Introduction
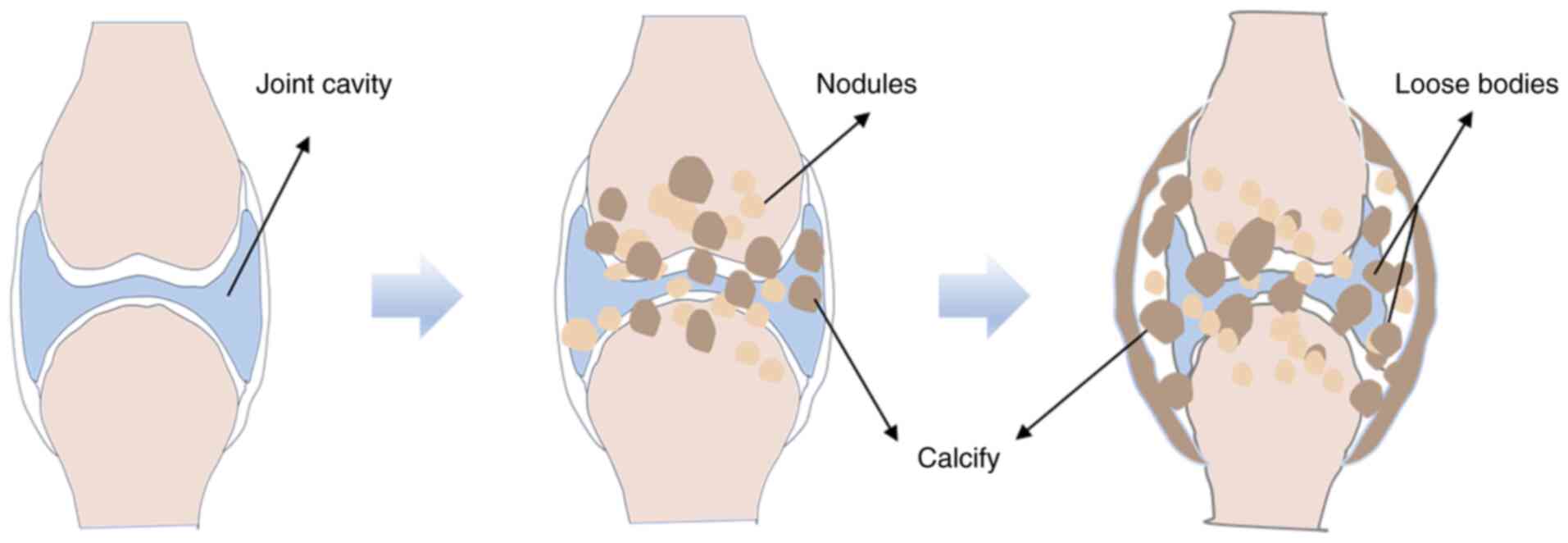
Synovial chondromatosis (SC), also known as synovial
osteochondromatosis, is a relatively rare benign synovial lesion.
The hallmark feature of SC is the formation of multiple cartilage
nodules within the synovium of joints, tendon sheaths and bursae.
Some of these nodules may detach into the joint cavity, forming
loose bodies (1,2) (Fig.
1). SC largely affects the knee joint, followed by the hip,
shoulder, elbow, ankle and wrist joints (3). It is less commonly observed in the
metacarpophalangeal, interphalangeal, acromioclavicular,
temporomandibular and intervertebral joints (4–6).
Extraglenoid SC is commonly observed in the hands, feet, wrists and
ankles, typically presenting as localized, painless masses
(7). Clinically, SC can range from
asymptomatic to symptomatic, with manifestations including joint
swelling, pain, limited range of motion, recurrent effusion,
crepitus and locking phenomena (3,8). In
certain cases, palpable masses may be detected and severe cases may
involve neurovascular compromise (3,8,9). SC
is generally classified into primary SC (PSC) or secondary SC (SSC)
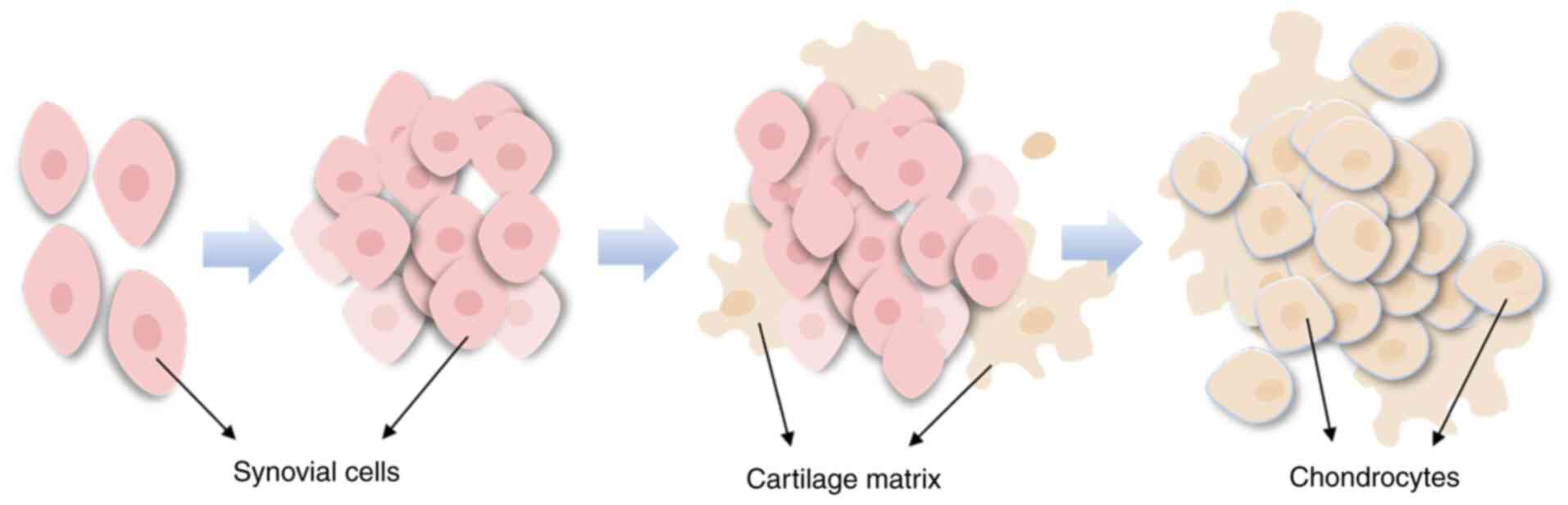
based on its etiology and histopathological features. PSC
originates from the normal synovium and is characterized by the
abnormal chondroid metaplasia of synovial cells (Fig. 2), with an increased number of loose
bodies that are widely distributed and a greater recurrence rate
(9,10). By contrast, SSC is often induced by
conditions such as osteoarthritis or joint trauma, with cartilage
fragments embedded in the synovium, triggering chondroid
metaplasia. SSC typically presents with fewer but larger loose
bodies and imaging studies often show characteristic changes
related to the underlying primary disease (10,11).
Although SC is a benign lesion, it has a high recurrence rate, and
certain cases may transform into low-grade synovial sarcoma (SS)
(9). Therefore, in-depth research
into the pathogenesis of SC is essential for optimizing clinical
diagnosis and treatment. The present review summarizes the latest
advancements in SC research, focusing on its epidemiological
trends, pathogenesis, clinical strategies and malignant potential.
The aim of the present review is to highlight avenues to improve
clinical recognition, optimize treatment plans and reduce the risk
of recurrence and malignant transformation.
Epidemiology of SC
Incidence rate
SC is a relatively rare condition with an incidence
of ~1 case per 100,000 individuals. It primarily affects adults
between the ages of 30 and 50 years, although both infants and the
elderly can also be affected (1,12,13).
Earlier studies suggested that the male-to-female ratio of SC was
1.8:1, indicating a higher prevalence in males (12,13).
However, other studies suggest that this ratio may range from 2:1
to 4:1 (14). Notably, in areas
such as the temporomandibular joint, hands and wrists, the
incidence in females may be higher compared with males (15). Furthermore, due to the
often-insidious symptoms of SC, the average time to diagnosis is ~5
years (15). However, with
advancements in imaging technology, the detection rate of SC has
markedly increased over the years (8). The higher incidence of SC in adults
may be associated with long-term joint stress and a history of
trauma, while the rarity of SC in children may be related to
protective mechanisms during skeletal development (14). Additionally, PSC and SSC exhibit
notable differences in epidemiology. PSC is generally more common
and has a higher recurrence rate, whereas SSC is primarily
associated with osteoarthritis or joint injuries, being relatively
rare and often confused with primary diseases (9–11,16).
Extraglenoid SC is rare, typically presenting as a painless mass
and there is no notable trend associated with sex or age in these
cases (14).
Recurrence rate and malignant
transformation rate
The recurrence rate of SC ranges from 0–31%, with
the recurrence rate being lower when loose body removal and
synovectomy are carried out via arthroscopy (~7.1%) (17,18).
However, if the synovectomy is incomplete, the recurrence rate may
rise to 20–30% (19). PSC has an
increased recurrence rate compared with SSC, suggesting that PSC
may exhibit more biological activity (9–11).
