Introduction
Transformation-associated alteration of the
carbohydrate structures in cellular glycoconjugates, including
glycolipids and glycoproteins, occurs frequently in various types
of cancer, mainly due to the aberrant expression of
glycosyltransferases (1). Detection
of these structures, including sialyl lacto-N-fucopentaose (CA19.9,
sialyl Lewis a), in sera was successfully applied for the clinical
diagnosis of epithelial cancer in gynecologic tissues and the
digestive tract (2). However, in
comparison to CA125, whose frequency in ovarian carcinomas is
higher than that of CA19.9, and the level of which is used for
preoperative surgical counseling and planning, the clinical
usefulness and cell biological properties of ovarian cancers with
CA19.9-carbohydrates have not been clearly elucidated yet (3). Since a number of carbohydrate
structures were shown to play a role in the ligands of animal
lectins, such as NeuAcα2-6Galβ1-4GlcNAc for CD22 (4), sialyl LeX for P-selectin
(5) and sialyl
6-sulfo-LeX for L-selectin (6), the expression of these structures and
their modifications may affect the lectin-mediated adhesion related
to the invasion and metastasis of cancer cells. Transfection of the
α1,2-fucosyltransferase gene into RMG-1 cells resulted in increases
in LeY and H-1 glycolipids, and a concomitant decrease
in sialylated glycolipids. The transfectants exhibited increased
adhesion with mesothelial cells and resistance against an
anticancer drug, 5-fluorouracil, in comparison to those of RMG-1
cells (7,8). In addition, significant changes in
glycolipids including Lewis-active ones were observed in ovarian
carcinoma-derived KF28 cells exhibiting anticancer drug-resistance
to cisplatin and taxol, probably due to an alteration of the
activities of transporter proteins in regard to the excretion of
drugs in glycolipid-rich membrane rafts (9,10).
These findings showed that the expression of fucosylated
glycolipids exhibiting Lewis- and H-antigenecities is closely
correlated to the malignancy of cancer cells, including increased
dissemination, metastatic potential and anticancer drug-resistance.
However, since Lewis-active glycolipids are constructed of more
than five carbohydrates, synthesis occurs through more than five
glycosyltransferase reactions, whose activities are regulated by
various epigenetic factors, including the concentrations of sugar
nucleotides and acceptor glycolipids, pH and divalent cations.
Notably, glycolipids in each step of the sequential multi-step
reaction serve as substrates for the following step, suggesting
that glycosyltransferase reactions determine the overall profile of
glycolipids, including cancer-associated ones. Accordingly, the
glycolipids in tissues from patients with ovarian carcinomas and
cell lines derived from them were quantitatively determined in
order to clarify their histologic classification-associated
alterations, including Lewis-active ones, and to apply them as
molecular markers for determining the malignancy of ovarian
carcinomas, similar to those for colorectal carcinomas (11,12).
Materials and methods
Tissue specimens
Histologic classification of the ovarian cancers was
performed using criteria defined by the World Health Organization.
The serous (7 cases) and mucinous (6 cases) cystadenocarcinoma,
clear cell adenocarcinoma (3 cases) and endometrioid carcinoma (3
cases) tissues were obtained from the National Saitama Hospital.
Written informed consent to use the specimens in this study was
obtained from the patients, and the experimental protocol was
approved by the local ethics committee.
Cell lines derived from ovarian
cancers
The cell lines used in this experiment were obtained
from patients with the following ovarian cancers: HAC-2 and RMG-1
from clear cell adenocarcinomas, 2008 and KF28 from serous
cystadenocarcinomas, HMKOA from mucinous cystadenocarcinomas and
HNOA from endometrioid carcinomas. The cell lines were cultured in
Dulbecco’s modified Eagle’s medium (Nissui, Tokyo, Japan)
supplemented with 10% fetal bovine serum (Nichirei Biosciences
Inc., Tokyo, Japan) under a humidified atmosphere containing 5%
CO2 at 37°C.
Materials
The glycolipids used in this experiment were
purified from various sources in our laboratory: GM3 and
IV3NeuAcα-nLc4Cer from human erythrocytes,
GD3 from bovine brain, LeX from human fetal brain and
Lc4Cer, Leb and
IV6NeuAcα-nLc4Cer from human meconium
(9,10).
Antisera
Monoclonal antibodies, Y916 and 5h6, were prepared
by the immunization of mice with gangliosides from bovine milk and
IV3NeuAcα-nLc4Cer from human erythrocytes,
respectively, and the hybridization of lymphocytes with myeloma
P3-X63-Ag8.653 in our laboratory (13,14).
