitivity is
fundamentally different from that of rods and cones (13). Melanopsin phototransduction is a
depolarizing cascade initiated by the activation of a Gq/11-class
G-protein, leading to the engagement of phospholipase Cβ4. This
enzyme hydrolyzes phosphatidylinositol 4,5-bisphosphate into
inositol trisphosphate and diacylglycerol. The subsequent opening
of specific transient receptor potential canonical (TRPC) channels,
primarily TRPC6 and TRPC7, permits a sustained influx of
Na+ and Ca2+, causing a prolonged membrane
depolarization and a tonic increase in the cell's action potential
firing rate (11). This sustained
response is ideal for encoding ambient irradiance over long
timescales, a critical feature for circadian signaling. A key
feature of melanopsin is its function as a bistable pigment; its
all-trans-retinal chromophore can be photoisomerized back to the
11-cis form by long-wavelength light, allowing for regeneration
within the cell, a stark contrast to the enzymatic recycling
pathway required for rod and cone opsins (16,17).
The ipRGC population is not monolithic. In mammals,
at least six subtypes (M1-M6) have been delineated based on
distinct morphological, molecular and functional characteristics
(13). M1 ipRGCs stratify their
dendrites in the OFF sublamina of the inner plexiform layer (IPL)
and are defined by expression of the transcription factor Brn3b.
They are the principal drivers of photoentrainment, forming the
primary afferents of the retinohypothalamic tract (RHT) to the SCN
(18). M2 and M4 cells stratify in
the ON sublamina of the IPL, co-express the transcription factor
Tbr2 and contribute markedly to the pupillary light reflex via
projections to the olivary pretectal nucleus. M4 cells, in
particular, have large dendritic fields and are also implicated in
contrast sensitivity for conscious vision (4). Other subtypes, such as M5 and M6,
project to a diverse array of over 40 distinct subcortical targets,
including limbic structures such as the amygdala, bed nucleus of
the stria terminalis and lateral habenula, providing a direct
anatomical substrate for light's influence on mood, fear and
motivation. This remarkable projection diversity means that the
retina disseminates parallel streams of photic information to
functionally distinct brain circuits (8).
Crucially, ipRGCs do not operate in isolation. They
are deeply embedded within the retinal circuitry, acting as complex
integrators of both their own intrinsic photoresponse and extrinsic
signals relayed from rods and cones through specific amacrine and
bipolar cell pathways. For example, ipRGCs receive significant
input from ON-cone bipolar cells, allowing them to respond robustly
to daylight. Rod signals are conveyed primarily through AII
amacrine cells and subsequent gap junctions, conferring high
sensitivity to light at night (19). This integration allows ipRGCs to
encode light information across an astonishing 12-log-unit range of
intensities. The spectral sensitivity of this integrated system is
complex, but the intrinsic (15)
response, with its peak sensitivity to blue light ~480 nm, is the
dominant driver of NIF functions such as circadian resetting and
melatonin suppression (20) This
specific wavelength dependency is the biophysical basis for the
potent effects of blue-enriched light from modern sources on human
physiology (21).
Ophthalmic diseases profoundly alter these intricate
mechanisms. In glaucoma, the progressive apoptosis of RGCs includes
the loss of ipRGCs. Studies indicate that different ipRGC subtypes
may have differential vulnerability, potentially explaining the
specific patterns of NIF deficits observed in patients, such as
impaired pupillary responses and severe sleep disturbances. In
retinal degenerations such as retinitis pigmentosa, the loss of
rods and cones functionally ‘unmasks’ the pure melanopsin-based
photosensitivity of surviving ipRGCs (22,23)
While these surviving cells can sustain circadian entrainment, the
absence of rod and cone input severely reduces the overall
sensitivity of the system and eliminates its ability to respond to
rapid light changes, often leading to poorly entrained or
free-running rhythms. These pathologies transform the retina into a
model system for understanding how specific disruptions in photic
signaling pathways lead to systemic neurobehavioral dysfunction
(15). The main cell types and
photopigments involved in NIF light perception are summarized in
Table I.
 | Table I.Key cell types and photopigments
involved in non-image-forming light perception. |
Table I.
Key cell types and photopigments
involved in non-image-forming light perception.
| Cell type |
Marker/photopigment | Main function | Projection
target(s) |
|---|
| Rods | Rhodopsin | Low-light vision,
support NIF via ipRGCs | Bipolar cells,
ipRGCs (indirect) |
| Cones | Photopsins | Color, daylight
vision, input to NIF | Bipolar cells,
ipRGCs (indirect) |
| M1 ipRGCs | Melanopsin
(OPN4) | Circadian
photoentrainment | SCN, OPN |
| M2-M6 ipRGCs | Melanopsin
(OPN4) | Pupillary reflex,
mood regulation, etc. | OPN, LHb, mPFC,
other targets |
| Amacrine/Bipolar
cells | Various | Intraretinal signal
integration | ipRGCs |
The intricate relationship between the
retina and circadian rhythms
The process of photoentrainment, the synchronization
of the endogenous circadian clock to the 24-h light/dark cycle, is
governed by a precise neuroanatomical and molecular dialogue
between the retina and the SCN. This section delves into the
detailed mechanisms of this critical interaction, from the specific
neurochemistry of the RHT to the molecular clockwork within SCN
neurons that is the target of retinal input (Fig. 1) (24,25).
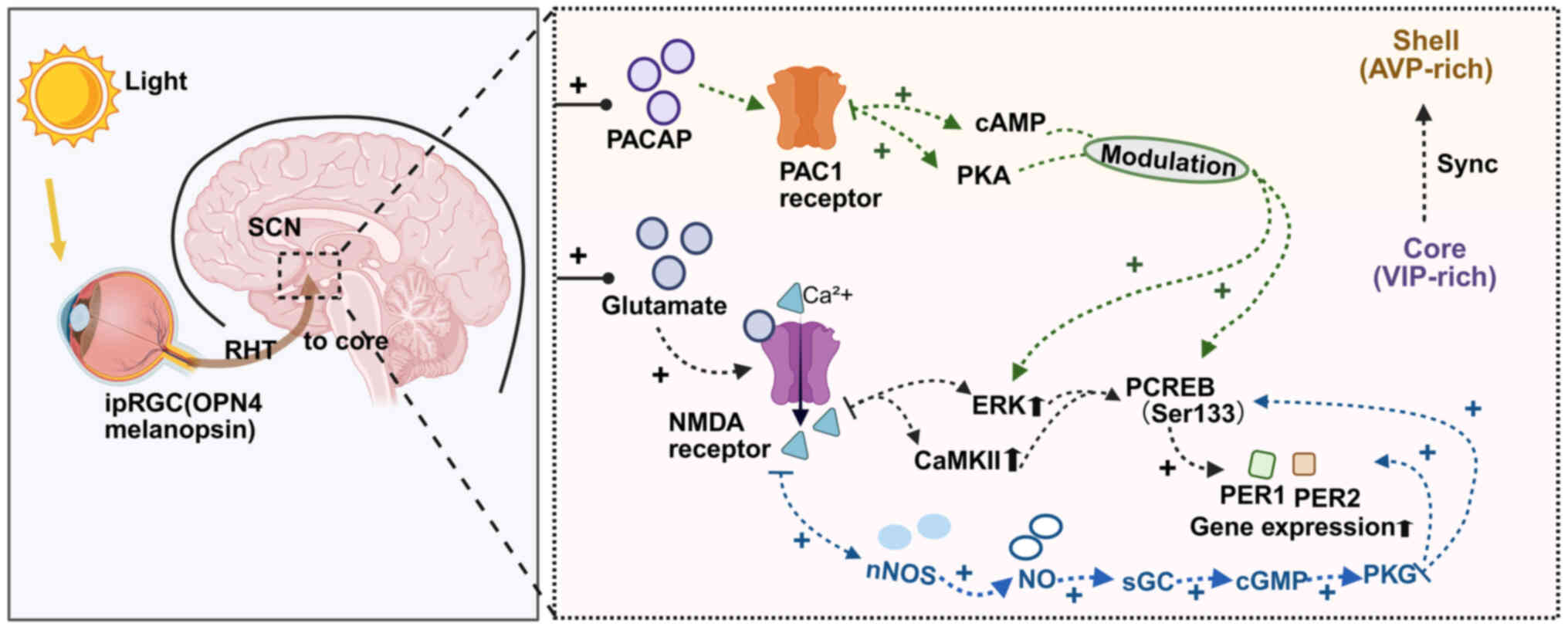 | Figure 1.Retinal input and intracellular
signaling underlying photic entrainment of the SCN. Light is
detected by melanopsin-expressing ipRGCs in the retina and conveyed
to the SCN via the RHT, which releases glutamate and PACAP. In SCN
neurons, glutamate activates NMDA receptors, elevating
Ca2+ and engaging CaMKII/ERK, which promotes CREB
(Ser133) phosphorylation and PER1/PER2 expression. In parallel,
Ca2+ activates nNOS and NO stimulates sGC to increase
cGMP, activating PKG that further supports CREB phosphorylation and
clock-gene induction. PACAP acting on PAC1 receptors modulates
these processes and stabilizes entrainment. SCN, suprachiasmatic
nucleus; ipRGCs, intrinsically photosensitive retinal ganglion
cells; RHT, retinohypothalamic tract; PACAP, pituitary adenylate
cyclase-activating polypeptide; NMDA, N-methyl-D-aspartate; CaMKII,
calmodulin-dependent protein kinase II; CREB, cAMP response
element-binding protein; period circadian protein homolog; nNOS,
neuronal nitric oxide synthase; NO, nitric oxide; sGC, soluble
guanylyl cyclase; cGMP, cyclic guanosine monophosphate; PKG,
protein kinase G; AVP, arginine vasopressin; VIP, vasoactive
intestinal polypeptide. |
The anatomical foundation of photoentrainment is the
RHT, a monosynaptic pathway originating from a subset of ipRGCs,
predominantly M1 cells, which terminates in the ventrolateral core
region of the SCN. The principal neurotransmitters released by RHT
terminals are glutamate and pituitary adenylate cyclase-activating
polypeptide (PACAP) (4). These
transmitters have distinct but synergistic roles. Glutamate, acting
on N-methyl-D-aspartate (NMDA) receptors, is the primary mediator
of the acute, phase-shifting effects of light. Its release is
proportional to the light intensity and duration, triggering rapid
intracellular signaling. PACAP, acting on PAC1 receptors, plays a
more modulatory role, enhancing the SCN's sensitivity to glutamate
and being crucial for entrainment to full light-dark cycles and for
the long-term stability of the clock (26).