Multiple recurrences may increase the risk of transformation into
SS. Overall, the risk of malignant transformation in SC is low,
typically ~5% (20). However, a
study has reported a malignant transformation rate of ≤11.1% in hip
joint SC cases (21), although
this value may be lower in actual clinical practice due to limited
follow-up times. Malignant transformation in SC is typically
observed in cases with a disease duration of >5 years or in
recurrent cases. Symptoms such as pain, swelling and local
destruction may occur, and certain patients may exhibit cartilage
matrix calcification and nuclear atypia, which resemble the
features of low-grade SS (14).
Compared with SSC, PSC carries a higher risk of malignant
transformation, and gene fusions such as FN1-ACVR2A and chromosomal
abnormalities have been detected in certain cases (22). However, to the best of our
knowledge, there are currently no clear predictive markers for
malignant transformation, emphasizing the need for long-term
follow-up and biological assessments.
In summary, SC has a low incidence, delayed
diagnosis and a higher prevalence in males. The recurrence rate of
PSC is higher than that of SSC, with stronger biological activity,
which may increase the risk of malignancy. The overall malignancy
rate of SC is low, but cases with long-term recurrence may have
potential for malignant transformation. Currently, epidemiological
data on SC are limited and the mechanisms of malignancy remain
unclear, with no effective predictive indicators. Further research
is required on its pathogenesis, diagnostic methods and long-term
prognosis.
Novel advances in the pathogenesis of
SC
Although the exact pathogenesis of SC remains
incompletely understood, recent research has revealed several
potential mechanisms underlying the disease. According to the 2020
edition of the World Health Organization classification, SC has
been categorized as a neoplastic disease due to its local
invasiveness and clonal chromosomal changes (23). Milgram (24) categorized the pathological
progression of SC into three stages: Synovial hyperplasia (Stage
I), free body formation (Stage II) and stabilization (Stage III),
although this staging system does not accurately predict disease
progression. By contrast, Gerard et al (25) classified SC into four stages based
on changes in synovial activity: Early chondrification, free body
formation, cartilage nodule maturation and synovial atrophy. This
staging system is more effective for evaluating disease activity
and determining the timing of surgical intervention (25).
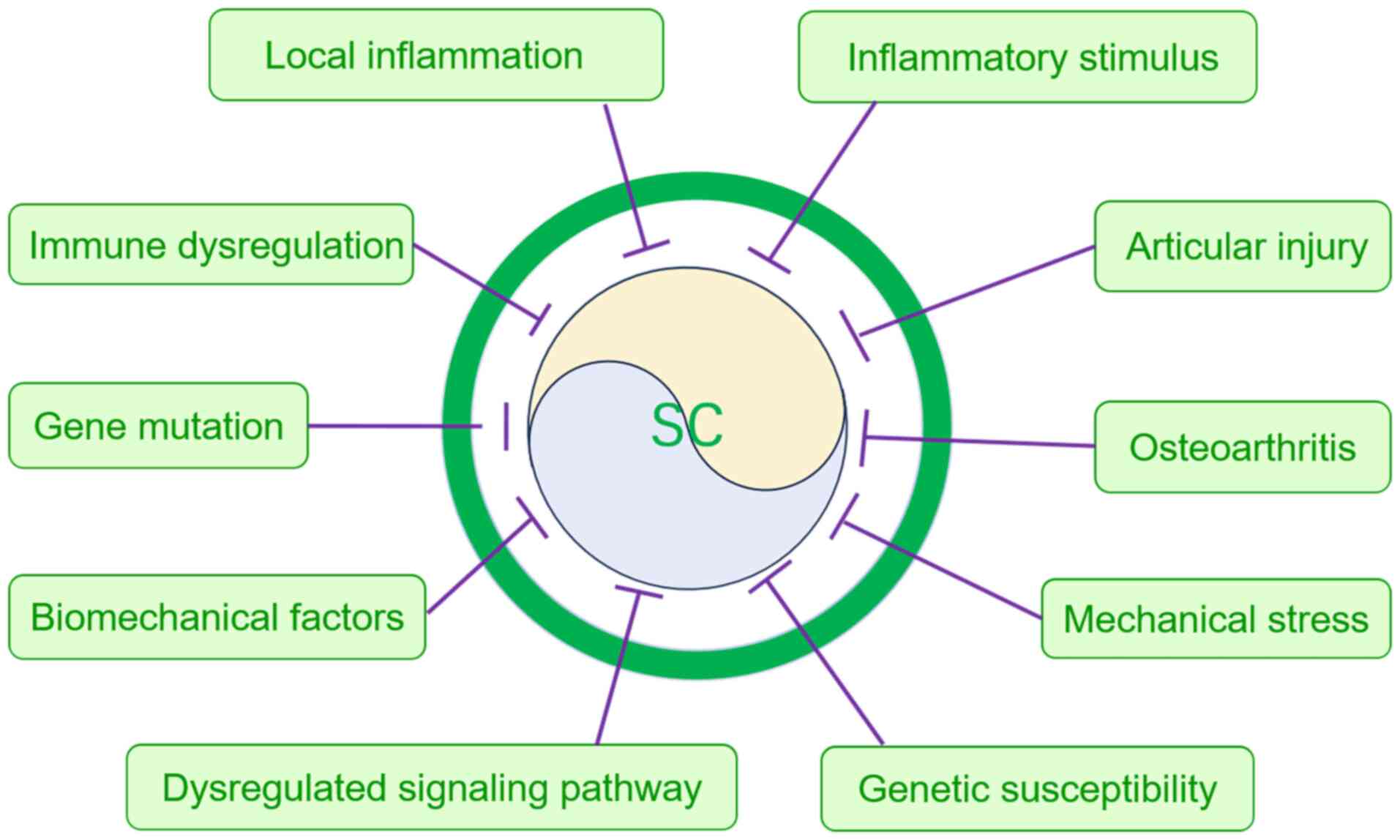
Regarding the pathogenesis of SC, current studies
primarily focus on the differential pathological origins of PSC and
SSC (7,11,26,27).
PSC is hypothesized to result from abnormal chondrification of
synovial cells, potentially associated with genetic mutations,
signaling pathway abnormalities, inflammatory stimuli and
mechanical stress (7,11,26,27).
By contrast, SSC is more commonly triggered by chronic stimuli such
as osteoarthritis or joint injury, primarily resulting from
prolonged exposure of the synovium to mechanical stress or the
embedding of cartilage fragments into the synovium (7,11).
In terms of risk factors, PSC is frequently associated with genetic
susceptibility, immune abnormalities and signaling pathway
activation, while SSC is more likely to be influenced by local
inflammation and biomechanical factors (Fig. 3) (26,27).
Recently, research on the pathogenesis of SC has shifted towards
the regulation of signaling pathways, changes in the synovial
microenvironment and genetic characteristics. Several potential
pathogenic mechanisms have been proposed in previous studies
(28–33). This section discusses the
pathogenesis of SC in terms of signaling pathway abnormalities,
inflammatory microenvironment and mechanical stress (Table I).
 | Table I.Key signaling pathways and their
mechanisms in synovial chondromatosis with experimental
evidence. |
Table I.
Key signaling pathways and their
mechanisms in synovial chondromatosis with experimental
evidence.
| Signaling
pathway | Key regulators and
effectors | Biological
function | Experimental
evidence | (Refs.) |
|---|
| Hedgehog | GLI1, PTC1, Gli3,
Cyclin D1, Col2A1 | Synovial cell
proliferation, accelerated chondrogenesis | PTC1 and GLI1 gene
expression in PSC tissues increased 8-fold and 6-fold,
respectively, compared with normal synovium tissue, in
Gli3-deficient mouse models, Hedgehog pathway inhibition reduced SC
lesion incidence by 50%. | (28) |
| TGF-β | TGF-β1, Smad2/3,
Aggrecan, Sox9 | Cartilage matrix
synthesis, enhanced cell migration | Animal models
confirmed TGF-β1 overexpression directly induces synovial
fibroblast differentiation into chondrocytes (223-fold increase in
type II collagen), TGF-β1 drives chondrometaplasia via MMP-13
(300-fold) and osteopontin (130-fold) upregulation. | (30) |
| FGF9/FGFR3 | FGF9, MAPK/ERK,
c-Myc, Bcl-2 | Chondrometaplasia,
apoptosis | FGF9 levels
elevated 3-5-fold in PSC tissues, FGFR3 inhibitors reduced
cartilage nodules by 62%. | (29,32) |
| IL-6 | IL-6, STAT3,
VEGF-A, MMPs | Angiogenesis,
cartilage degradation | IL-6 concentration
in SC synovial fluid was 150-fold higher than the controls, VEGF-A
levels in the lesion group were 60-fold higher than in
controls. | (31) |
| Mechanical
stress | TNF-α, CD105,
Ca2+ | Adaptive synovial
hyperplasia, osteophyte formation | Stress stimulation
upregulates TNF-α/CD105. | (31,33) |
The role of signaling pathways in the
pathogenesis of SC
Occurrence of SC involves multiple signaling
pathways, with the Hedgehog, FGF9/FGFR3 and TGF-β pathways being
considered the most key (28–30)
(Fig. 4). Overactivation of the
Hedgehog signaling pathway induces abnormal proliferation of
synovial cells and promotes their differentiation into
chondrocytes. A study has shown that elevated expression of PTC1
and GLI1 genes in PSC patient tissues suggests that the Hedgehog
signaling pathway may carry out a pivotal role in SC pathogenesis
(28). Furthermore, the FGF9/FGFR3
pathway promotes chondrification of synovial cells through the
abnormal activation of the MAPK/ERK signaling axis, accelerating
disease progression. The notably elevated FGF9 levels in tissues of
patients with PSC further corroborate this mechanism (29). The abnormal activation of the TGF-β
signaling pathway also carries out a significant role in the
pathogenesis of SC, as TGF-β1 promotes synovial cell proliferation
and cartilage matrix formation via both Smad-dependent and
independent pathways, exacerbating disease progression (30). In addition to these pathways,
TGF-β3, FGF2 and IL-6 may also contribute to SC development
(31,32,34).