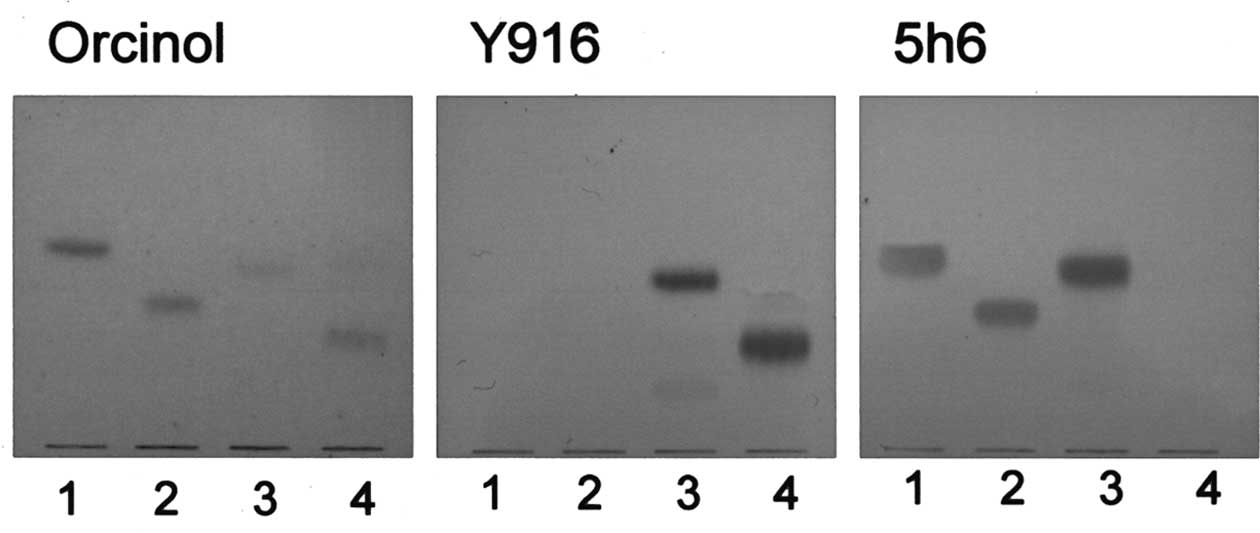
As shown in Fig. 1, Y916 reacted
with GD3 and IV6NeuAcα-nLc4Cer, and 5h6 with
GM3, GD3 and IV3NeuAcα-nLc4Cer, indicating
that structural isomers, IV6NeuAcα- and
IV3NeuAcα-nLc4Cer, are identified with Y916
and 5h6. Moreover, GM3 and GD3 were identified by their mobility on
thin layer chromatograpy (TLC) and their reactions with Y916 and
5h6. The following monoclonal antibodies were kindly donated:
NCC-LU-279 for LeX and NCC-ST-433 for LeY by
Dr S. Hirohashi, National Cancer Center, Tokyo, Japan, MSN-1 for
Leb by Dr S. Nozawa, Keio University, Tokyo, Japan, and
3C11 for sialyl Lea by Dr K. Matsumoto, Mikuri Immunol.
Lab., Kyoto, Japan.
Analysis of lipids
The neutral and acidic glycolipids derived from the
tissues and cells were examined by TLC and TLC-immunostaining with
the development solvents, chloroform/methanol/water (65:35:8, by
volume) for neutral glycolipids and chloroform/methanol/0.5%
CaCl2 (55:45:10, by volume) for gangliosides, as
previously described (10,13). Known amounts (0.1–1.5 μg) of
glycolipids, such as GM3, Lc4Cer,
IV3NeuAcα-nLc4Cer,
IV6NeuAcα-nLc4Cer and GD3, were developed on
the same TLC plates for the preparation of standard curves. The
densities of spots on TLC plates were determined by image analysis
using NIH image.
α2,3- and α2,6-sialyltransferases
The cancer tissues were homogenized using a
homogenizer (Polytron; Kinematica, Luzern, Switzerland) in 0.25 M
sucrose, and the microsomal fractions were prepared by
centrifugation as previously described (15). The standard assay mixture for
microsomal α2,3- and α2,6-sialyltransferases comprised 7.6 nmol
nLc4Cer, 10 mM MgCl2, 5 mM CaCl2,
10 mM CMP-sialic acid, 0.3% Triton CF54, 50 mM 4-morpholinoethane
sulfonic acid-NaOH buffer (pH 6.4), and 50 μg enzyme protein, in a
final volume of 50 μl (16,17). After incubation at 37°C for 3 h, the
reaction was terminated with 100 μl of ethanol. Then, 50 μl
aliquots of the solution were developed on two TLC plates with
chloroform/methanol/0.5% CaCl2 in water (55:45:10, by
volume), detection being performed with 5h6 for one and with Y916
for the other. Known amounts of
IV3NeuAcα-nLc4Cer and
IV6NeuAcα-nLc4Cer (5–100 ng) were stained on
the same plates, and the densities of the spots were determined by
image analysis using NIH image. The amounts of endogenous
gangliosides in the microsomes were subtracted from the values
after the enzyme reactions.