Upon light stimulation, the release of glutamate and
PACAP in the SCN initiates a complex intracellular signaling
cascade within postsynaptic SCN neurons (27). Glutamate-induced NMDA receptor
activation leads to a significant influx of Ca2+, which
in turn activates multiple downstream pathways, including
calcium/calmodulin-dependent protein kinase II (CaMKII) and the
Ras/MAPK/ERK pathway. These kinases converge on the phosphorylation
of the transcription factor cAMP response element-binding protein
(CREB) at its Serine-133 residue (28). Phosphorylated (p-)CREB then
translocates to the nucleus and binds to cAMP response elements in
the promoters of the core clock genes Per1 and Per2,
rapidly inducing their transcription (29). This light-driven surge in
Per expression is the molecular event that resets the phase
of the SCN clock. A light pulse in the early subjective night
advances the accumulation of PER protein, causing a phase delay,
while a pulse in the late subjective night or early morning
accelerates the declining phase of PER, leading to a phase advance
(30).
The SCN itself possesses a sophisticated internal
structure, comprising a ventrolateral ‘core’ and a dorsomedial
‘shell.’ The RHT innervates the core, which is rich in neurons
expressing vasoactive intestinal polypeptide. These core neurons
are the primary recipients of light information. They then
synchronize the much larger population of neurons in the shell,
which express arginine vasopressin and are considered the primary
output neurons of the SCN (31).
This core-shell network architecture allows the SCN to integrate
the photic signal, generate a robust, consolidated rhythm and then
broadcast this timing information to the rest of the brain and body
(32).
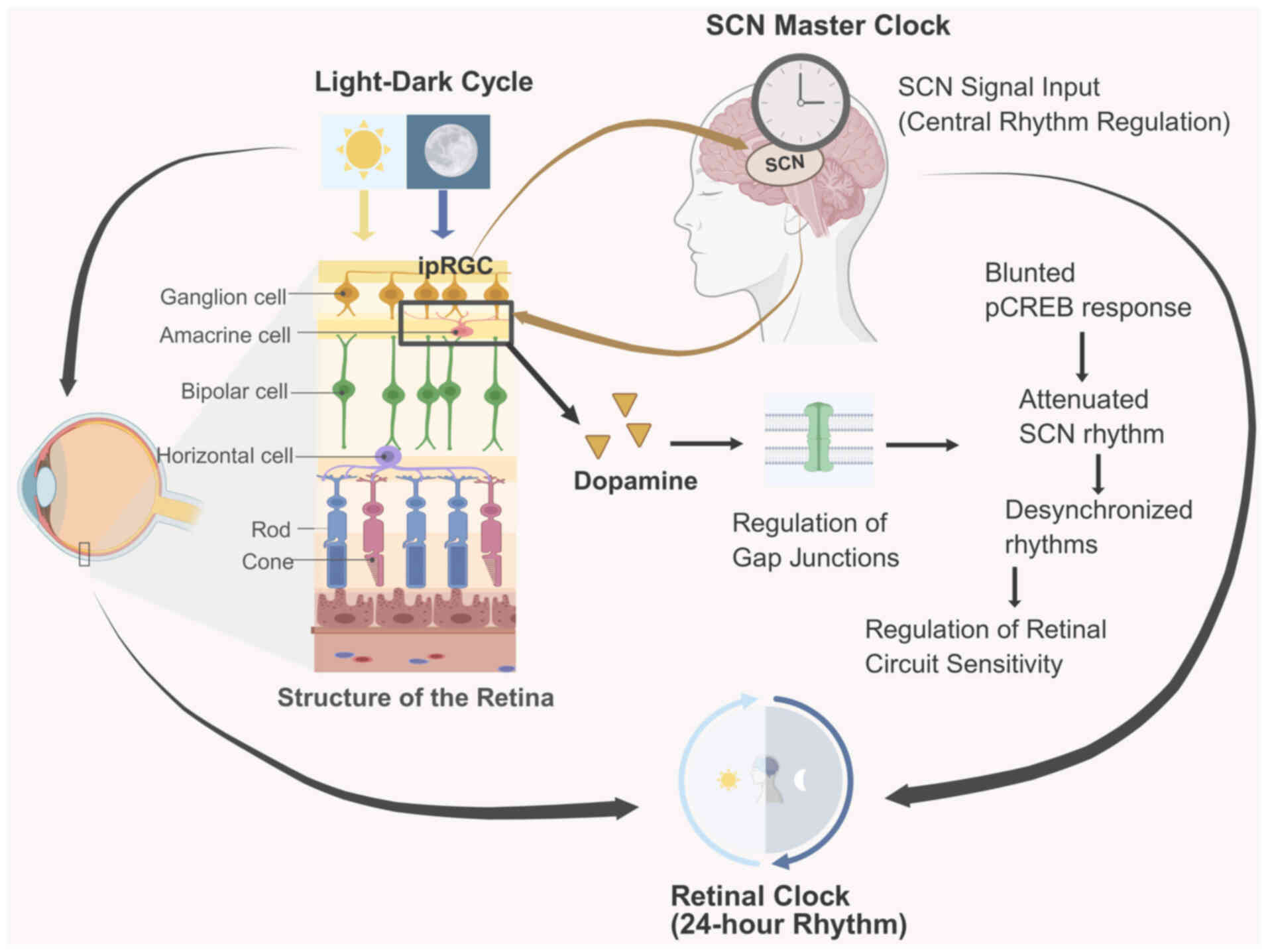
Disruptions to this system via retinal pathology
have profound consequences. In glaucoma models, the reduction in
ipRGC density leads to a quantifiable decrease in RHT input to the
SCN (33). This manifests as a
blunted pCREB response to light pulses, attenuated amplitude of the
SCN's overall electrical activity rhythm and, ultimately, the
desynchronization of behavioral and hormonal rhythms, such as
locomotor activity and melatonin secretion. In humans with advanced
glaucoma, these central deficits are correlated with clinical
reports of non-24-h sleep-wake disorder and depression (34). In cases of retinitis pigmentosa
where cone and rod function is lost but ipRGCs survive, the SCN can
still be entrained (35). However,
the system's sensitivity is markedly reduced, requiring much higher
irradiances of blue-enriched light to elicit a phase shift. This
highlights the critical contribution of the classical
photoreceptors in sensitizing the NIF system under normal
conditions (36).
Furthermore, the retina itself harbors an autonomous
circadian clock (Fig. 2). Clock
genes are rhythmically expressed in various retinal cell types,
including photoreceptors and dopaminergic amacrine cells,
regulating local physiological processes such as dopamine release,
gene expression and metabolic activity in a 24-h cycle (37). This retinal clock is synchronized
by both systemic signals from the SCN and the local light-dark
cycle. Dopamine, released rhythmically during the day, acts as a
key intraretinal signal that modulates gap junction coupling and
adjusts the sensitivity of retinal circuits. This local timekeeping
mechanism adds another layer of complexity, suggesting a
bidirectional communication system where the retina not only
signals time to the brain but also possesses its own intrinsic
temporal organization that fine-tunes its function across the
day-night cycle (38).