Although these signaling pathway abnormalities have been confirmed,
their specific roles in different stages and subtypes of SC require
further investigation (28–32,34).
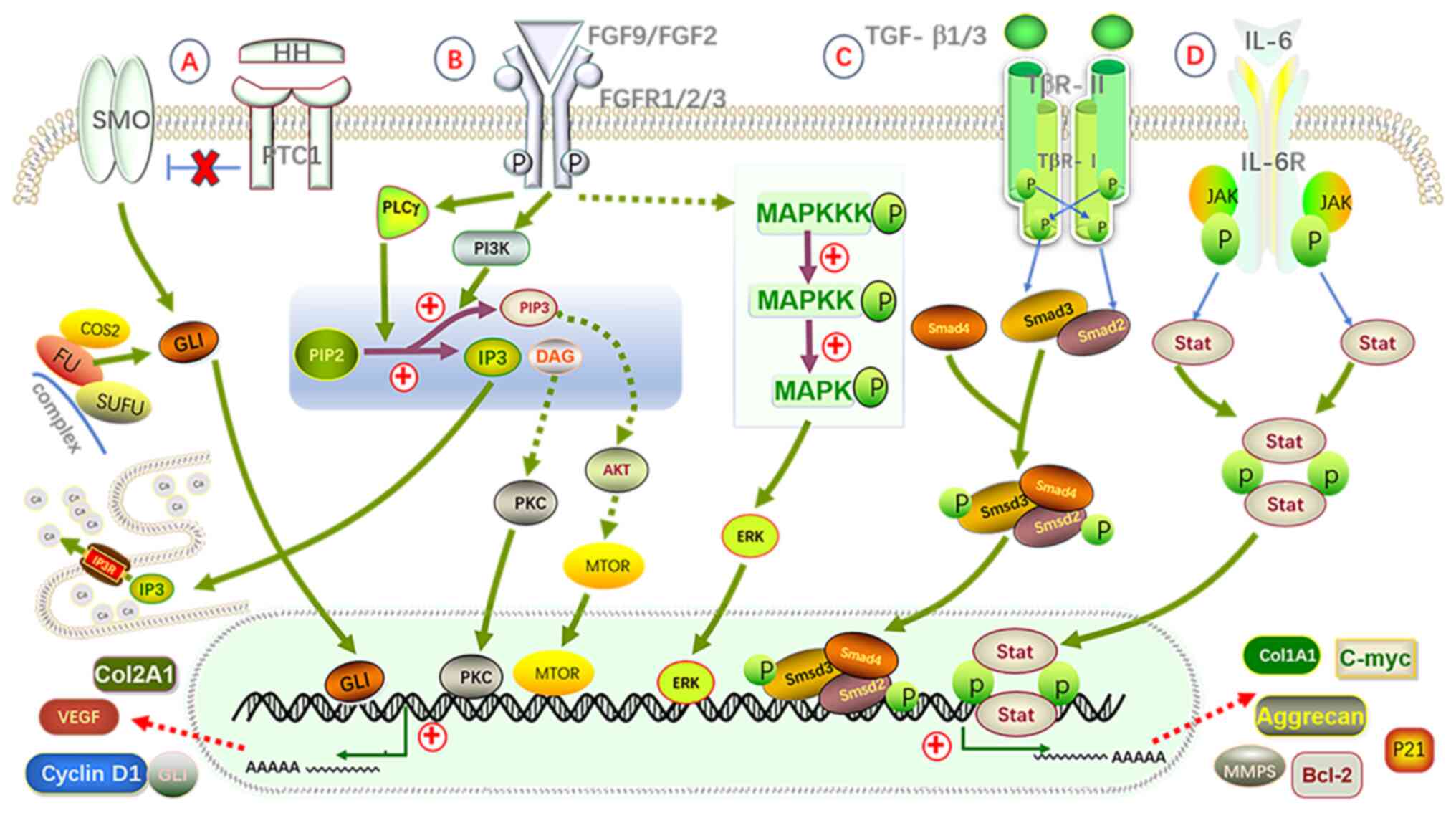 | Figure 4.Schematic diagram of the roles of
four major signaling pathways (Hedgehog, FGF, TGF-β and IL-6) in
the pathogenesis of SC. (A) Hedgehog pathway: The HH ligand binds
to the PTC1 receptor, relieving the inhibition of the SMO receptor,
which activates Gli proteins and causes them to dissociate from the
complex formed by COS2, FU and SUFU. This activation subsequently
upregulates the expression of genes such as Cyclin D1, Col2A1 and
Aggrecan. (B) FGF pathway: After the FGF ligand binds to the FGFR
receptor, it activates the IP3-DAG-PKC, MAPK/ERK and PI3K/Akt
signaling pathways, leading to the expression of genes including
calcium ions, Cyclin D1, Bcl-2 and c-Myc. (C) TGF-β pathway: The
TGF-β ligand binds to the TGF-βRII receptor, activating the
tyrosine phosphorylation of the TGF-βRI receptor, which in turn
activates the Smad2/3 signaling pathway. The activated Smad complex
then enters the nucleus, regulating genes associated with
chondrogenesis and cell proliferation, such as Col2A1, Aggrecan,
Sox9 and Cyclin D1. (D) IL-6 pathway: IL-6 binds to its receptor
IL-6R, activating the JAK/STAT signaling pathway, which promotes
the expression of Cyclin D1, c-Myc, Bcl-2 and VEGF. Ultimately, the
upregulation of these genes promotes cell proliferation, inhibits
apoptosis, accelerates synovial cell differentiation and
chondrogenesis, and thus facilitates the formation of SC. Bcl-2,
B-cell lymphoma 2; c-Myc, cellular Myc; Col2A1, collagen type IIα1;
DAG, diacylglycerol; FGFR, fibroblast growth factor receptor; FGF,
fibroblast growth factor; Gli, Glioma-associated oncogene homolog;
HH, Hedgehog; IL-6, interleukin 6; IL-6R, IL-6 receptor; IP3,
inositol 1,4,5-trisphosphate; IP3R, inositol 1,4,5-trisphosphate
receptor; JAK/STAT, Janus kinase/signal transducer and activator of
transcription; MAPK/ERK, mitogen-activated protein
kinase/extracellular signal-regulated kinase; MMPs, matrix
metalloproteinases; mTOR, mechanistic target of rapamycin;
PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; PIP2,
phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PTC1,
Patched 1; SC, synovial chondromatosis; Smad2/3/4, Smad family
proteins 2/3/4; Sox9, SRY-box 9; SMO, Smoothened; SUFU, suppressor
of FU; TGF-βRI, transforming growth factor-β receptor I; VEGF,
vascular endothelial growth factor; COS2, Costal 2; FU, Fused. |
Role of the inflammatory
microenvironment in SC pathogenesis
The development of SC is associated with its
inflammatory microenvironment (Fig.
5). A study has shown that IL-6 and VEGF-A levels are
considerably elevated in the synovial fluid of patients with SC,
where IL-6 enhances inflammation via the JAK/STAT signaling pathway
and VEGF-A promotes angiogenesis, potentially leading to chronic
inflammation and accelerating disease progression (31). In patients with PSC, an increase in
CD68+ synovial macrophages and human leukocyte antigen-DR-positive
cells indicates that immune dysregulation carries out a key role in
the pathogenesis of PSC (35). At
the molecular level, the co-expression of COL3A1 and CD90
synergistically promotes the synthesis of glycosaminoglycans and
COL2A1, enhancing chondrocyte migration and proliferation. These
molecules contribute to the pathogenesis of SC through immune and
metabolic pathways and may serve as potential diagnostic markers to
distinguish PSC from SSC (36).
Additionally, the overlapping expression of biomarkers such as
PCNA, CD90, CD105, IGF-1 and TGF-β1 in PSC cartilage nodules
highlights the complex interactions between cell proliferation,
mesenchymal stem cells and cartilage formation (37). Elevated expression of BMP-2 and
BMP-4 in SC lesions further supports their key role in
chondrification (38), while
overexpression of C-erbB-2 in certain PSC tissue samples may be
associated with synovial cell proliferation and disruptions in
signaling pathways (39).