RT-PCR analysis
Total RNA extracted from the tissues with Isogen
(Nippongene, Toyama, Japan) was reverse-transcribed to cDNA with
reverse transcriptase (M-MuLV; Takara, Kyoto, Japan) and oligo
dT-primers, and then subjected to PCR with 0.5 units of Taq DNA
polymerase (GoTaq; Promega, Kyoto, Japan) under the following
conditions: LacCer sialyltransferase (GM3 synthase, AB018356),
sense primer, atttgagcacaggtatagc, antisense primer,
gatgtcaaaggcagtctct; GM3 sialyltransferase (GD3 synthase, D26360),
sense primer, acaaatggaagactgctgcga, antisense primer, tggctctgt
tcctgtcttcat; α2,6-sialyltransferase (BC031476), sense primer,
tgcgtcctggtctttcttct, antisense primer, tctgcactgaacttgatgcc;
α2,3-sialyltransferase (BC010645), sense primer, atctcccg
ggaagacaggta, antisense primer, ccatgaagaaggggttgaga; and
α1,3-fucosyltransferase 3 (FUT3, NM1097640), sense primer,
tggtggctgtgtgtttcttc, antisense primer, ggctccaagttgaaccagat; 35
cycles of 95°C for 15 sec, 54–64°C for 30 sec and 72°C for 40 sec.
The primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
were used as controls. The resulting PCR products were
electrophoresed on a 1.5% agarose gel, stained with ethidium
bromide, and examined using a UV trans-illuminator (15).
Results
Glycolipids in ovarian carcinoma
tissues
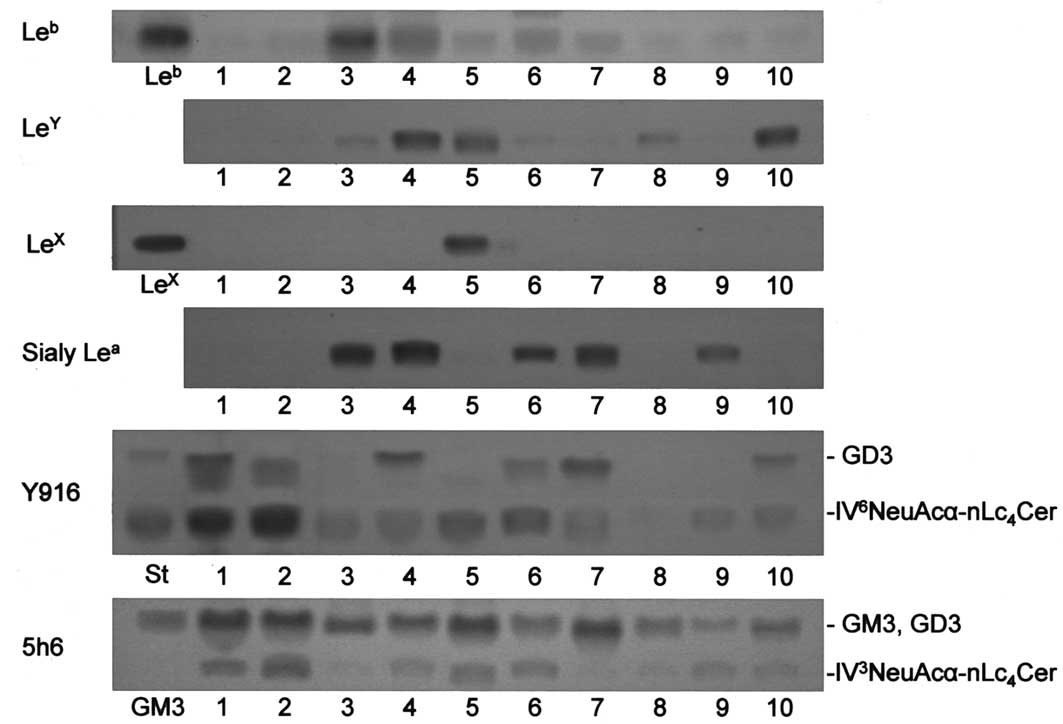
Fig. 2 shows
TLC-immunostaining of lipids from a number of ovarian carcinoma and
uterine endometrial carcinoma tissues. In agreement with our
previous results (7),
Leb in mucinous cystadenocarcinomas was present in
higher amounts than those in the other carcinomas (Table I). Among the Lewis glycolipids
examined, Leb, LeY, LeX and sialyl
Lea, whether one or a number of them, were detected in
all tissues other than ovarian serous cystadenocarcinoma ones, in
which they were not present or only in a trace amount.