Light-evoked glutamatergic input from the RHT
elevates postsynaptic Ca2+ in SCN neurons via NMDA
receptors, which activates neuronal nitric oxide (NO) synthase
(nNOS) and drives NO production (39). NO stimulates soluble guanylyl
cyclase (sGC) to increase cyclic guanosine monophosphate (cGMP),
engaging protein kinase G (PKG) pathways that facilitate
phosphorylation of CREB and the rapid induction of
Per1/Per2. Pharmacological inhibition of NOS or sGC
diminishes light-induced phase shifts and Per transcription,
whereas NO donors or membrane-permeant cGMP analogs enhance them
(40). Thus, NO functions as a
critical second-messenger amplifier that couples the initial RHT
glutamatergic signal to the transcriptional machinery that resets
the circadian clock. In parallel, intraretinal NO-produced by
amacrine and other interneurons-modulates gap junction coupling and
photoreceptor/bipolar signaling, shaping the irradiance code
ultimately delivered by ipRGCs to the SCN (41).
Retinal light input and the neurobiology of
sleep regulation
The regulation of the sleep-wake cycle is a complex
interplay between a homeostatic process, which increases sleep
pressure with time spent awake and the circadian process, which
dictates the timing of sleep and wakefulness. Retinal light
perception is the most powerful modulator of the circadian process,
influencing not only the timing of sleep but also its internal
architecture and consolidation through both acute alerting effects
and chronic entrainment of the central clock (42).
The SCN orchestrates the timing of sleep through a
network of efferent projections. One of the most critical pathways
is a multisynaptic circuit to the pineal gland. The SCN projects to
the paraventricular nucleus of the hypothalamus, which in turn
sends signals down to preganglionic sympathetic neurons in the
intermediolateral cell column of the spinal cord (43). These neurons drive postganglionic
fibers from the superior cervical ganglion that innervate the
pineal gland, controlling the synthesis and release of melatonin.
During the day, the SCN is active and inhibits this pathway. At
night, SCN activity wanes, disinhibiting the pathway and allowing
for robust melatonin production. Light exposure at night, detected
by retinal ipRGCs, rapidly reactivates the SCN, which clamps this
pathway shut, acutely suppressing melatonin synthesis. The spectral
sensitivity of this suppression reflex mirrors that of melanopsin,
with short-wavelength blue light being the most potent stimulus
(44).
In addition to its chronobiotic role, light exerts a
direct and immediate alerting effect on the brain. This is mediated
by ipRGC projections to key arousal centers, bypassing the SCN.
These projections innervate the lateral hypothalamic area, home to
the orexin/hypocretin neurons that are critical for maintaining
consolidated wakefulness, as well as monoaminergic arousal centers
in the brainstem, such as the noradrenergic locus coeruleus
(45). Activation of these
pathways by light can directly antagonize sleep-promoting signals.
For example, the SCN also regulates the ventrolateral preoptic
nucleus (VLPO), a key sleep-promoting center containing GABAergic
and galaninergic neurons that inhibit arousal systems (46). The SCN promotes VLPO activity
during the biological night. Light at night can therefore disrupt
this balance, both by directly stimulating arousal centers and by
suppressing the SCN's drive onto the VLPO, leading to fragmented
sleep and increased awakenings (47).
The differential effect of light spectra on sleep is
a direct consequence of the biophysics of retinal photoreceptors.
Short-wavelength blue light (~480 nm), through its potent
activation of the melanopsin system in ipRGCs, has the most
significant effects on sleep regulation (11). Evening exposure to blue-enriched
light has been demonstrated to not only suppress melatonin and
delay sleep onset but also to alter subsequent sleep architecture,
typically by reducing the amount of restorative slow-wave sleep,
characterized by high-amplitude delta (0.5-4 Hz)
electroencephalogram waves and decreasing the duration of rapid eye
movement sleep (45). By contrast,
evening exposure to long-wavelength red light, which minimally
stimulates ipRGCs, has little to no effect on melatonin or
circadian phase and preserves sleep architecture. This principle
underpins the strategy of using ‘circadian lighting’ or
blue-blocking filters to create a transition to sleep (44,48).
The clinical relevance of these mechanisms is most
evident in individuals with disrupted retinal input. Patients with
glaucoma or advanced diabetic retinopathy often suffer from severe
sleep fragmentation and insomnia, which correlate with the degree
of ipRGC damage as measured by objective tests such as the
pupillary light reflex (49). In
totally blind individuals without any light perception (that is,
with complete optic nerve destruction or bilateral enucleation),
the sleep-wake cycle often ‘free-runs’ with a period slightly
longer than 24 h, leading to a condition known as non-24-h
sleep-wake disorder (50).
However, in blind individuals who retain functional ipRGCs, timed
light administration can successfully entrain their rhythms,
highlighting the therapeutic potential of targeting this residual
NIF pathway. The integrity of the retinal photic input pathway is
therefore a fundamental determinant of an individual's ability to
maintain a stable and restorative sleep-wake cycle (12).
Systemic health consequences of retinal
light perception
The influence of retinal light perception extends
far beyond visual processing and sleep regulation, acting as the
primary synchronizing agent for the entire organism's physiology.
Through the RHT and other projections, photic information entrains
the central SCN pacemaker, which in turn orchestrates a hierarchy
of peripheral clocks in virtually every tissue (51). This establishes a direct and
profound link between ocular health, environmental light patterns
and systemic well-being. Consequently, circadian disruption,
initiated by aberrant retinal signaling from either pathological
conditions or unnatural light exposure, is now understood as a
fundamental pathogenic mechanism. It contributes to a spectrum of
chronic diseases by desynchronizing the tightly regulated temporal
expression of clock-controlled genes that govern metabolism, cell
cycle and immune function. Furthermore, direct retinal projections
to non-circadian brain centers inextricably link light perception
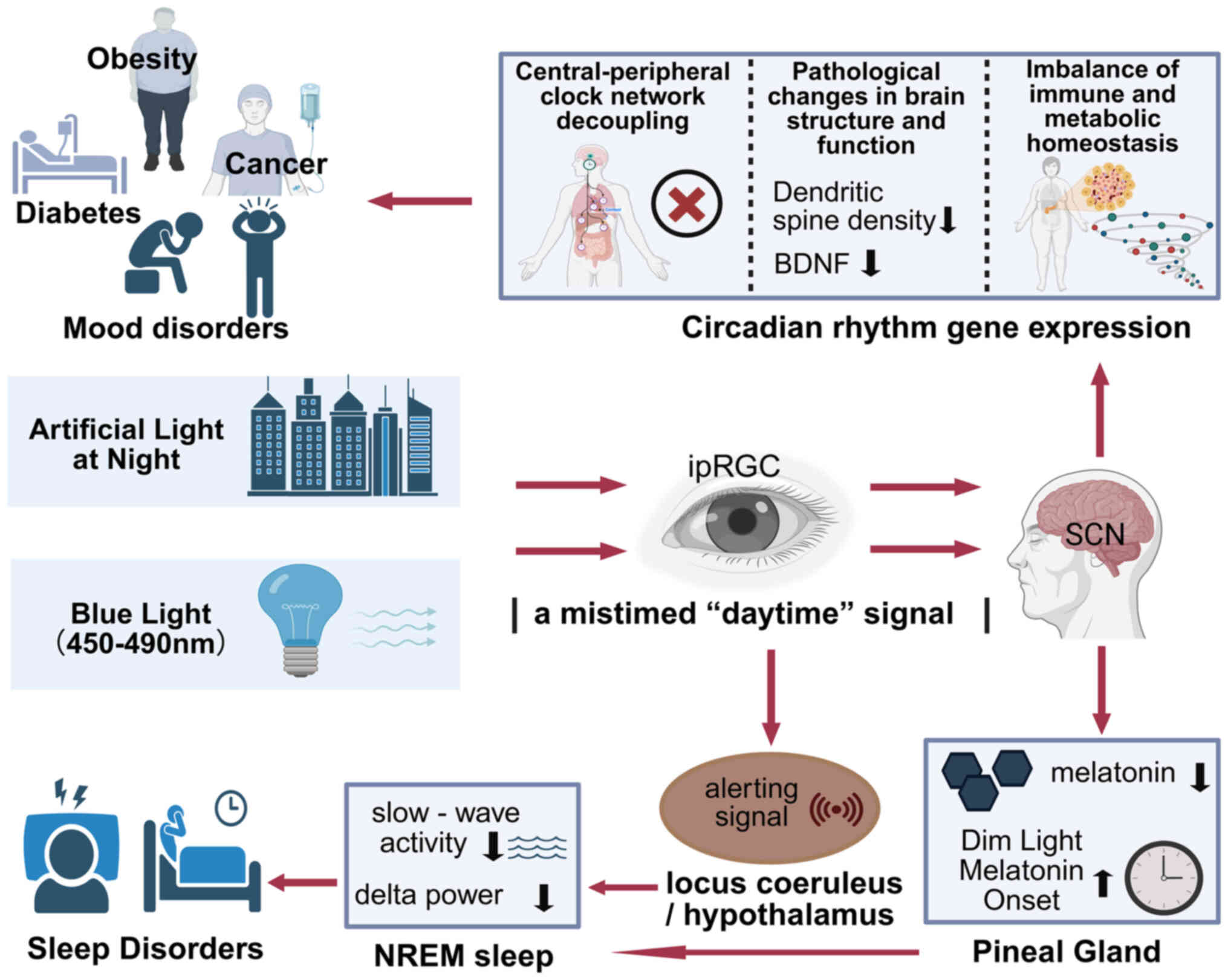
to the neurobiology of mood and mental health (Fig. 3) (12).