Moreover, abnormal proliferation of CD34+ progenitor cells, in
conjunction with TWIST1, TGF-β1 and Harmane, may promote osteogenic
differentiation, accelerating chondrification and calcification
processes (40). Synovial
hyperplasia, angiogenesis and proteoglycan deposition are key
pathological features of SC lesions (31). By contrast, the inflammatory
microenvironment in SSC is primarily driven by joint damage, with
its inflammatory state closely associated with the progression of
the primary disease. Furthermore, synovial macrophages, through
M1/M2 polarization, carry out a key role in cartilage degradation
and regeneration. M1 macrophages promote inflammation and cartilage
degradation, while M2 macrophages support cartilage repair and
regeneration (35).
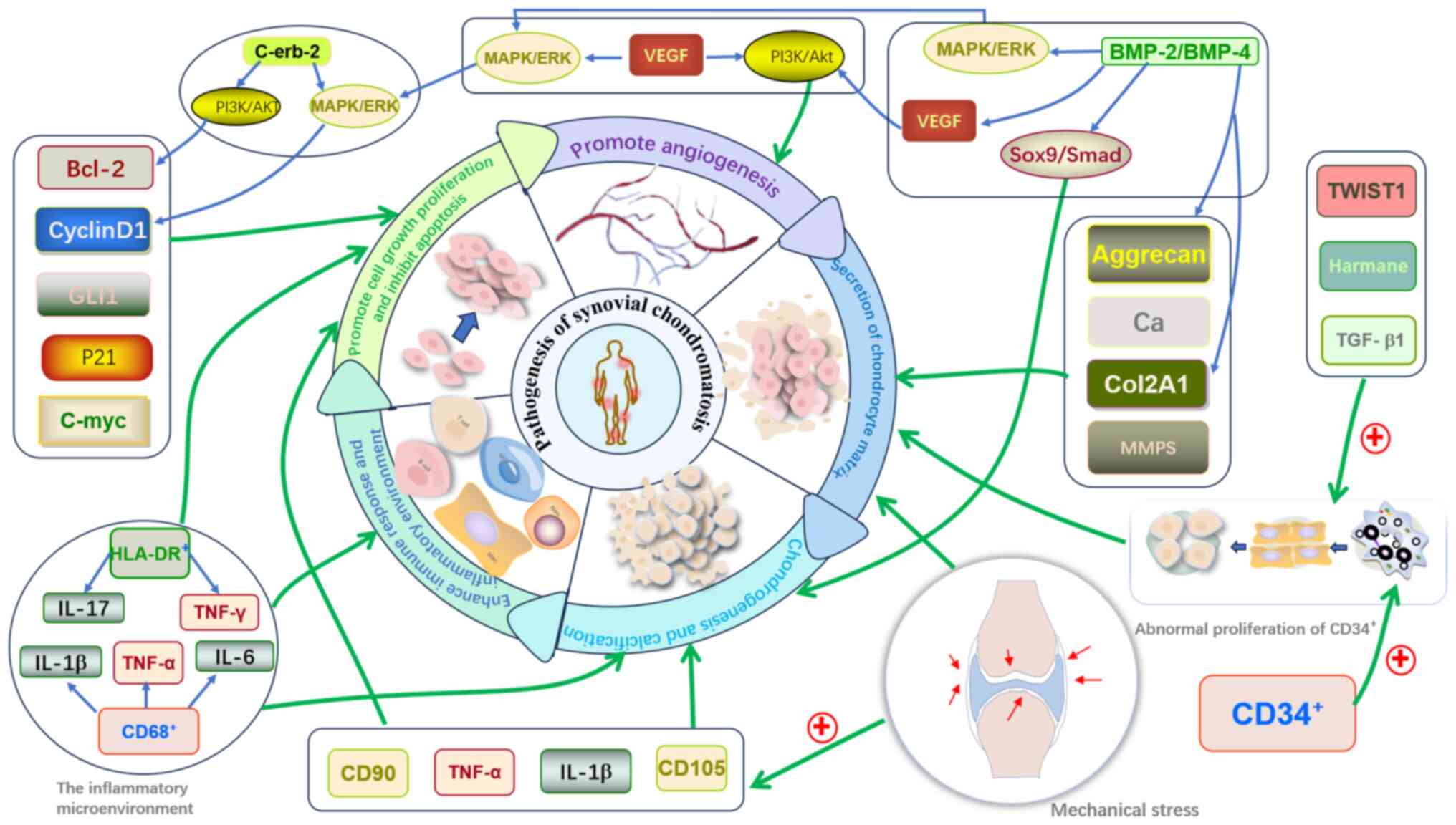 | Figure 5.Schematic diagram of the impact of
signaling pathway regulatory products, inflammatory
microenvironment and mechanical stress on the pathogenesis of SC.
i) Impact of factors: Bcl-2, Cyclin D1, GLI1, P21 and c-Myc
primarily promote the growth and proliferation of synovial cells
and inhibit apoptosis. Aggrecan, calcium ions, Col2A1 and MMPs
promote the synthesis of cartilage matrix and cartilage repair.
VEGF-A promotes cell proliferation and angiogenesis through the
MAPK/ERK and PI3K/Akt pathways. ii) Impact of an inflammatory
microenvironment: CD+macrophages and
HLA-DR+cells secrete inflammatory cytokines, such as
IL-1β, TNF-α, IL-6, IL-17 and IFN-γ. These cytokines enhance
synovial inflammation, strengthen immune responses and accelerate
synovial cell proliferation. iii) Impact of mechanical stress:
Mechanical stress upregulates the expression of TNF-α, IL-1β, CD105
and CD90, promoting synovial cell proliferation, cartilage matrix
synthesis, chondrogenesis and calcification, further driving the
formation of chondromas. iv) Impact of other factors: Other
factors, such as C-erbB-2, promote the proliferation of synovial
cells through the MAPK/ERK and PI3K/Akt pathways and inhibit
apoptosis. The abnormal proliferation of CD34+progenitor
cells provides a source of cells for chondrogenesis and
calcification. TWIST1, TGF-β1 and harmine act synergistically to
further promote the formation and progression of SC. These factors
and mechanisms interact through different signaling pathways,
collectively driving the occurrence and development of SC. Bcl-2,
B-cell lymphoma 2; BMP-2/4, bone morphogenetic protein 2/4; c-Myc,
cellular Myc; Col2A1, collagen type IIα1; erbB-2, receptor
tyrosine-protein kinase ErbB-2; Gli1, glioma-associated oncogene
homolog 1; HLA-DR+, human leukocyte antigen-DR;
IL-6/17/1β, interleukin 6/17/1β; MAPK/ERK, mitogen-activated
protein kinase/extracellular signal-regulated kinase; MMPs, matrix
metalloproteinases; PI3K/Akt, phosphoinositide 3-kinase/protein
kinase B; SC, synovial chondromatosis; Smad, Smad family protein;
Sox9, SRY-box 9; TGF-βRI, transforming growth factor-β receptor I;
TNF-α/γ, tumor necrosis factor-α/γ; TWIST1, twist family BHLH
transcription factor 1; VEGF, vascular endothelial growth
factor. |
Role of mechanical stress in the
pathogenesis of SC
Mechanical stress carries out a key role in the
pathogenesis of SSC (Fig. 5).
Prolonged mechanical stress can induce synovial cell proliferation
and promote chondrification through the regulation of CD105 and
CD90 (33,41). Moreover, mechanical stimulation may
further enhance the expression of pro-inflammatory factors such as
TNF-α and IL-1β, promoting synovial cell proliferation and
chondrification (31). Under
mechanical stress, synovial stem cells may differentiate into
chondrocytes, ultimately leading to SC lesions. Patients with SSC
often exhibit subchondral bone sclerosis, joint space narrowing and
osteophyte formation in affected areas, suggesting that SSC may be
an adaptive proliferative response of the synovium to prolonged
mechanical stress (41).
Overall, PSC is primarily driven by signaling
pathway abnormalities, while SSC is more influenced by inflammation
and mechanical stress. Factors such as IL-6 and VEGF-A may promote
disease progression, but the specific roles of these mechanisms in
the disease process require further clarification. To the best of
our knowledge, there is currently no unified pathogenic model and
future research should focus on in-depth exploration of these
mechanisms and their key regulatory factors. A deeper understanding
of the pathogenesis of SC will provide theoretical support for
clinical molecular biological diagnosis and targeted drug
therapy.
Diagnosis and differential diagnosis of
SC
Diagnosis
SC manifests with a range of clinical symptoms. The
most common include persistent joint pain, typically rated between
5 and 7 on the VAS scale, which intensifies as the disease
progresses (3,8,9).
With SC, 75–85% of patients experience mechanical dysfunctions,
such as joint popping, locking and a reduced range of motion, often
associated with intra-articular loose bodies. A total of 85–90% of
patients present with recurrent joint swelling and increased local
skin temperature, indicating an inflammatory response. In patients
with a disease duration >3 years, nerve compression symptoms
(such as numbness in the branches of the trigeminal nerve) and
secondary osteoarthritis may develop. These symptoms not only
reflect the severity of the disease but also provide key clues for
clinical diagnosis and management (3,8,9).