Alternatively, serous cystadenocarcinomas contained
IV6NeuAcα-nLc4Cer in significantly higher
amounts than in the other carcinomas (Fig. 2). The amounts of
IV6NeuAcα-nLc4Cer in serous
cystadenocarcinomas were >9 times higher than those in the other
carcinomas, while no significant differences were observed in the
amounts of IV3NeuAcα-nLc4Cer among the
various types of ovarian carcinomas (Fig. 2 and Table I). The expression of
IV6NeuAcα-nLc4Cer and Leb was
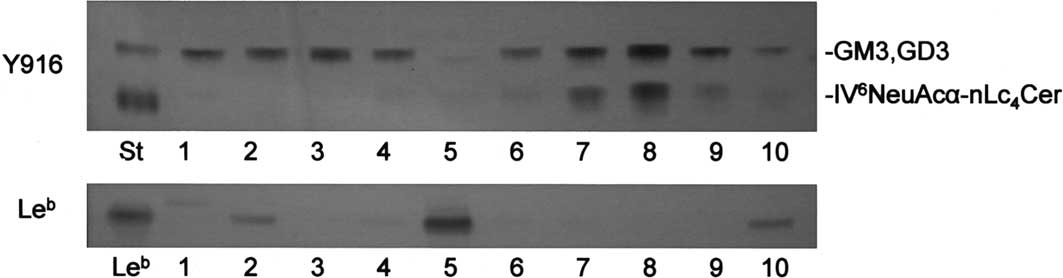
further examined in an additional 5 cases of ovarian serous and
mucinous cystadenocarcinomas, respectively. As shown in Fig. 3 and Table II,
IV6NeuAcα-nLc4Cer was present in 3/5 serous
cystadenocarcinomas in amounts >0.1 μg per mg of dry weight, but
not in mucinous cystadenocarcinomas. Conversely, Leb was
detected in 4/5 mucinous cystadenocarcinomas and in one of the
serous cystadenocarcinomas. Thus, the frequencies of expression of
IV6NeuAcα-nLc4Cer and Lewis-active
glycolipids were significantly high in serous cystadenocarcinomas
and the other ovarian carcinoma tissues, respectively.
 | Table IGlycolipids in human ovarian
carcinoma tissues. |
Table I
Glycolipids in human ovarian
carcinoma tissues.
| No. | Histological
classification | Specimen Case | LeX | LeY | Leb | Sialyl
Lea | GM3 | GD3 | α2,6 | α2,3 |
|---|
| 1 | Ovarian serous
cystadenocarcinoma | 1 | – | – | – | – | 1.19 | 0.11 | 0.45 | 0.04 |
| 2 | | 2 | – | – | – | – | 1.25 | 0.08 | 0.45 | 0.06 |
| 3 | Ovarian mucinous
cystadenocarcinoma | 1 | – | 0.01 | 0.53 | 0.04 | 0.54 | – | 0.01 | – |
| 4 | Endometrial
adenocarcinoma | 1 | – | 0.30 | 0.06 | 0.04 | 0.48 | 0.08 | 0.02 | 0.02 |
| 5 | Ovarian
endometrioid carcinoma | 1 | 0.09 | 0.09 | 0.02 | – | 1.33 | – | 0.04 | 0.03 |
| 6 | | 2 | – | tr | 0.03 | 0.02 | 0.50 | 0.01 | 0.05 | 0.02 |
| 7 | | 3 | – | – | 0.02 | 0.04 | 0.90 | 0.07 | 0.01 | tr |
| 8 | Ovarian clear cell
adenocarcinoma | 1 | – | 0.01 | 0.01 | – | 0.37 | – | – | tr |
| 9 | | 2 | – | tr | 0.01 | 0.01 | 0.15 | tr | 0.02 | 0.01 |
| 10 | | 3 | – | 0.20 | tr | – | 0.22 | 0.02 | 0.02 | 0.01 |
 | Table IIAmounts of
IV6NeuAcα-nLc4Cer and Leb in
ovarian serous and mucinous cystadenocarcinomas. |
Table II
Amounts of
IV6NeuAcα-nLc4Cer and Leb in
ovarian serous and mucinous cystadenocarcinomas.