Metabolic dysregulation and
disease
The circadian system imposes a temporal order on
metabolism, ensuring that anabolic and catabolic processes are
aligned with feeding/fasting and activity/rest cycles. This
coordination is achieved by the SCN synchronizing peripheral clocks
within key metabolic organs. Chronic circadian disruption,
epitomized by shift work or exposure to light-at-night, severs this
synchrony, leading to internal temporal chaos with severe metabolic
consequences (52).
In the liver, the local clock, driven by rhythmic
transcription of Bmal1 and Clock, directly regulates hundreds of
genes involved in glucose and lipid homeostasis. For instance, the
clock-controlled protein REV-ERBα acts as a potent rhythmic
repressor of key gluconeogenic genes, including
glucose-6-phosphatase and phosphoenolpyruvate carboxykinase,
suppressing hepatic glucose output during the inactive/fasting
phase (53,54). Simultaneously, the liver clock
gates lipid metabolism by controlling the expression of sterol
regulatory element-binding protein 1c and its downstream targets
such as fatty acid synthase, restricting de novo lipogenesis
to the active/feeding phase (55)
Mistimed light exposure and consequent behavioral shifts (such as
eating at night) create a conflict between the central SCN signal
and metabolic substrate availability, uncoupling these genetic
programs and leading to pathological states such as insulin
resistance and non-alcoholic fatty liver disease due to incessant
gluconeogenesis and lipogenesis (56,57).
In the pancreas, the clock within islet β-cells is
critical for normal insulin function. BMAL1/CLOCK heterodimers
directly regulate the transcription of genes essential for every
step of insulin secretion, from glucose transport (Slc2a2/GLUT2)
and ATP production to the function of the ATP-sensitive potassium
channels that trigger membrane depolarization. Circadian
misalignment impairs glucose-stimulated insulin secretion and
reduces insulin sensitivity in peripheral tissues, creating a
direct pathway to the development of type 2 diabetes (58,59).
In adipose tissue, the local clock governs
adipogenesis and lipid storage, partly through the rhythmic control
of the master adipogenic transcription factor Pparg by BMAL1. This
clock also controls the rhythmic secretion of key adipokines. The
normal nocturnal peak of leptin, a satiety hormone, is blunted or
shifted by mistimed light and sleep, disrupting hunger signals and
contributing directly to obesity (60).
Circadian disruption and cancer
pathogenesis
The link between circadian disruption and cancer is
supported by robust epidemiological data and grounded in precise
molecular mechanisms that couple the clock to cell proliferation
and immune surveillance. The International Agency for Research on
Cancer has classified shift work involving circadian disruption as
a probable human carcinogen (3,61).
The core clock machinery is physically and
functionally integrated with the cell cycle. Key clock proteins,
such as period circadian protein homolog (PER)1 and PER2, can bind
to and inhibit critical cell cycle promoters such as Cyclin D1 and
the proto-oncogene c-Myc, acting as tumor suppressors. Conversely,
BMAL1/CLOCK rhythmically regulate the expression of the cell cycle
checkpoint kinase Wee1, which controls entry into mitosis.
Circadian disruption dismantles this temporal gating, permitting
uncontrolled cell proliferation (62).
A primary mechanistic link is the suppression of
nocturnal melatonin by light at night. Melatonin is a potent
oncostatic agent with pleiotropic anti-cancer effects. Through its
receptors, MT1 and MT2, melatonin can inhibit signaling pathways
crucial for cancer growth, such as the MAPK/ERK and PI3K/Akt
pathways (63,64). It also has powerful
receptor-independent functions as a free radical scavenger,
protecting DNA from oxidative damage. Light-at-night exposure
effectively removes this endogenous anti-cancer brake, a mechanism
strongly implicated in the increased risk of hormone-sensitive
cancers such as estrogen receptor-positive breast cancer and
prostate cancer among night-shift workers (65).
Furthermore, the circadian clock regulates the
body's anti-tumor immune response. The trafficking and cytotoxic
function of immune cells, including natural killer (NK) cells and
cytotoxic T lymphocytes, exhibit robust daily rhythms. For example,
NK cell infiltration into tumors and their lytic activity are
markedly higher during the early resting phase. Circadian
disruption flattens these rhythms, leading to impaired immune
surveillance and creating a permissive environment for tumor cells
to evade detection and elimination (66).
Direct retinal pathways and mental
health
The neurobiological connections between light and
mental health are increasingly understood to be mediated by direct
retinal projections to limbic and cortical circuits, independent of
the SCN. Specific ipRGC subtypes, project to non-circadian centers
that are fundamental to affective regulation (13). A critical pathway involves
projections to the lateral habenula (LHb), a nucleus that encodes
negative valence and aversive signals and is pathologically
hyperactive in depression (67).
The LHb exerts inhibitory control over midbrain monoaminergic
systems, including the ventral tegmental area (VTA) for dopamine
and the dorsal raphe nucleus (DRN) for serotonin. Preclinical
studies show that acute light exposure can rapidly suppress the
firing of LHb neurons via this retinal input, thereby disinhibiting
VTA and DRN neurons. This provides a direct, fast-acting mechanism
by which light can enhance motivation and elevate mood (5,68).
Another key target is the medial prefrontal cortex
(mPFC), essential for executive function and the cognitive
regulation of emotion. Light enhances activity and strengthens
glutamatergic synaptic plasticity in the mPFC, probably via a
multisynaptic ipRGC-thalamus-cortex pathway (69). This strengthens top-down control
over subcortical structures such as the amygdala, which is involved
in processing fear and anxiety.
The efficacy of bright light therapy for Seasonal
Affective Disorder (SAD) is a clinical manifestation of these
pathways. SAD is characterized by a phase-delayed circadian rhythm
and a hypothesized ‘retinal subsensitivity’ to light (70). High-intensity light therapy acts as
a potent retinal stimulus that both corrects the underlying
circadian misalignment and directly modulates these mood-relevant
circuits. The high comorbidity of depression in ophthalmic diseases
such as glaucoma and retinitis pigmentosa is also explained by
these mechanisms (71). The
progressive loss of ipRGCs in these conditions severs the photic
input to the SCN, LHb and mPFC, creating a state of ‘biological
darkness’ that fosters a neurochemical and network-level state
conducive to affective disorders, a pathology that transcends the
psychological burden of vision loss (72). This suggests that therapies aimed
at activating any residual ipRGC function could have significant
mental health benefits even in low-vision patients.
Compromised adaptive capacity due to
age and disease
The circadian system's ability to adapt to
environmental changes, such as trans-meridian travel, depends on
the robustness of the retinal signal relayed to the SCN. This
adaptive capacity is markedly compromised by both normal aging and
ocular disease. With aging, the crystalline lens naturally yellows,
acting as a short-pass filter that blocks a significant portion of
the blue light (460–480 nm) required to optimally stimulate
melanopsin (73). This, combined
with senile miosis (pupil constriction), dampens the photic signal
reaching the retina, contributing to the flattened circadian
amplitude, advanced sleep phase and fragmented sleep common in the
elderly (11,74). Cataract surgery, by replacing the
yellowed lens with a clear intraocular lens that transmits blue
light, has been shown to robustly improve circadian entrainment and
sleep consolidation, highlighting the clinical importance of
retinal light hygiene. In glaucoma, the apoptotic loss of ipRGCs
creates a permanent and progressive deficit in the NIF system,
impairing the gain of the system's response to light and
making it profoundly difficult for patients to entrain their
rhythms or adapt to environmental changes (8,75).
The retina and the challenge of modern light
exposure
The contemporary human light environment represents
a dramatic departure from the evolutionary conditions under which
our visual and circadian systems evolved. The transition from a
natural regimen of bright, full-spectrum sunlight during the day
and profound darkness at night to a life spent predominantly
indoors under dim, spectrally-narrow electric light, coupled with
pervasive exposure to artificial light at night (ALAN), presents a
significant physiological challenge (76). The retina, as the first point of
contact, is at the epicenter of this challenge and understanding
its response to modern lighting is critical for public health
(77).