They offer clinicians essential insights to assess the severity of
the disease and progression. In practice, doctors typically devise
personalized treatment plans based on both the patient's subjective
symptoms and imaging results.
The clinical presentation of early-stage SC is
characterized by a lack of specificity, which poses considerable
difficulties for accurate diagnosis. Ultrasonography (US), x-ray,
computed tomography (CT), magnetic resonance imaging (MRI) and
pathological examinations form a diagnostic system that progresses
from basic to advanced methods, complementing each other. Among
these, MRI is regarded as the most optimal imaging modality,
capable of thoroughly evaluating synovial lesions and surrounding
tissue involvement. However, pathological examination remains the
definitive diagnostic tool (42).
Furthermore, other diagnostic methods have become increasingly
important in diagnosing SC.
US
US is primarily used for early screening and
postoperative monitoring. It can detect synovial thickening,
effusion and free bodies and dynamically assess the activity of the
lesions (7,42). However, US has limited sensitivity
for detecting non-calcified lesions and is inadequate in accurately
determining the size and number of cartilage nodules, particularly
in deep joints such as the hip, thus limiting its role in final
diagnosis (14).
X-ray
X-ray remains the first choice for imaging in SC,
effectively screening for lesions and providing preliminary staging
information (14). The typical
X-ray features of PSC include multiple spherical or ring-shaped
calcifications within the joint cavity, while SSC typically
involves calcification patterns that are irregular and may overlap
with osteoarthritis calcifications, complicating the diagnosis
(7,42). Additionally, x-ray is less
sensitive for detecting extra-articular SC and soft tissue
calcifications may be misdiagnosed as tendon calcifications.
Non-calcified SC or early-stage cases may appear normal on x-rays,
necessitating further evaluation with CT or MRI (43).
CT
CT carries out a key role in the early diagnosis of
SC, especially in evaluating fine calcifications and bone
destruction (7). The high
resolution of CT allows clear visualization of small calcification
points, ring-like calcifications and bone erosion, making it
suitable for complex lesions or cases involving bone destruction
(42,44). Furthermore, the multi-planar
reconstruction function of CT helps analyze the relationship
between calcified nodules, bone, synovium and soft tissues, which
is especially advantageous in areas such as the hip, shoulder and
ankle (44,45). However, CT is less effective for
assessing synovial thickening and non-calcified cartilage nodules
and MRI remains necessary for further analysis (7,45).
MRI
MRI is considered the gold standard for imaging
diagnosis of SC, as it provides a comprehensive assessment of
lesion extent, free body characteristics, soft tissue involvement
and potential malignant transformation risks (7,46).
MRI can detect non-calcified free bodies and clearly visualize
synovial proliferation and cartilage matrix structure,
demonstrating excellent diagnostic capability for both PSC, SSC and
extra-articular SC (8). Compared
with x-ray and CT, MRI offers considerable advantages in early
detection of lesions, synovial activity assessment and
postoperative follow-up (39). A
study by Kramer et al (47)
categorized the MRI presentation of SC into three patterns: Type A,
typical calcified type, high T2 signal with focal low-signal areas,
corresponding to Milgram Stage II; Type B, non-calcified type, high
T2 signal without focal low-signal areas, corresponding to Milgram
Stage I; and Type C, advanced ossification type, high signal
intensity areas resembling fat, indicating cartilage matrix
enrichment or malignant potential. This classification aids in
determining the disease stage and selecting the most appropriate
treatment (Table II).
Additionally, MRI can help distinguish SC from SS, PVNS and other
lesions, improving diagnostic accuracy (48). In early-stage SC, extra-articular
SC and suspected malignant cases, the diagnostic value of MRI is
irreplaceable. However, the high cost and longer examination time
may necessitate complementary CT or X-ray evaluations for certain
cases.
 | Table II.Milgram staging of synovial
chondromatosis with corresponding Kramer MRI types and recommended
surgical strategies (24,47). |
Table II.
Milgram staging of synovial
chondromatosis with corresponding Kramer MRI types and recommended
surgical strategies (24,47).
| Stage | Stage name | Pathological
features | MRI findings
(Kramer type) | Recommended
surgical strategy | (Refs.) |
|---|
| Stage I | Synovial
proliferation phase | Active
cartilaginous metaplasia of the synovium without loose body
formation. May be misdiagnosed as synovitis. | Kramer Type B: High
T2 signal without low-signal calcification. | Early arthroscopic
synovectomy is recommended to prevent disease progression and
reduce recurrence risk. | (24,25,47,66,67) |
| Stage II | Transitional phase
with loose bodies | Persistent synovial
activity with formation of intra-articular loose bodies. | Kramer Type A: High
T2 signal with focal low-signal calcifications. | Arthroscopic
removal of loose bodies. Synovectomy as needed depending on
synovial activity. |
|
| Stage III | Loose body dominant
phase | Synovial activity
has diminished. Numerous mature loose bodies are present, often
causing mechanical symptoms. | Kramer Type C:
Mixed high signals with fat-like or erosive features. | Focus on loose body
removal. Synovectomy is usually unnecessary and reserved for
specific symptoms. |
|
Histopathology
Although imaging carries out a key role in
diagnosing SC, the final diagnosis depends on pathological
analysis, which includes gross pathological and microscopic
evaluations (29). Gross pathology
in PSC typically reveals multiple translucent blue-white cartilage
nodules with smooth surfaces; by contrast, SSC presents as
localized cartilaginous metaplasia accompanied by chronic synovial
inflammation and fibrosis (49,50).
Microscopic examination of PSC reveals abundant undifferentiated
chondrocytes within the synovium, rich matrix and uniform cellular
arrangement, while SSC exhibits inflammatory cell infiltration,
cartilage fragment deposition and fibrous tissue proliferation
(49,50).
In pathological diagnosis, the gene fusion of
FN1-ACVR2A and the overexpression of C-erbB-2 protein can indicate
the clonal proliferation characteristics of PSC (22,38).
This biomarker has considerable clinical value: In terms of
diagnosis, the sensitivity of FN1-ACVR2A fusion for identifying SS
is 92%, and the specificity is 100% (22); in terms of prognosis, patients with
positive fusion have a 2.3-fold increased risk of recurrence, and
the overexpression of C-erbB-2 is negatively associated with
chemotherapy response (38). In
terms of treatment, patients with positive fusion are recommended
to undergo extensive synovectomy and extended follow-up for ≥5
years, while those with negative fusion can undergo local resection
combined with routine 3-year follow-up (22,38).
Although FN1-ACVR2A has not been included in the diagnostic
criteria for malignancy, its status should be noted in the
pathological report and integrated with imaging and clinical
manifestations for risk-stratification management (23).
Other diagnostic methods
In addition to imaging and pathological examination,
several auxiliary methods can aid in the diagnosis of SC. Electron
microscopy can be used to observe the ultrastructural features of
SC tissues, such as bone-cartilage differentiation, but its
clinical application remains limited (51). Arthroscopy allows direct assessment
of synovial proliferation and free bodies but is unable to
precisely measure synovial thickness and the extent of soft tissue
infiltration (10). Artificial
intelligence (AI)-assisted diagnosis has shown promise in SC
imaging analysis, particularly in using deep learning techniques to
identify non-calcified SC, assess recurrence risks and predict
malignant tendencies. However, this technology is still in the
research phase and requires further validation before clinical
implementation (52).
In summary, the diagnosis of SC should combine
multiple diagnostic methods. US is useful for early screening and
postoperative follow-up, while x-ray is the preferred method to
identify calcified lesions, although non-calcified cases may be
missed. CT can precisely assess calcification patterns and bone
destruction, while MRI provides a comprehensive evaluation of
synovial lesions, loose bodies and soft tissue involvement, making
it suitable for early diagnosis and the assessment of malignancy.
Definitive diagnosis still requires pathological analysis. The
diagnosis of SC relies on a comprehensive evaluation of imaging and
pathology, and AI-assisted diagnosis shows potential, but further
research is needed for validation.
Differential diagnosis
SC must be differentiated from all diseases that may
cause intra-articular free bodies or synovial proliferation,
including crystal deposition diseases (such as calcific tendinitis
and hydroxyapatite deposition), osteochondral free bodies (such as
osteochondritis dissecans), arthritis-related lesions (such as
rheumatoid arthritis, degenerative arthritis and osteoarthritis),
synovial proliferative diseases [such as pigmented villonodular
synovitis (PVNS) or synovial hemangiomas], and other conditions
(such as giant cell tumors of the tendon sheath, elbow joint
tuberculosis, tumor-induced calcification and periarticular
scleroderma) (27,53,54).
The majority of these diseases can be distinguished through
clinical presentation, imaging and pathological examination.
However, the differential diagnosis between PSC and SS is
particularly notable, as misdiagnosis can lead to unnecessary
overtreatment, while failure to diagnose may delay treatment and
compromise the outcome (52).
Imaging differentiation
Imaging findings are the cornerstone for
differentiating SC from SS (27).