| No. | Histological
classification | Specimen Case | α2,6 | Leb |
|---|
| 1 | Ovarian serous
cystadenocarcinoma | 3 | 0.06 | 0.01 |
| 2 | | 4 | 0.23 | — |
| 3 | | 5 | 0.37 | — |
| 4 | | 6 | 0.11 | — |
| 5 | | 7 | 0.01 | 0.13 |
| 6 | Ovarian mucinous
cystadenocarcinoma | 2 | — | 0.01 |
| 7 | | 3 | — | 0.12 |
| 8 | | 4 | — | 0.02 |
| 9 | | 5 | 0.01 | 0.08 |
| 10 | | 6 | — | 0.48 |
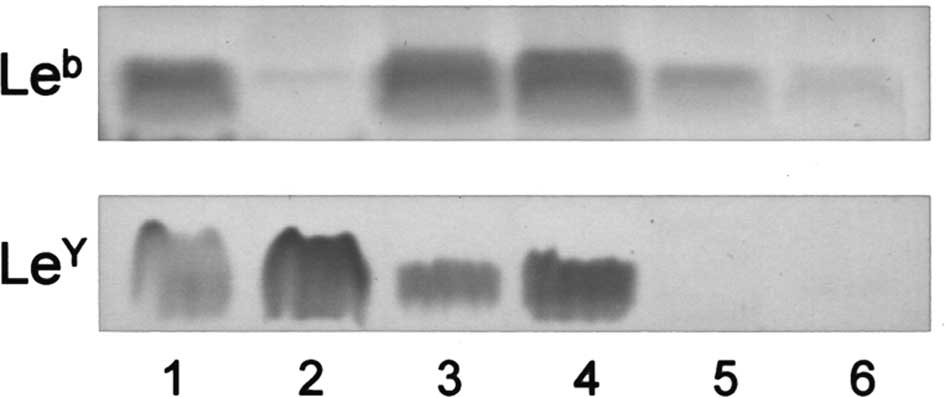
Glycolipids in ovarian carcinoma-derived
cells
The expression of
IV6NeuAcα-nLc4Cer and Lewis-active
glycolipids was examined in cell lines established from various
types of ovarian carcinomas. Although
IV6NeuAcα-nLc4Cer was not detected in any
cell line, Lewis-active glycolipids were present in clear cell
adenocarcinoma-derived RMG-1 and HAC-2, mucinous
cystadenocarcinoma-derived HMKOA and endometrioid carcinoma-derived
HNOA, in amounts higher than those in serous
cystadenocarcinoma-derived 2008 and KF28. Their presence shows that
relatively low and high amounts of Lewis-active glycolipids in
serous cystadenocarcinomas and the other ovarian carcinoma tissues,
respectively, are retained in the respective cell lines (Fig. 4).
Enzyme activities and gene expression of
α2,3- and α2,6-sialyltransferases in ovarian carcinoma tissues
To examine the enzymatic and genetic backgrounds of
the expression of IV6NeuAcα-nLc4Cer and
Lewis-active glycolipids, the specific activities of α2,3- and
α2,6-sialyltransferases were determined by detection of the
products, IV3NeuAcα-nLc4Cer and
IV6NeuAcα-nLc4Cer, and the gene expression by
RT-PCR.
As shown in Fig. 5
and Table III, although the
specific activities of α2,3-sialyltransferase with
nLc4Cer as the substrate in the tissues were not
correlated with the amounts of
IV3NeuAcα-nLc4Cer, or with the histological