The circadian potency of modern light
sources
The advent and ubiquity of solid-state lighting,
particularly ‘white’ light-emitting diodes (LEDs) and the back-lit
screens of electronic devices have fundamentally altered the
spectral diet of the modern eye. Unlike the smooth, black-body
radiation curve of incandescent light, numerous white LEDs operate
on a ‘blue pump-yellow phosphor’ principle (78). This creates a spectral power
distribution (SPD) with a narrow, high-energy peak in the
short-wavelength blue portion of the spectrum (typically 450–490
nm) to excite a phosphor that re-emits a broader band of
longer-wavelength light. This blue peak aligns almost perfectly
with the maximal spectral sensitivity of melanopsin in ipRGCs,
making these sources a powerful, albeit often unintended, stimulus
for the non-image-forming system (44).
When encountered in the evening, this blue-enriched
light transmits a potent, mistimed ‘daytime’ signal to the SCN and
other brain centers. The neurobiological consequences are
quantifiable and significant. Exposure to typical indoor light
levels can acutely suppress the amplitude and duration of the
nocturnal melatonin profile and markedly delay its onset (the Dim
Light Melatonin Onset, or DLMO), a key marker of circadian phase
(79). This disruption directly
affects sleep architecture by suppressing slow-wave activity and
delta power during subsequent non-rapid eye movement sleep, a
process vital for synaptic homeostasis and memory consolidation
(80,81). The direct alerting effect, which
counteracts homeostatic sleep pressure, is mediated by ipRGC
projections to the noradrenergic locus coeruleus and the
orexinergic neurons of the lateral hypothalamus. This highlights
the inadequacy of traditional photometry, based on photopic lux,
which is weighted towards cone sensitivity for vision (~555 nm) and
fails to capture the biological potency of light (82). Consequently, the concept of α-opic
lux, particularly Melanopic Equivalent Daylight Illuminance (MEDI),
has emerged. MEDI quantifies light based on its activation
potential for the melanopsin channel, providing a far more accurate
metric for predicting circadian, neuroendocrine and alerting
responses and it is becoming a critical tool for evidence-based
lighting design (83).
Urban light pollution and chronic
circadian disruption
Beyond personal devices, broad-scale urban light
pollution constitutes a chronic and pervasive source of circadian
disruption. ALAN from streetlights, buildings and advertising can
infiltrate sleeping environments, creating nocturnal light levels
that, while seemingly low (often ranging from <1 to >10 lux),
are well within the activation threshold of the highly sensitive
ipRGC system, especially when integrated over a number of hours
(84). Large-scale epidemiological
studies, leveraging satellite data to quantify outdoor ALAN, have
found strong correlations between residence in brightly lit areas
and increased odds ratios for obesity, type 2 diabetes, mood
disorders and certain cancers (85–87).
Causal mechanisms have been elucidated in controlled
animal models. Chronic exposure to dim light at night (~5 lux) is
sufficient to markedly dampen the amplitude of the SCN's rhythmic
molecular clock gene expression. This central disruption cascades
to peripheral systems and directly impacts brain structures crucial
for mood and cognition (88). For
example, in rodents, dim ALAN has been shown to reduce dendritic
spine density in the CA1 region of the hippocampus, decrease levels
of brain-derived neurotrophic factor and impair performance on
hippocampus-dependent spatial memory tasks such as the Morris water
maze (48). These structural and
functional deficits are the neurobiological underpinnings of the
depressive-such as behaviors, such as increased immobility in the
forced swim test, observed in these animals. The retina's ipRGCs
are the unequivocal mediators of this effect, transmitting a
persistent, low-level disruptive signal to the brain throughout the
biological night (44).
Ophthalmic and behavioral intervention
strategies
In response to these challenges, several
intervention strategies based on the neurophysiology of the NIF
system have been developed. Blue-light filtering lenses aim to
attenuate the evening circadian stimulus (89). These range from lenses with
blue-reflecting coatings to short-wavelength-absorbing filters
(which appear yellow or amber). Studies show that amber lenses,
which markedly block light below ~500 nm, can effectively prevent
light-induced melatonin suppression and preserve the natural timing
of the DLMO (90). However, the
clinical efficacy remains debated, as it is highly dependent on the
precise spectral characteristics and the total amount of light
being blocked. Furthermore, indiscriminate use during the day could
be counterproductive, dampening the necessary alerting and
entraining signals (91).
A more proactive approach is structured light
therapy, which uses the light Phase Response Curve as a guiding
principle. Morning bright light therapy (typically 10,000 photopic
lux, which is high in melanopic content, for 30 min upon awakening)
is a first-line treatment for SAD and delayed sleep-wake phase
disorder because it provides a powerful, timed phase-advancing
signal to the SCN (92). For
patients with retinal diseases, these protocols can be
personalized. For instance, in a patient with retinitis pigmentosa
who has lost cone function but retains ipRGCs, a therapeutic device
could use monochromatic blue light (~480 nm) to selectively and
efficiently activate the residual melanopsin system for
entrainment, a wavelength that would be useless for image formation
(43).
The future of lighting lies in dynamic,
human-centric (or integrative) lighting systems. These systems use
multi-channel LED engines (such as combining red, green, blue and
white/amber LEDs) to actively sculpt the SPD of indoor light
throughout the day (93). The goal
is to create a biomimetic light cycle: high-intensity, high-CCT
(Correlated Color Temperature) light with a high MEDI (>250
melanopic lux) in the morning and midday to maximize alertness,
performance and circadian entrainment; followed by a gradual
transition to low-intensity, low-CCT light with a markedly reduced
MEDI (<10 melanopic lux) in the evening, thereby creating a
state of ‘circadian darkness’ that protects the melatonin rhythm
and facilitates sleep (37,94).
A summary of clinical conditions, their NIF deficits and possible
interventions is provided in Table
II.
 | Table II.Effect of ophthalmic diseases and
modern lighting on NIF functions and intervention strategies. |
Table II.
Effect of ophthalmic diseases and
modern lighting on NIF functions and intervention strategies.
|
Condition/Factor | NIF system
deficit | Main symptoms | Potential
intervention |
|---|
| Glaucoma | ipRGC loss, reduced
RHT signaling | Sleep disturbance,
mood disorders | IOP control,
structured light therapy, pupil response tests |
| Retinitis
pigmentosa | Loss of rods/cones,
ipRGC preserved | Poor circadian
entrainment, insomnia | Blue-light therapy,
time-restricted lighting |
| Aging/cataract | Reduced blue light
transmission | Advanced sleep
phase, fragmented sleep | Cataract surgery,
daylight exposure |
| Urban ALAN (light
pollution) | Excess blue light
at night | Circadian
misalignment, metabolic risk | Blue-blocking
filters, smart home lighting |
| Shift work | Chronic circadian
disruption | Insomnia, metabolic
disease, cancer | Scheduled
light/dark exposure, chronotherapy |
Future research directions and unanswered
questions
Despite monumental progress in understanding the
retina's role in NIF functions, numerous critical questions and
technological challenges remain. Future research must pursue a
multi-pronged approach, combining molecular genetics, systems
neuroscience and clinical investigation to further unravel the
complexities of retinal light perception and translate this
knowledge into effective clinical and public health strategies
(95).
A primary frontier is the deeper molecular and
functional characterization of ipRGC diversity. While the M1-M6
classification has provided a valuable framework, it is probably an
oversimplification. Single-cell RNA sequencing and spatial
transcriptomics are poised to reveal a more granular taxonomy of
ipRGC subtypes, identifying unique molecular signatures,
developmental lineages and signaling pathways for each (96). A key goal is to precisely map the
full ‘projectome’ of each subtype, that is, to identify all of its
downstream brain targets, and to functionally interrogate the role
of each specific projection in behavior and physiology using
advanced techniques such as optogenetics and chemogenetics in
animal models. Understanding the differential vulnerability of
these specific subtypes in diseases such as glaucoma is a critical
clinical question that could lead to more targeted diagnostics and
neuroprotective strategies. Furthermore, the detailed biophysical
properties of melanopsin itself, including its signaling dynamics
and regeneration cycle under various lighting conditions and in
pathological states, warrant continued investigation (97).
The development of personalized light therapy and
precision light exposure management represents a significant
translational goal (98). The
current ‘one-size-fits-all’ approach to light therapy does not
account for the vast inter-individual variability in light
sensitivity, chronotype, genetics (such as polymorphisms in clock
genes), age, or the health status of the retina. Future research
should focus on developing objective biomarkers, perhaps based on
pupillometry, melatonin profiles, or electroretinography, to
quantify an individual's NIF function. These data could then be
integrated with genetic information and lifestyle monitoring from
wearable sensors into machine learning algorithms to generate truly
personalized light ‘prescriptions’ (99). This would involve specifying the
optimal dose (intensity), spectrum, timing and duration of light
needed to achieve a desired therapeutic outcome, whether it be
advancing a delayed sleep phase, alleviating depressive symptoms,
or bolstering circadian rhythms in an elderly individual or a
patient with retinal disease. The integration of such intelligent
systems with smart home and workplace lighting environments could
enable the seamless, automated delivery of optimal light exposure
throughout the 24-h day (100).