SC typically presents with cartilage nodules on the synovial
surface, multiple free bodies in the joint and mild synovial
thickening (2). By contrast, SS
appears as an irregular soft tissue mass around the joint, with
fewer free bodies (47). The
calcification patterns in SC are typically ring-like, clustered or
lobulated, which are characteristic of cartilage calcification
(7,42). Conversely, SS presents as diffuse,
patchy or punctate calcification with uneven distribution (52). SC lesions are generally confined to
the synovium and rarely infiltrate deep tissues, whereas SS is more
prone to invasion of muscles, nerves and blood vessels, with
widespread edema and soft tissue infiltration visible on MRI
(21,52). The degree of local tissue
destruction also differs. SC shows mild bone erosion on the joint
surface, with localized bone defects visible on CT and preserved
bone cortex, while SS tends to cause bone destruction, periosteal
reaction and marrow infiltration, indicating a higher degree of
aggressiveness (7,21,52).
Histopathological differentiation
Histopathological examination further aids in
distinguishing SC from SS. Microscopically, PSC shows translucent
cartilage nodules with a regular arrangement of chondrocytes and
few signs of atypia, necrosis or abnormal mitosis. By contrast, SS
is characterized by high cellular density, disorganized arrangement
of small round cells, active mitotic figures and may exhibit a
biphasic pattern (epithelial-like and spindle cells) (42,55,56).
Molecular markers can provide more definitive diagnostic evidence.
In PSC, Bcl-2 protein expression is low, whereas SS shows high
Bcl-2 expression. Additionally, SYT-SSX gene fusion [t
(X;18)(p11.2; q11.2)] is a specific marker for SS, which is not
present in SC (Table III)
(55).
 | Table III.Comparative analysis of SC and
SS. |
Table III.
Comparative analysis of SC and
SS.
| Category | SC | SS | (Refs.) |
|---|
| Clinical
features | Benign lesion, rare
malignant transformation (5%), predominantly affects large joints
(knees/hips), presenting with joint pain, crepitus and limited
mobility, peak incidence: Middle-aged adults (30–50 years). | Malignant tumor
with aggressive behavior, typically occurs in deep soft tissues
near joints, presenting as a rapidly growing mass with neuropathic
pain/numbness, peak incidence: Young adults (15–40 years; median 25
years). | (1,12–14,20) |
| Imaging
findings | MRI: Joint space
expansion with homogeneous T2-hyperintense nodules/target sign of
loose bodies, CT: Multiple calcified/ossified loose bodies. | MRI:
Heterogeneously enhancing soft tissue mass with
hemorrhage/necrosis, CT: Minimal calcification, frequent bone
invasion. | (7,21,42,46,47,52) |
| Histopathology | Synovial
chondrometaplasia forming hyaline cartilage nodules, no cellular
atypia, low proliferative activity, IHC: Strong VEGF-A
expression. | Biphasic
(epithelioid + spindle) or monophasic spindle patterns, Marked
cellular atypia with frequent mitoses, IHC: EMA/CK/BCL-2 positive,
SS18-SSX fusion+. | (29,42,49,50,55,56) |
| Molecular
markers | No specific
mutations, potential Hedgehog/TGF-β pathway involvement. | Pathognomonic
t(X;18)(p11.2;q11.2) translocation with SS18-SSX fusion (>95%
cases). | (28,29,30,55) |
| Management and
prognosis | Surgical excision
of loose bodies + synovectomy, low recurrence (<10%), excellent
prognosis. | Wide resection +
chemo/radiotherapy, high local recurrence, 5-year survival 50–80%,
metastatic risk (lungs/bones). | (1,17,21,55) |
In summary, the typical imaging features of SC
include loose bodies, multiple calcifications and localized
synovial thickening, which are indicative of a benign mass, while
SS is characterized by aggressive growth, soft tissue infiltration,
bone destruction and specific gene fusions, suggesting malignancy.
A combined approach of imaging, pathology and molecular testing can
help improve diagnostic accuracy, reduce misdiagnosis and optimize
clinical management. Although SC and SS show notable differences in
imaging and molecular features, diagnosis remains challenging due
to overlapping imaging characteristics with other diseases and the
need for improved sensitivity and specificity of molecular markers.
Future research should focus on optimizing the combined use of
imaging diagnostics and molecular testing, as well as exploring the
potential of AI-assisted diagnosis to enhance early diagnostic
accuracy and efficiency.
Treatment of SC
The treatment of SC should be individualized based
on the extent of the lesion, symptom severity, and the functional
requirements of the patient. Although SC has a certain degree of
self-limitation, the mechanical irritation from free bodies and
secondary joint damage can exacerbate symptoms, necessitating
active intervention. Current treatment options include conservative
and surgical approaches, with surgery remaining the primary
treatment modality (57). However,
the necessity of synovectomy continues to be debated (58).
Non-surgical treatment is appropriate for patients
with mild or no symptoms, primarily involving non-steroidal
anti-inflammatory drugs, activity modification and physical therapy
to alleviate pain and delay disease progression (59,60).
Additionally, biological agents such as FGF9 signaling pathway
inhibitors and TNF inhibitors may provide novel strategies for
non-surgical management of SC, although large-scale clinical
validation is lacking (61,62).
Currently, chemotherapy and radiotherapy lack clear evidence of
efficacy in SC and are rarely used clinically, showing no
significant advantages pre- or postoperatively. By contrast,
radioactive synovectomy, especially with 188 rhenium-sulfide
colloids, has shown potential in lesion removal and may reduce
postoperative recurrence risk (63,64).
Surgical treatment remains the first-line option for
patients with notable symptoms, impaired joint function or
secondary joint damage (58).
Arthroscopic surgery is widely used in cases with localized lesions
and small joint cavities due to its minimally invasive nature,
rapid recovery and preservation of post-operative function
(65). Open surgery is suitable
for patients with extensive synovial hyperplasia or involvement
both inside and outside the joint, as it allows for more complete
lesion removal and reduces the risk of recurrence, particularly in
severe cases of knee SC (57,58).
The necessity of synovectomy in surgical treatment
remains contested. Certain studies suggest that removing only the
free cartilage bodies may suffice to control the condition, while
others argue that combining synovectomy can reduce the recurrence
rate (64,66,67).
The Milgram staging system (24)
may help explain this controversy: Stage I, where there is no free
body, may necessitate synovectomy; Stage II, with free body
formation, allows for arthroscopic removal of cartilage bodies
while preserving the synovium; Stage III, with multiple free bodies
that are no longer forming, prioritizes free body removal with less
emphasis on synovectomy (66,67).
Furthermore, while synovectomy can reduce recurrence rates, it may
increase postoperative complications and decisions should be based
on the stage of the lesion and individual risk of recurrence. Joint
replacement surgery is indicated for patients with severe joint
destruction and widespread osteoarthritis (68). As SC may continue to form free
bodies, the long-term stability of joint replacements remains an
area for further research (69).
In summary, the treatment of SC primarily involves
surgery, with arthroscopy as the preferred method; open surgery is
considered for extensive or recurrent cases. The necessity of
synovectomy depends on the assessment of the stage of the disease
and the risk of recurrence. In the future, SC treatment may move
towards precision medicine, including molecular targeted therapy,
imaging-assisted decision-making and novel surgical strategies, to
optimize clinical management and improve long-term prognosis.
Conclusion
Despite pronounced progress in understanding the
pathogenesis and therapeutic strategies of SC, challenges remain in
elucidating the mechanisms of pathological transformation and in
the pursuit of precision medicine for this condition. The
pathogenesis of PSC is primarily driven by abnormalities in the
Hedgehog, FGF9/FGFR3 and TGF-β signaling pathways, while SSC is
more influenced by inflammatory microenvironments and mechanical
stress. However, the molecular mechanisms underlying these
processes are not yet fully understood and the risk of malignancy
and the biological basis of SC remain inconclusive. Although MRI is
considered the gold standard for diagnostic imaging, and
AI-assisted diagnosis shows promising potential, further clinical
validation is required for widespread implementation. Surgical
intervention remains the cornerstone of treatment, with arthroscopy
being suitable for localized lesions, while open surgery is
preferred for extensive or recurrent cases. The necessity of
synovectomy varies depending on the stage of the disease, and no
unified standard currently exists. Current research is primarily
focused on the molecular mechanisms of SC, predictive models for
recurrence and risk of malignancy, exploration of molecular
diagnostic techniques and the application of AI in early diagnosis
and personalized treatment. Future research should continue to
concentrate on the hierarchical regulation of signaling pathways,
the role of the inflammatory microenvironment in disease
progression and the optimization of precision treatment strategies
to enhance early diagnosis, reduce the risk of recurrence and
improve long-term patient outcomes.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Technology Innovation
Leading Program of Shaanxi (grant no. 2023KXJ-095), the Shaanxi
Provincial People's Hospital Science and Technology Talent Support
Program for Elite Talents (grant no. 2021JY-50) and the Shaanxi
Provincial People's Hospital Science and Technology Development
Incubation Foundation (grant no. 2023YJY-39).
Availability of data and materials
Not applicable.
Authors' contributions
HL conceived the topic of study. XD made a
substantial contribution to data interpretation and analysis and
wrote and prepared the draft of the manuscript. HL supervised the
present review and provided key revisions. XD, SL and HL
contributed to manuscript revision and have read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, ChatGPT-4.0 by
OpenAI and DeepSeek-R1 were used for native language editing and
proofreading for improving readability. AI tools were not used for
any other purposes. The manuscript was subsequently revised and
edited by the authors. The authors take full responsibility for the
final content of this manuscript.