classification, those of α2,6-sialyltransferase were positively
correlated with the relative amounts of
IV6NeuAcα-nLc4Cer in the tissues, indicating
that the high amounts of IV6NeuAcα-nLc4Cer in
serous cystadenocarcinomas are due to the higher specific activity
of α2,6-sialyltransferase. However, the α2,3- and
α2,6-sialyltransferase genes were ubiquitously expressed in all of
the tissues examined and their relative intensities were not
positively correlated with the enzymatic activities, or with the
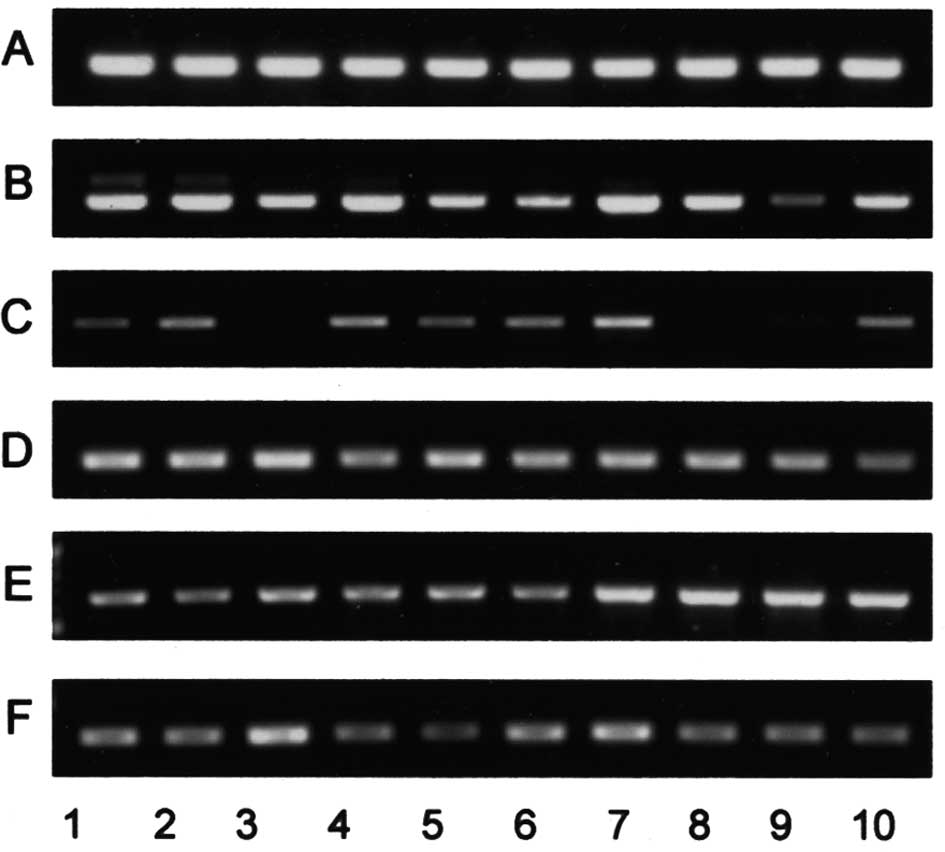
amounts of glycolipids (Fig. 6).
Similarly, expression of the FUT3 gene encoding an α1,3/4
fucosyltransferase responsible for the synthesis of Lewis antigen
was not correlated with the amounts of Leb,
LeX, LeY and sialyl Lea. In
contrast to the gene expression of sugar transferases for
neolacto-series glycolipids, the relative intensities of the GM3
and GD3 synthase genes were positively correlated with the amounts
of GM3 and GD3 (Fig. 6).
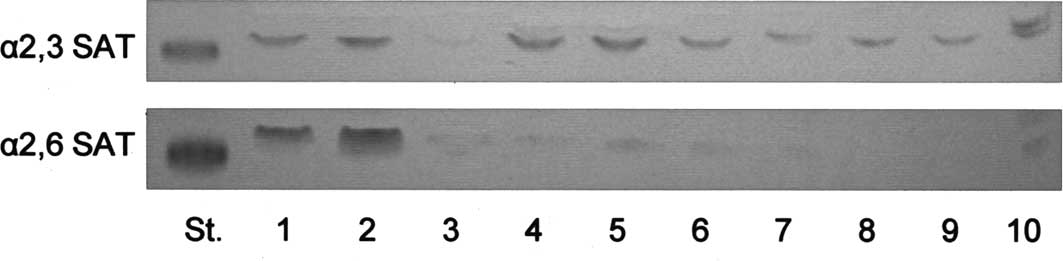 | Figure 5TLC-immunostaining of products
following reactions of α2,3- and α2,6-sialyltransferases (SAT). The
products following the reactions with 50 μg of enzyme proteins were
developed with chloroform/methanol/0.5% CaCl2 in water
(55:45:10, by volume), and detected by TLC-immunostaining with
monoclonal antibodies 5h6 for
IV3NeuAcα-nLc4Cer (α2,3SAT) and Y916 for
IV6NeuAcα-nLc4Cer (α2,6SAT), respectively.
Standard glycolipids IV3NeuAcα-nLc4Cer for
α2,3SAT and IV6NeuAcα-nLc4Cer for α2,6SAT,
respectively. The specimen numbers are the same as those in
Fig. 2 and Table I. |
 | Table IIISpecific activities of α2,3- and
α2,6-sialyltransferases with nLc4Cer as the
substrate. |
Table III
Specific activities of α2,3- and
α2,6-sialyltransferases with nLc4Cer as the
substrate.