Finally, progress in this field will be critically
dependent on enhanced interdisciplinary collaboration. The study of
retinal light perception sits at the crossroads of ophthalmology,
neuroscience, sleep medicine, psychiatry and engineering.
Large-scale, longitudinal studies are needed to move beyond
correlation and establish causality between specific light exposure
patterns and long-term health outcomes, such as the incidence of
metabolic disease or cognitive decline. Collaboration between
ophthalmologists and neuroscientists is essential for elucidating
the precise circuit-level mechanisms by which retinal diseases lead
to systemic symptoms and for co-developing novel therapies that
target the NIF system (101).
Similarly, partnerships with sleep physicians and psychiatrists
will be crucial for designing and implementing rigorous clinical
trials to expand the application of light therapy to a broader
range of disorders and to optimize its combination with
pharmacological or psychological treatments (102). By integrating multi-modal data
from genomics, neuroimaging and wearable physiological sensors into
comprehensive computational models, the scientific community can
hope to build a holistic, systems-level understanding of how light,
as perceived by the eye, shapes human health and well-being
(80).
Conclusion
The retina's functional scope extends far beyond its
role as an organ of sight; it is a critical neuroendocrine gateway
that translates environmental light into the fundamental language
of biological time. Retinal dysfunction, whether caused by disease
or injury, not only leads to visual impairment but is frequently
accompanied by a debilitating constellation of non-image-forming
deficits, including circadian arrhythmia and sleep disorders, which
underscores the imperative of maintaining retinal health for
systemic physiological stability. Concurrently, the unprecedented
light exposure patterns of modern life, dominated by indoor living
and nocturnal blue-light exposure, pose a continuous challenge to
the retina's photosensory mechanisms, exacerbating the risk of
circadian misalignment. The confluence of these pathological and
environmental factors profoundly affects the retina's ability to
regulate circadian rhythms and sleep, highlighting the pressing
need for scientifically-guided strategies to manage light exposure
and preserve retinal function. A deep and mechanistic understanding
of retinal photobiology, particularly the function of the
melanopsin-ipRGC system, provides a robust foundation for designing
precise and personalized light-based interventions (103). A clearer appreciation of the
NO-cGMP pathway in the SCN, alongside melanopsin-driven
glutamatergic and PACAP signaling, may refine light-based
interventions and improve interpretation of circadian vulnerability
in ophthalmic disease. Advanced light therapies and intelligent
lighting technologies hold immense promise for improving sleep
quality, stabilizing mood and preventing a host of rhythm-related
pathologies. The continued synergy of ophthalmology, neuroscience
and sleep medicine will drive further discovery, fostering the
development of novel diagnostic and therapeutic paradigms that
leverage the power of light to optimize human health.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Open Fund of the Key
Laboratory for Hubei Province (grant no. 2023KFZZ026).
Availability of data and materials
Not applicable.
Authors' contributions
MY conceived the study, conducted the literature
search, drafted the manuscript and designed the figures. HL
contributed to literature screening, data extraction and
verification, literature review and critical manuscript revision.
RZ participated in the literature search, manuscript drafting and
data analysis. YL collected literature, prepared tables and revised
the manuscript. SS supervised the overall project, was responsible
for manuscript writing and critical revision. AY supervised the
study and revised the manuscript. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Campbell I, Sharifpour R and Vandewalle G:
Light as a modulator of non-image-forming brain functions-positive
and negative impacts of increasing light availability. Clocks
Sleep. 5:116–140. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abbott KS, Queener HM and Ostrin LA: The
ipRGC-driven pupil response with light exposure, refractive error
and sleep. Optom Vis Sci. 95:323–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lazzerini Ospri L, Prusky G and Hattar S:
Mood, the circadian system and melanopsin retinal ganglion cells.
Annu Rev Neurosci. 40:539–556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stinchcombe AR, Hu C, Walch OJ, Faught SD,
Wong KY and Forger DB: M1-Type, but Not M4-Type, melanopsin
ganglion cells are physiologically tuned to the central circadian
clock. Front Neurosci. 15:6529962021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagle M, Zarei M, Lovett-Barron M, Poston
KT, Xu J, Ramey V, Pollard KS, Prober DA, Schulkin J, Deisseroth K
and Guo S: Brain-wide perception of the emotional valence of light
is regulated by distinct hypothalamic neurons. Mol Psychiatry.
27:3777–3793. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gooley JJ, Ho Mien I, St Hilaire MA, Yeo
SC, Chua EC, van Reen E, Hanley CJ, Hull JT, Czeisler CA and
Lockley SW: Melanopsin and rod-cone photoreceptors play different
roles in mediating pupillary light responses during exposure to
continuous light in humans. J Neurosci. 32:14242–14253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt TM, Chen SK and Hattar S:
Intrinsically photosensitive retinal ganglion cells: many subtypes,
diverse functions. Trends Neurosci. 34:572–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matynia A, Recio BS, Myers Z, Parikh S,
Goit RK, Brecha NC and Perez de Sevilla Muller L: Preservation of
intrinsically photosensitive retinal ganglion cells (ipRGCs) in
Late adult mice: Implications as a potential biomarker for early
onset ocular degenerative diseases. Invest Ophthalmol Vis Sci.
65:282024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ksendzovsky A, Pomeraniec IJ, Zaghloul KA,
Provencio JJ and Provencio I: Clinical implications of the
melanopsin-based non-image-forming visual system. Neurology.
88:1282–1290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mure LS: Intrinsically photosensitive
retinal ganglion cells of the human Retina. Front Neurol.
12:6363302021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang G, Liu YF, Yang Z, Yu CX, Tong Q,
Tang YL, Shao YQ, Wang LQ, Xu X, Cao H, et al: Short-term acute
bright light exposure induces a prolonged anxiogenic effect in mice
via a retinal ipRGC-CeA circuit. Sci Adv. 9:eadf46512023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vartanian GV, Zhao X and Wong KY: Using
flickering light to enhance nonimage-forming visual stimulation in
humans. Invest Ophthalmol Vis Sci. 56:4680–4688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aranda ML and Schmidt TM: Diversity of
intrinsically photosensitive retinal ganglion cells: Circuits and
functions. Cell Mol Life Sci. 78:889–907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moore RY: The suprachiasmatic nucleus and
the circadian timing system. Prog Mol Biol Transl Sci. 119:1–28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Do MTH: Melanopsin and the intrinsically
photosensitive retinal ganglion cells: Biophysics to behavior.
Neuron. 104:205–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mure LS, Hatori M, Ruda K, Benegiamo G,
Demas J and Panda S: Sustained melanopsin photoresponse is
supported by specific roles of β-Arrestin 1 and 2 in deactivation
and regeneration of photopigment. Cell Rep. 25:2497–2509.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lucas RJ: Chromophore regeneration:
Melanopsin does its own thing. Proc Natl Acad Sci USA.
103:10153–10154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SK, Sonoda T and Schmidt TM: M1
intrinsically photosensitive retinal ganglion cells integrate rod
and melanopsin inputs to signal in low light. Cell Rep.
29:3349–3355. e22019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lazarus C, Soheilypour M and Mofrad MR:
Torsional behavior of axonal microtubule bundles. Biophys J.
109:231–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
St Hilaire MA, Amundadottir ML, Rahman SA,
Rajaratnam SMW, Ruger M, Brainard GC, Czeisler CA, Andersen M,
Gooley JJ and Lockley SW: The spectral sensitivity of human
circadian phase resetting and melatonin suppression to light
changes dynamically with light duration. Proc Natl Acad Sci USA.
119:e22053011192022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Melelli A, Jamme F, Beaugrand J and
Bourmaud A: Evolution of the ultrastructure and polysaccharide
composition of flax fibres over time: When history meets science.
Carbohydr Polym. 291:1195842022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshikawa T, Obayashi K, Miyata K, Saeki K
and Ogata N: Association between postillumination pupil response
and glaucoma severity: A cross-sectional analysis of the LIGHT
study. Invest Ophthalmol Vis Sci. 63:242022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatori M and Panda S: The emerging roles
of melanopsin in behavioral adaptation to light. Trends Mol Med.