Glossary
Abbreviations
Abbreviations:
|
Bcl-2
|
B-cell lymphoma 2
|
|
BMP-2/4
|
bone morphogenetic protein 2/4
|
|
C-erbB-2
|
receptor tyrosine-protein kinase
ErbB-2
|
|
Col2A1
|
collagen type IIα1
|
|
Col3A1
|
collagen type IIIα1
|
|
C-Myc
|
cellular Myc
|
|
FGF2/9
|
fibroblast growth factor 2/9
|
|
FGFR1/2/3
|
fibroblast growth factor receptor
1/2/3
|
|
GLI1
|
GLI family zinc finger 1
|
|
HLA-DR+
|
human leukocyte antigen-DR
|
|
IFN-γ
|
interferon γ
|
|
IGF-1
|
insulin-like growth factor 1
|
|
IL-6/17/1β
|
interleukin 6/17/1β
|
|
IL-6R
|
IL-6 receptor
|
|
JAK/STAT
|
Janus kinase/signal transducer and
activator of transcription
|
|
MAPK/ERK
|
mitogen-activated protein
kinase/extracellular signal-regulated kinase
|
|
MMPs
|
matrix metalloproteinases
|
|
MTOR
|
mechanistic target of rapamycin
|
|
PI3K/Akt
|
phosphoinositide 3-kinase/protein
kinase B
|
|
PTC1
|
Patched 1
|
|
Smad2/3/4
|
Smad family proteins 2/3/4
|
|
Sox9
|
SRY-box 9
|
|
TGF
|
transforming growth factor
|
|
TNF
|
tumor necrosis factor
|
|
TWIST1
|
twist family BHLH transcription
factor 1
|
|
VEGF-A
|
vascular endothelial growth factor
A
|
|
SC
|
synovial chondromatosis
|
|
PSC
|
primary SC
|
|
SSC
|
secondary SC
|
|
US
|
ultrasonography
|
|
MRI
|
magnetic resonance imaging
|
|
AI
|
artificial intelligence
|
References
|
1
|
Etemad-Rezaie A, Tarchala M and Howard A:
Primary synovial osteochondromatosis of the shoulder in pediatric
patient: Case report and review of the literature. Arch Bone Jt
Surg. 10:1060–1064. 2022.PubMed/NCBI
|
|
2
|
Braun S, Flevas DA, Sokrab R, Ricotti RG,
Rojas Marcos C, Pearle AD and Sculco PK: De novo synovial
chondromatosis following primary total knee arthroplasty: A case
report. Life (Basel). 13:13662023.PubMed/NCBI
|
|
3
|
Wei JN, Ghislain NA, Li YG and Wu XT:
Disseminated knee synovial chondromatosis treated by arthroscopy
and combined anterior and posterior approaches. J Clin Exp Orthop.
1:12015. View Article : Google Scholar
|
|
4
|
Fajardo M: Recurrent synovial
chondromatosis of the finger. Orthopedics. 44:e454–e457. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirza FA and Vasquez RA: Surgical
management of multi-level cervical spine synovial chondromatosis.
Asian J Neurosurg. 16:367–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pirmorad S, Pirmorad S, Rab KZ and Komath
D: 69Incidental intra-operative finding of synovial chondromatosis
of the temporomandibular joint observed through an arthroscope. Br
J Oral Maxillofac Surg. 62:e792024. View Article : Google Scholar
|
|
7
|
Murphey MD, Vidal JA, Fanburg-Smith JC and
Gajewski DA: Imaging of synovial chondromatosis with
radiologic-pathologic correlation. Radiographics. 27:1465–1488.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wengle LJ, Hauer TM, Chang JS and
Theodoropoulos J: Systematic arthroscopic treatment of synovial
chondromatosis of the knee. Arthrosc Tech. 10:e2265–e2270. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neumann JA, Garrigues GE, Brigman BE and
Eward WC: Synovial chondromatosis. JBJS Rev. 4:e22016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Afzal S, Kazemian G, Baroutkoub M,
Amouzadeh Omrani F, Ahmadi A and Tavakoli Darestani R: Arthroscopic
management of ankle primary synovial chondromatosis (Reichel's
syndrome): A case report with literature review. Int J Surg Case
Rep. 111:1088322023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang BG, Huh KH, Kang JH, Kim JE, Yi WJ,
Heo MS and Lee SS: Imaging features of synovial chondromatosis of
the temporomandibular joint: A report of 34 cases. Clin Radiol.
76:627.e1–627.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tripathy SR, Parida MK, Thatoi PK, Mishra
A and Das BK: Primary synovial chondromatosis (Reichel syndrome).
Lancet Rheumatol. 2:e5762020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alamiri N, Alfayez SM, Marwan Y, Groszman
L, Al Farii H and Burman M: Arthroscopic management of knee
synovial chondromatosis: A systematic review of outcomes and
recurrence. Int Orthop. 49:1037–1045. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko E, Mortimer E and Fraire AE:
Extraarticular synovial chondromatosis: Review of epidemiology,
imaging studies, microscopy and pathogenesis, with a report of an
additional case in a child. Int J Surg Pathol. 12:273–280. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee LM, Zhu YM, Zhang DD, Deng YQ and Gu
Y: Synovial chondromatosis of the temporomandibular joint: A
clinical and arthroscopic study of 16 cases. J Craniomaxillofac
Surg. 47:607–610. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans S, Boffano M, Chaudhry S, Jeys L and
Grimer R: Synovial chondrosarcoma arising in synovial
chondromatosis. Sarcoma. 2014:6479392014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Memon F, Pawar ED, Gupta D and Yadav AK:
Diagnosis arthroscopic treatment of synovial chondromatosis of
glenohumeral joint: A case report. J Orthop Case Rep. 11:59–62.
2021.PubMed/NCBI
|
|
18
|
Gatt T and Portelli M: Recurrence of
primary synovial chondromatosis (Reichel's Syndrome) in the ankle
joint following surgical excision. Case Rep Orthop.
2021:99226842021.PubMed/NCBI
|
|
19
|
de Sa D, Horner NS, MacDonald A, Simunovic
N, Ghert MA, Philippon MJ and Ayeni OR: Arthroscopic surgery for
synovial chondromatosis of the hip: A systematic review of rates
and predisposing factors for recurrence. Arthroscopy.
30:1499–1504.e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davis RI, Hamilton A and Biggart JD:
Primary synovial chondromatosis: A clinicopathologic review and
assessment of malignant potential. Hum Pathol. 29:683–688. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCarthy C, Anderson WJ, Vlychou M,
Inagaki Y, Whitwell D, Gibbons CL and Athanasou NA: Primary
synovial chondromatosis: A reassessment of malignant potential in
155 cases. Skeletal Radiol. 45:755–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amary F, Perez-Casanova L, Ye H, Cottone
L, Strobl AC, Cool P, Miranda E, Berisha F, Aston W, Rocha M, et
al: Synovial chondromatosis, soft tissue chondroma: Extraosseous
cartilaginous tumor defined by FN1 gene rearrangement. Mod Pathol.
32:1762–1771. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JH and Ro JY: The 2020 WHO
classification of tumors of soft tissue: Selected changes and new
entities. Adv Anat Pathol. 28:44–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milgram JW: Synovial osteochondromatosis:
A histopathological study of thirty cases. J Bone Joint Surg Am.
59:792–801. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gerard Y, Shall A and Ameil M: Synovial
osteochondromatosis. Therapeutic indications based on a
histological classification. Chirurgie. 119:190–194. 1993.(In
French). PubMed/NCBI
|
|
26
|
Villacin AB, Brigham LN and Bullough PG:
Primary, secondary synovial chondrometaplasia: Histopathologic and
clinicoradiologic differences. Hum Pathol. 10:439–451. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Habusta SF, Mabrouk A and Tuck JA:
Synovial chondromatosis. StatPearls [Internet]. StatPearls
Publishing; Treasure Island, FL: 2017
|
|
28
|
Hopyan S, Nadesan P, Yu C, Wunder J and
Alman BA: Dysregulation of hedgehog signalling predisposes to
synovial chondromatosis. J Pathol. 206:143–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ichikawa J, Imada H, Kawasaki T and Haro
H: Opinion: The nature of primary and secondary synovial
chondromatosis: Importance of pathological findings. Front Oncol.