| No. |
α2,3-sialyltransferase |
α2,6-sialyltransferase |
|---|
| 1 | 7.4 | 57.2 |
| 2 | 8.5 | 105.2 |
| 3 | 1.0 | 21.7 |
| 4 | 11.6 | 12.4 |
| 5 | 12.0 | 7.5 |
| 6 | 5.5 | 10.9 |
| 7 | 5.3 | nd |
| 8 | 5.2 | nd |
| 9 | 3.9 | nd |
| 10 | 11.0 | 16.1 |
Discussion
Among ovarian carcinoma tissues with different
histologic classifications, serous cystadenocarcinomas have been
shown to express IV6NeuAcα-nLc4Cer at a
higher frequency than the other carcinomas, and in compensation,
the expression of Lewis-related glycolipids in serous
cystadenocarcinomas was lower than in the other carcinomas. As
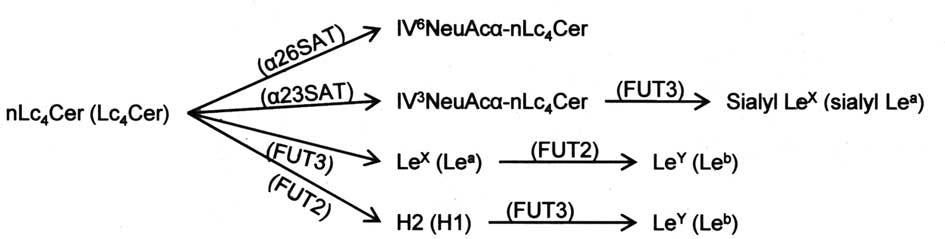
shown in Fig. 7, since the
syntheses of IV6NeuAcα-nLc4Cer and
Lewis-active glycolipids occur with the same substrate at the
branch of the lacto-series pathway, our findings suggest that the
synthesis of lacto-series glycolipids is influenced by the
availability of substrate glycolipids in individual steps of
glycosyltransferase reactions. As reported in our previous paper
(8), when the activity of
α1,2-fucosyltransferase in ovarian carcinoma-derived RMG-1 cells
increased to 20–30 fold that in the original cells on transfection
with the α1,2-fucosyltransferase gene, the amount of LeY
increased to 10-fold of the original level. On the other hand,
sialylated glycolipids in the original cells, including sialyl
LeX and IV3NeuAcα-nLc4Cer, were
absent in the transfectants, suggesting that the enhanced
fucosylation of LeX and nLc4Cer at the
terminal step of glycosylation inhibits their sialylation through
deprivation of the substrate. Similarly, the high specific activity
of α2,6-sialyltransferase in serous cystadenocarcinomas was
considered to cause the increased amount of
IV6NeuAcα-nLc4Cer (Lc4Cer) and the
absence of the Lewis antigen due to the consumption of
nLc4Cer (Lc4Cer) for the syntheses of
LeX (Lea) by α1,3/4-fucosyltransferase
(FUT3), blood group H-glycolipid by α1,2-fucosyltransferase (FUT2)
and IV6NeuAcα-nLc4Cer by
α2,3-sialyltransferase (α2,3SAT), whose mRNAs were expressed in all
of the tissues examined (18).
Therefore, the epigenetic regulation of enzymatic activities in the
individual steps of the neolacto- and lacto-series pathways may be
involved in the determination of the mode of expression of
sialylated and fucosylated glycolipids, including Lewis antigens,
irrespective of the expression of glycosyltransferase mRNA. In
contrast, the amounts of GM3 with shorter carbohydrate chains were
closely correlated to the relative intensities of GM3-and
GD3-synthase mRNAs, whose expression may directly lead to the
active syntheses of GM3 and GD3 with a sufficient supply of
substrate LacCer in proportion to their enzymatic activities.
However, among ovarian carcinoma-derived cells, Lewis-related
glycolipids were present in mucinous cystadenocarcinoma-, clear
cell adenocarcinoma- and endometrioid carcinoma-derived cells in
significantly higher amounts than in serous
cystadenocarcinoma-derived cells, suggesting that the synthetic
potential as regards to Lewis glycolipids in the tissues is
maintained in the cultured cell lines. On the other hand,
IV6NeuAcα-nLc4Cer was present in 5/7 serous
cystadenocarcinoma tissues in amounts of more than 0.1 μg per mg
dried tissue, while the amounts in the other carcinomas, if
present, were less than 0.05 μg per mg dry weight, indicating that
the expression of IV6NeuAcα-nLc4Cer in serous
cystadenocarcinomas occurs at a higher frequency than in the other
carcinomas. In agreement with our results, the frequency of
detection of sialyl Lea (CA19.9) in sera of patients
with ovarian serous cystadenocarcinomas was reported to be low in
comparison to those in the other carcinomas (3). In the case of a murine lymphoblastoid
cell line, cells with IV6NeuAcα-nLc4Cer were
shown to exhibit a low metastatic potential in comparison to those
without it, probably due to the attenuated expression of
adhesion-related Lewis structures, due to enhanced
α2,6-sialyltransferase activity (19). Transfection of the
α2,6-sialyltransferase gene into cell lines with Lewis glycolipids
is currently under investigation to demonstrate the modification of
the Lewis glycolipid expression. In addition, since serous
cystadenocarcinomas generally show a favorable prognosis, it can be
suggested that IV6NeuAcα-nLc4Cer is a useful
marker for the benign properties of cancer cells. To demonstrate
the value of screening for IV6NeuAcα-nLc4Cer
and Lewis-active glycolipids for the diagnosis of ovarian
carcinomas, amounts of these glycolipids in sera of patients with
serous cystadenocarcinomas are now being determined in comparison
to those in tissues in our laboratory.