16:435–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt C, Collette F, Reichert CF, Maire
M, Vandewalle G, Peigneux P and Cajochen C: Pushing the Limits:
Chronotype and time of day modulate working memory-dependent
cerebral activity. Front Neurol. 6:1992015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blume C and Münch M: Effects of light on
biological functions and human sleep. Handb Clin Neurol. 206:3–16.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan D, Wang Z, Chen Y and Cao J:
Melanopsin-mediated optical entrainment regulates circadian rhythms
in vertebrates. Commun Biol. 6:10542023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Purrier N, Engeland WC and Kofuji P: Mice
deficient of glutamatergic signaling from intrinsically
photosensitive retinal ganglion cells exhibit abnormal circadian
photoentrainment. PLoS One. 9:e1114492014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Wu H, Liu N, Cao X, Yang Z, Lu B, Hu
R, Wang X and Wen J: Melatonin exerts an inhibitory effect on
insulin gene transcription via MTNR1B and the downstream Raf-1/ERK
signaling pathway. Int J Mol Med. 41:955–961. 2018.PubMed/NCBI
|
|
29
|
Brenna A, Ripperger JA, Saro G, Glauser
DA, Yang Z and Albrecht U: PER2 mediates CREB-dependent light
induction of the clock gene Per1. Sci Rep. 11:217662021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee B, Li A, Hansen KF, Cao R, Yoon JH and
Obrietan K: CREB influences timing and entrainment of the SCN
circadian clock. J Biol Rhythms. 25:410–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ono D, Weaver DR, Hastings MH, Honma KI,
Honma S and Silver R: The suprachiasmatic nucleus at 50: Looking
back, then looking forward. J Biol Rhythms. 39:135–165. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blume C, Cajochen C, Schollhorn I, Slawik
HC and Spitschan M: Effects of calibrated blue-yellow changes in
light on the human circadian clock. Nat Hum Behav. 8:590–605. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gubin D and Weinert D: Melatonin,
circadian rhythms and glaucoma: current perspective. Neural Regen
Res. 17:1759–1760. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao J, Provencio I and Liu X:
Intrinsically photosensitive retinal ganglion cells in glaucoma.
Front Cell Neurosci. 16:9927472022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Wang H, Wu S, Liu Q and Wang N:
Regulation of reentrainment function is dependent on a certain
minimal number of intact functional ipRGCs in rd Mice. J
Ophthalmol. 2017:68048532017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucas RJ: Mammalian inner retinal
photoreception. Curr Biol. 23:R125–R133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bilu C, Einat H, Zimmet P, Vishnevskia-Dai
V and Kronfeld-Schor N: Beneficial effects of daytime
high-intensity light exposure on daily rhythms, metabolic state and
affect. Sci Rep. 10:197822020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rahman SA, Brainard GC, Czeisler CA and
Lockley SW: Spectral sensitivity of circadian phase resetting,
melatonin suppression and acute alerting effects of intermittent
light exposure. Biochem Pharmacol. 191:1145042021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Starnes AN and Jones JR: Inputs and
outputs of the mammalian circadian clock. Biology (Basel).
12:5082023.PubMed/NCBI
|
|
40
|
Reghunandanan V and Reghunandanan R:
Neurotransmitters of the suprachiasmatic nuclei. J Circadian
Rhythms. 4:22006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ashton A, Foster RG and Jagannath A:
Photic entrainment of the circadian system. Int J Mol Sci.
23:7292022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Z, Beier C, Weil T and Hattar S: The
retinal ipRGC-preoptic circuit mediates the acute effect of light
on sleep. Nat Commun. 12:51152021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fernandez DC, Fogerson PM, Lazzerini Ospri
L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A,
Zhao H, et al: Light affects mood and learning through distinct
retina-brain pathways. Cell. 175:71–84. e182018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ostrin LA, Abbott KS and Queener HM:
Attenuation of short wavelengths alters sleep and the ipRGC pupil
response. Ophthalmic Physiol Opt. 37:440–450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rupp AC, Ren M, Altimus CM, Fernandez DC,
Richardson M, Turek F, Hattar S and Schmidt TM: Distinct ipRGC
subpopulations mediate light's acute and circadian effects on body
temperature and sleep. Elife. 8:e443582019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peplow M: Structure: The anatomy of sleep.
Nature. 497:S2–S3. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pilorz V, Tam SK, Hughes S, Pothecary CA,
Jagannath A, Hankins MW, Bannerman DM, Lightman SL, Vyazovskiy VV,
Nolan PM, et al: Melanopsin regulates both sleep-promoting and
arousal-promoting responses to light. PLoS Biol. 14:e10024822016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pun TB, Phillips CL, Marshall NS, Comas M,
Hoyos CM, D'Rozario AL, Bartlett DJ, Davis W, Hu W, Naismith SL, et
al: The effect of light therapy on electroencephalographic sleep in
sleep and circadian rhythm disorders: A scoping review. Clocks
Sleep. 4:358–373. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang C, Yang W, Chen Y, Cheng Q and Chen
W: Improving renoprotective effects by adding piperazine ferulate
and angiotensin receptor blocker in diabetic nephropathy: A
meta-analysis of randomized controlled trials. Int Urol Nephrol.
54:299–307. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Atan YS, Subasi M, Guzel Ozdemir P and
Batur M: The effect of blindness on biological rhythms and the
consequences of circadian rhythm disorder. Turk J Ophthalmol.
53:111–119. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Besharse JC and McMahon DG: The retina and
other light-sensitive ocular clocks. J Biol Rhythms. 31:223–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Poggiogalle E, Jamshed H and Peterson CM:
Circadian regulation of glucose, lipid and energy metabolism in
humans. Metabolism. 84:11–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Verlande A, Chun SK, Goodson MO, Fortin
BM, Bae H, Jang C and Masri S: Glucagon regulates the stability of
REV-ERBα to modulate hepatic glucose production in a model of lung
cancer-associated cachexia. Sci Adv. 7:eabf38852021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Papazyan R, Damle M, Fang B,
Jager J, Feng D, Peed LC, Guan D, Sun Z and Lazar MA: The hepatic
circadian clock fine-tunes the lipogenic response to feeding
through RORα/γ. Genes Dev. 31:1202–1211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sengupta S, Tang SY, Devine JC, Anderson
ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, López CB
and FitzGerald GA: Circadian control of lung inflammation in
influenza infection. Nat Commun. 10:41072019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Montanari T, Boschi F and Colitti M:
Comparison of the effects of browning-inducing capsaicin on two
murine adipocyte models. Front Physiol. 10:13802019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reutrakul S, Crowley SJ, Park JC, Chau FY,
Priyadarshini M, Hanlon EC, Danielson KK, Gerber BS, Baynard T, Yeh
JJ and McAnany JJ: Relationship between intrinsically
photosensitive ganglion cell function and circadian regulation in
diabetic retinopathy. Sci Rep. 10:15602020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee J, Moulik M, Fang Z, Saha P, Zou F, Xu
Y, Nelson DL, Ma K, Moore DD and Yechoor VK: Bmal1 and β-cell clock
are required for adaptation to circadian disruption and their loss
of function leads to oxidative stress-induced β-cell failure in
mice. Mol Cell Biol. 33:2327–2338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wheaton KL, Hansen KF, Aten S, Sullivan
KA, Yoon H, Hoyt KR and Obrietan K: The Phosphorylation of CREB at
serine 133 is a key event for circadian clock timing and
entrainment in the suprachiasmatic nucleus. J Biol Rhythms.
33:497–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Figueiro MG, Plitnick B and Rea MS: Light
modulates leptin and ghrelin in sleep-restricted adults. Int J
Endocrinol. 2012:5307262012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Papantoniou K, Devore EE, Massa J,
Strohmaier S, Vetter C, Yang L, Shi Y, Giovannucci E, Speizer F and
Schernhammer ES: Rotating night shift work and colorectal cancer
risk in the nurses' health studies. Int J Cancer. 143:2709–2717.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zeng Y, Guo Z, Wu M, Chen F and Chen L:
Circadian rhythm regulates the function of immune cells and
participates in the development of tumors. Cell Death Discov.
10:1992024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mayo JC, Hevia D, Quiros-Gonzalez I,
Rodriguez-Garcia A, Gonzalez-Menendez P, Cepas V, Gonzalez-Pola I
and Sainz RM: IGFBP3 and MAPK/ERK signaling mediates
melatonin-induced antitumor activity in prostate cancer. J Pineal
Res. Nov 9–2017.(Epub ahead of print). View Article : Google Scholar
|
|
64
|
Chen K, Zhu P, Chen W, Luo K, Shi XJ and
Zhai W: Melatonin inhibits proliferation, migration and invasion by
inducing ROS-mediated apoptosis via suppression of the
PI3K/Akt/mTOR signaling pathway in gallbladder cancer cells. Aging
(Albany NY). 13:22502–22515. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Davis S, Mirick DK and Stevens RG: Night
shift work, light at night and risk of breast cancer. J Natl Cancer
Inst. 93:1557–1562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rayatdoost E, Rahmanian M, Sanie MS,
Rahmanian J, Matin S, Kalani N, Kenarkoohi A, Falahi S and Abdoli
A: Sufficient sleep, time of vaccination and vaccine efficacy: A
systematic review of the current evidence and a proposal for
COVID-19 vaccination. Yale J Biol Med. 95:221–235. 2022.PubMed/NCBI
|
|
67
|
Liu X, Li H, Ma R, Tong X, Wu J, Huang X,
So KF, Tao Q, Huang L, Lin S and Ren C: Burst firing in
output-defined parallel habenula circuit underlies the
antidepressant effects of bright light treatment. Adv Sci (Weinh).