13:12818902023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alcaraz MJ, Megías J, García-Arnandis I,
Clérigues V and Guillén MI: New molecular targets for the treatment
of osteoarthritis. Biochem Pharmacol. 80:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wake M, Hamada Y, Kumagai K, Tanaka N,
Ikeda Y, Nakatani Y, Suzuki R and Fukui N: Up-regulation of
interleukin-6 and vascular endothelial growth factor-A in the
synovial fluid of temporomandibular joints affected by synovial
chondromatosis. Br J Oral Maxillofac Surg. 51:164–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Cai H, Fang W, Meng Q, Li J, Deng M
and Long X: Fibroblast growth factor 2 involved in the pathogenesis
of synovial chondromatosis of temporomandibular joint. J Oral
Pathol Med. 43:388–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mansor Y, Perets I, Close MR, Mu BH and
Domb BG: In search of the spherical femoroplasty: Cam overresection
leads to inferior functional scores before and after revision hip
arthroscopic surgery. Am J Sports Med. 46:2061–2071. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, El Mozen LA, Cai H, Fang W, Meng Q,
Li J, Deng M and Long X: Transforming growth factor beta 3 involved
in the pathogenesis of synovial chondromatosis of temporomandibular
joint. Sci Rep. 5:88432015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Zhou Y, Wang Y, Crawford R and Xiao
Y: Synovial macrophages in cartilage destruction and
regeneration-lessons learnt from osteoarthritis and synovial
chondromatosis. Biomed Mater. 17:0120012022. View Article : Google Scholar
|
|
36
|
Chen WK, Zhang HJ, Liu J, Dai Z and Zhan
XL: COL3A1 is a potential diagnostic biomarker for synovial
chondromatosis and affects the cell cycle and migration of
chondrocytes. Int Immunopharmacol. 127:1114162024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshitake H, Kayamori K, Wake S, Sugiyama
K and Yoda T: Biomarker expression related to chondromatosis in the
temporomandibular joint. Cranio. 39:362–366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakanishi S, Sakamoto K, Yoshitake H, Kino
K, Amagasa T and Yamaguchi A: Bone morphogenetic proteins are
involved in the pathobiology of synovial chondromatosis. Biochem
Biophys Res Commun. 379:914–919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Davis RI, Foster H and Biggart DJ: C-erb
B-2 staining in primary synovial chondromatosis: A comparison with
other cartilaginous tumours. J Pathol. 179:392–395. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Sun H, Li D, Cai Z, Xu J and Ma R:
CD34+ synovial fibroblasts exhibit high osteogenic potential in
synovial chondromatosis. Cell Tissue Res. 397:37–50. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sink J, Bell B and Mesa H: Synovial
chondromatosis of the temporomandibular joint: Clinical, cytologic,
histologic, radiologic, therapeutic aspects, and differential
diagnosis of an uncommon lesion. Oral Surg Oral Med Oral Pathol
Oral Radiol. 117:e269–e274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vasudevan R, Jayakaran H, Ashraf M and
Balasubramanian N: Synovial chondromatosis of the knee joint:
Management with arthroscopy-assisted ‘sac of pebbles’ extraction
and synovectomy. Cureus. 16:e693782024.PubMed/NCBI
|
|
43
|
Tekaya AB, Hamdi O, Bellil M, Saidane O,
Rouached L, Bouden S, Tekaya R, Salah MB, Mahmoud I and Abdelmoula
L: Synovial chondromatosis of the shoulder in rheumatoid arthritis:
A case report and brief review of the literature. Curr Rheumatol
Rev. 19:362–366. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee HJ, Han W and Kim K: Secondary
synovial chondromatosis of the subacromial subdeltoid bursa with
coexisting glenohumeral osteoarthritis: Case report. Medicine
(Baltimore). 100:e277962021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu Y, Gao G, Luan S, Wu K, Wang H, Zhang
Y, Zhang X, Wang J and Xu Y: Longitudinal assessment of clinical
outcomes after arthroscopic treatment for hip synovial
chondromatosis, the effect of residual loose bodies: Minimum 4-year
and 8-year follow-up. Am J Sports Med. 52:2306–2313. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Terra BB, Moraes EW, de Souza AC, Cavatte
JM, Teixeira JC and De Nadai A: Arthroscopic treatment of synovial
osteochondromatosis of the elbow. Case report and literature
review. Rev Bras Ortop. 50:607–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kramer J, Recht M, Deely DM, Schweitzer M,
Pathria MN, Gentili A, Greenway G and Resnick D: MR appearance of
idiopathic synovial osteochondromatosis. J Comput Assist Tomogr.
17:772–776. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Poutoglidou F, Metaxiotis D and
Mpeletsiotis A: Pigmented villonodular synovitis of the knee joint
in a 10-year-old patient treated with an all-arthroscopic
synovectomy: A case report. Cureus. 12:e119292020.PubMed/NCBI
|
|
49
|
Yadav SK, Rajnish RK, Kumar D, Khera S,
Elhence A and Choudhary A: Primary synovial chondromatosis of the
ankle in a child: A rare case presentation and review of
literature. J Orthop Case Rep. 13:5–10. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Han W, Luo H, Zhao Y, He Z, Guo C and Meng
J: Retrospective study of synovial chondromatosis of the
temporomandibular joint: Clinical and histopathologic analysis and
the early-stage imaging features. Oral Surg Oral Med Oral Pathol
Oral Radiol. 137:215–223. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Beger AW, Millard JA, Bresnehan A, Dudzik
B and Kunigelis S: Primary synovial chondromatosis: An elemental
investigation of a rare skeletal pathology. Folia Morphol (Warsz).
81:685–693. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang S, Yang J, Fong S and Zhao Q:
Artificial intelligence in cancer diagnosis, prognosis:
Opportunities and challenges. Cancer Lett. 471:61–71. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou N, Fang K, Arthur VDT, Yi R, Xiang F,
Wen J and Xiao S: Synovial chondromatosis combined with synovial
tuberculosis of knee joint: A case report. BMC Pediatr. 22:82022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chraibi O, Rajaallah A, Lamris MA, El
Kassimi CE, Rafaoui A and Rafai M: Concurrent arboreal lipoma,
synovial chondromatosis in an osteoarthritic knee: Insights from a
rare case study-A surgical case report. Int J Surg Case Rep.
119:1097862024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sperling BL, Angel S, Stoneham G, Chow V,
McFadden A and Chibbar R: Synovial chondromatosis, chondrosarcoma:
A diagnostic dilemma. Sarcoma. 7:69–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Furtado C, Zeitoun R, Remedios ID, Wilkes
J, Sumathi V and Tony G: Secondary chondrosarcoma arising in
synovial chondromatosis of wrist joint. J Orthop Case Rep.
13:30–36. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park JH, Noh HK, Bada LP, Wang JH and Park
JW: Arthroscopic treatment for synovial chondromatosis of the
subacromial bursa: A case report. Knee Surg Sports Traumatol
Arthrosc. 15:1258–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rai AK, Bansal D, Bandebuche AR, Rahman
SH, Prabhu RM and Hadole BS: Extensive synovial chondromatosis of
the knee managed by open radical synovectomy: A case report with
review of literature. J Orthop Case Rep. 12:19–22. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jung KA, Kim SJ and Jeong JH: Arthroscopic
treatment of synovial chondromatosis that possibly developed after
open capsular shift for shoulder instability. Knee Surg Sports
Traumatol Arthrosc. 15:1499–1503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Goyal S, Shrivastav S, Ambade R, Pundkar
A, Lohiya A and Naseri S: Unveiling the dance of crystals: A
surgical odyssey in the open excision of synovial chondromatosis in
the right knee. Cureus. 16:e569012024.PubMed/NCBI
|
|
61
|
Robinson D, Hasharoni A, Evron Z, Segal M
and Nevo Z: Synovial chondromatosis: The possible role of FGF 9 and
FGF receptor 3 in its pathology. Int J Exp Pathol. 81:183–189.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu S, Wu M, Zhou G, Ishikawa T, Liang J,
Nallapothula D, Singh RR, Wang Q and Wang M: Potential utility of
anti-TNF drugs in synovial chondromatosis associated with
ankylosing spondylitis. Int J Rheum Dis. 22:2073–2079. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vadi SK, Chouhan DK, Gorla AKR, Shukla J,
Sood A and Mittal BR: Potential adjunctive role of radiosynovectomy
in primary synovial osteochondromatosis of the knee: A case report.
Nucl Med Mol Imaging. 51:252–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dheer S, Sullivan PE, Schick F, Karanjia
H, Taweel N, Abraham J and Jiang W: Extra-articular synovial
chondromatosis of the ankle: Unusual case with
radiologic-pathologic correlation. Radiol Case Rep. 15:445–449.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Odluyurt M, Orhan Ö, Sezgin EA and Kanatli
U: Synovial chondromatosis in unusual locations treated with
arthroscopy: A report of three cases. Joint Dis Relat Surg Case
Rep. 1:063–066. 2022. View Article : Google Scholar
|
|
66
|
Fuerst M, Zustin J, Lohmann C and Rüther
W: Synovial chondromatosis. Orthopade. 38:511–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Reddivari AKR and Mehta P: Gastroparesis.
StatPearls [Internet]. StatPearls Publishing; Treasure Island, FL:
2024
|
|
68
|
Houdek MT, Wyles CC, Rose PS, Stuart MJ,
Sim FH and Taunton MJ: High rate of local recurrence and
complications following total knee arthroplasty in the setting of
synovial chondromatosis. J Arthroplasty. 32:2147–2150. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deinum J and Nolte PA: Total knee
arthroplasty in severe synovial osteochondromatosis in an
osteoarthritic knee. Clin Orthop Surg. 8:218–222. 2016. View Article : Google Scholar : PubMed/NCBI
|