11:e24010592024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang L, Xi Y, Peng Y, Yang Y, Huang X, Fu
Y, Tao Q, Xiao J, Yuan T, An K, et al: A visual circuit related to
habenula underlies the antidepressive effects of light therapy.
Neuron. 102:128–142. e82019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lazzerini Ospri L, Zhan JJ, Thomsen MB,
Wang H, Komal R, Tang Q, Messanvi F, du Hoffmann J, Cravedi K,
Chudasama Y, et al: Light affects the prefrontal cortex via
intrinsically photosensitive retinal ganglion cells. Sci Adv.
10:eadh92512024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hirakawa H, Terao T, Muronaga M and Ishii
N: Adjunctive bright light therapy for treating bipolar depression:
A systematic review and meta-analysis of randomized controlled
trials. Brain Behav. 10:e018762020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Burns AC, Saxena R, Vetter C, Phillips
AJK, Lane JM and Cain SW: Time spent in outdoor light is associated
with mood, sleep and circadian rhythm-related outcomes: A
cross-sectional and longitudinal study in over 400,000 UK Biobank
participants. J Affect Disord. 295:347–352. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zangen E, Hadar S, Lawrence C, Obeid M,
Rasras H, Hanzin E, Aslan O, Zur E, Schulcz N, Cohen-Hatab D, et
al: Prefrontal cortex neurons encode ambient light intensity
differentially across regions and layers. Nat Commun. 15:55012024.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen G, Chen P, Yang Z, Ma W, Yan H, Su T,
Zhang Y, Qi Z, Fang W, Jiang L, et al: Increased functional
connectivity between the midbrain and frontal cortex following
bright light therapy in subthreshold depression: A randomized
clinical trial. Am Psychol. 79:437–450. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Marsden WN: Synaptic plasticity in
depression: Molecular, cellular and functional correlates. Prog
Neuropsychopharmacol Biol Psychiatry. 43:168–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bedrosian TA and Nelson RJ: Timing of
light exposure affects mood and brain circuits. Transl Psychiatry.
7:e10172017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Khodasevich D, Tsui S, Keung D, Skene DJ,
Revell V and Martinez ME: Characterizing the modern light
environment and its influence on circadian rhythms. Proc Biol Sci.
288:202107212021.PubMed/NCBI
|
|
77
|
Lee J, Liu R, de Jesus D, Kim BS, Ma K,
Moulik M and Yechoor V: Circadian control of β-cell function and
stress responses. Diabetes Obes Metab. 17 (Suppl 1):S123–S133.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tosini G, Ferguson I and Tsubota K:
Effects of blue light on the circadian system and eye physiology.
Mol Vis. 22:61–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hanifin JP, Lockley SW, Cecil K, West K,
Jablonski M, Warfield B, James M, Ayers M, Byrne B, Gerner E, et
al: Randomized trial of polychromatic blue-enriched light for
circadian phase shifting, melatonin suppression and alerting
responses. Physiol Behav. 198:57–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stefani O, Schollhorn I and Munch M:
Towards an evidence-based integrative lighting score: A proposed
multi-level approach. Ann Med. 56:23812202024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
He M, Chen H, Li S, Ru T, Chen Q and Zhou
G: Evening prolonged relatively low melanopic equivalent daylight
illuminance light exposure increases arousal before and during
sleep without altering sleep structure. J Sleep Res. 33:e141132024.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vethe D, Drews HJ, Scott J, Engstrøm M,
Heglum HSA, Grønli J, Wisor JP, Sand T, Lydersen S, Kjørstad K, et
al: Evening light environments can be designed to consolidate and
increase the duration of REM-sleep. Sci Rep. 12:87192022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Russart KLG, Chbeir SA, Nelson RJ and
Magalang UJ: Light at night exacerbates metabolic dysfunction in a
polygenic mouse model of type 2 diabetes mellitus. Life Sci.
231:1165742019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lam C and Chung MH: Dose-response effects
of light therapy on sleepiness and circadian phase shift in shift
workers: A meta-analysis and moderator analysis. Sci Rep.
11:119762021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mason IC, Qian J, Adler GK and Scheer
FAJL: Impact of circadian disruption on glucose metabolism:
Implications for type 2 diabetes. Diabetologia. 63:462–472. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Engin A: Misalignment of circadian rhythms
in diet-induced obesity. Adv Exp Med Biol. 1460:27–71. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Barber LE, VoPham T, White LF, Roy HK,
Palmer JR and Bertrand KA: Circadian disruption and colorectal
cancer incidence in black women. Cancer Epidemiol Biomarkers Prev.
32:927–935. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fonken LK, Aubrecht TG, Melendez-Fernandez
OH, Weil ZM and Nelson RJ: Dim light at night disrupts molecular
circadian rhythms and increases body weight. J Biol Rhythms.
28:262–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liset R, Gronli J, Henriksen RE, Henriksen
TEG, Nilsen RM and Pallesen S: A randomized controlled trial on the
effect of blue-blocking glasses compared to partial blue-blockers
on melatonin profile among nulliparous women in third trimester of
the pregnancy. Neurobiol Sleep Circadian Rhythms. 12:1000742021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Esaki Y, Kitajima T, Ito Y, Koike S, Nakao
Y, Tsuchiya A, Hirose M and Iwata N: Wearing blue light-blocking
glasses in the evening advances circadian rhythms in the patients
with delayed sleep phase disorder: An open-label trial. Chronobiol
Int. 33:1037–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Arora S, Houdek P, Cajka T, Dockal T,
Sladek M and Sumova A: Chronodisruption that dampens output of the
central clock abolishes rhythms in metabolome profiles and elevates
acylcarnitine levels in the liver of female rats. Acta Physiol
(Oxf). 241:e142782025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hester L, Dang D, Barker CJ, Heath M,
Mesiya S, Tienabeso T and Watson K: Evening wear of blue-blocking
glasses for sleep and mood disorders: A systematic review.
Chronobiol Int. 38:1375–1383. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ru T, Kompier ME, Chen Q, Zhou G and
Smolders KC: Temporal tuning of illuminance and spectrum: Effect of
a full-day dynamic lighting pattern on well-being, performance and
sleep in simulated office environment. Building and Environment.
228:1098422023. View Article : Google Scholar
|
|
94
|
Grant LK, Crosthwaite PC, Mayer MD, Wang
W, Stickgold R, St Hilaire MA, Lockley SW and Rahman SA:
Supplementation of ambient lighting with a task lamp improves
daytime alertness and cognitive performance in sleep-restricted
individuals. Sleep. 46:zsad0962023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Oh AJ, Amore G, Sultan W, Asanad S, Park
JC, Romagnoli M, La Morgia C, Karanjia R, Harrington MG and Sadun
AA: Pupillometry evaluation of melanopsin retinal ganglion cell
function and sleep-wake activity in pre-symptomatic Alzheimer's
disease. PLoS One. 14:e02261972019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Berg DJ, Kartheiser K, Leyrer M, Saali A
and Berson DM: Transcriptomic signatures of postnatal and adult
intrinsically photosensitive ganglion cells. eNeuro.
6:ENEURO.0022–19.2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liberman AR, Kwon SB, Vu HT, Filipowicz A,
Ay A and Ingram KK: Circadian Clock Model Supports Molecular Link
Between PER3 and Human Anxiety. Sci Rep. 7:98932017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chellappa SL: Individual differences in
light sensitivity affect sleep and circadian rhythms. Sleep.
44:zsaa2142021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Schollhorn I, Stefani O, Lucas RJ,
Spitschan M, Epple C and Cajochen C: The impact of pupil
constriction on the relationship between melanopic EDI and
melatonin suppression in young adult males. J Biol Rhythms.
39:282–294. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Juda M, Liu-Ambrose T, Feldman F, Suvagau
C and Mistlberger RE: Light in the senior home: Effects of dynamic
and individual light exposure on sleep, cognition and well-being.
Clocks Sleep. 2:557–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Windred DP, Burns AC, Rutter MK, Ching
Yeung CH, Lane JM, Xiao Q, Saxena R, Cain SW and Phillips AJK:
Personal light exposure patterns and incidence of type 2 diabetes:
Analysis of 13 million hs of light sensor data and 670,000
person-years of prospective observation. Lancet Reg Health Eur.
42:1009432024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zauner J, Udovicic L and Spitschan M:
Power analysis for personal light exposure measurements and
interventions. PLoS One. 19:e03087682024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Stewart D and Albrecht U: Beyond vision:
Effects of light on the circadian clock and mood-related
behaviours. NPJ Biol Timing Sleep. 2:122025. View Article : Google Scholar : PubMed/NCBI
|