References
|
1
|
Roseman S: Reflections on glycobiology. J
Biol Chem. 276:41527–41542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koprowski H, Herlyn M, Steplewski Z and
Sears HF: Specific antigen in serum of patients with colon
carcinoma. Science. 212:53–55. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Canney PA, Wilkinson PM, James RD and
Moore M: CA19-9 as a marker for ovarian cancer: alone and in
comparison with CA125. Br J Cancer. 52:131–133. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sgroi D, Varki A, Braesch-Andersen S and
Stamenkovic I: CD22, a B cell-specific immunoglobulin superfamily
member, is a sialic acid-binding lectin. J Biol Chem.
268:7011–7018. 1993.PubMed/NCBI
|
|
5
|
Springer TA and Lasky LA: Cell adhesion.
Sticky sugars for selectins. Nature. 349:196–197. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imai Y, Singer MS, Fennie C, Lasky LA and
Rosen SD: Identification of a carbohydrate-based endothelial ligand
for a lymphocyte homing receptor. J Cell Biol. 113:1213–1221. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiguchi K, Takamatsu K, Tanaka J, Nozawa
S, Iwamori M and Nagai Y: Glycosphingolipids of various human
ovarian tumors: a significantly high expression of
I3SO3GalCer and Lewis antigen in mucinous
cystadenocarcinoma. Cancer Res. 52:416–421. 1992.PubMed/NCBI
|
|
8
|
Iwamori M, Tanaka K, Kubushiro K, Lin B,
Kiguchi K, Ishiwata I, Tsukazaki K and Nozawa S: Alterations in the
glycolipid composition and cellular properties of ovarian
carcinoma-derived RMG-1 cells on transfection of the
alpha1,2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiguchi K, Iwamori Y, Suzuki N, Kobayashi
Y, Ishizuka B, Ishiwata I, Kita T, Kikuchi Y and Iwamori M:
Characteristic expression of globotriaosyl ceramide in human
ovarian carcinoma-derived cells with anticancer drug resistance.
Cancer Sci. 97:1321–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwamori M, Iwamori Y, Kubushiro K,
Ishiwata I and Kiguchi K: Characteristic expression of
Lewis-antigenic glycolipids in human ovarian carcinoma-derived
cells with anticancer drug-resistance. J Biochem. 141:309–317.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goupille C, Marionneau S, Bureau V,
Hallouin F, Meichenin M, Rocher J and Le Pendu J:
alpha1,2-Fucosyltransferase increases resistance to apoptosis of
rat colon carcinoma cells. Glycobiology. 10:375–382. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baldus SE, Hanisch FG, Pütz C, Flucke U,
Mönig SP, Schneider PM, Thiele J, Hölscher AH and Dienes HP:
Immunoreactivity of Lewis blood group and mucin peptide core
antigens: correlations with grade of dysplasia and malignant
transformation in the colorectal adenoma-carcinoma sequence. Histol
Histopathol. 17:191–198. 2002.
|
|
13
|
Iwamori M, Takamizawa K, Momoeda M,
Iwamori Y and Taketani Y: Gangliosides in human, cow and goat milk,
and their abilities as to neutralization of cholera toxin and
botulinum type A neurotoxin. Glycoconj J. 25:675–683. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin B, Kubushiro K, Akiba Y, Cui Y,
Tsukazaki K, Nozawa S and Iwamori M: Alteration of acidic lipids in
human sera during the course of pregnancy: characteristic increase
in the concentration of cholesterol sulfate. J Chromatogr B Biomed
Sci Appl. 704:99–104. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka K, Kubushiro K, Iwamori Y, Okairi
Y, Kiguchi K, Ishiwata I, Tsukazaki K, Nozawa S and Iwamori M:
Estrogen sulfotransferase and sulfatase: roles in the regulation of
estrogen activity in human uterine endometrial carcinomas. Cancer
Sci. 94:871–876. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikehara Y, Shimizu N, Kono M, Nishihara S,
Nakanishi H, Kitamura T, Narimatsu H, Tsuji S and Tatematsu M: A
novel glycosyltransferase with a polyglutamine repeat; a new
candidate for GD1alpha synthase (ST6GalNAc V)(1). FEBS Lett.
463:92–96. 1999.PubMed/NCBI
|
|
17
|
Yoshiki J, Kubushiro K, Tsukazaki K,
Udagawa Y, Nozawa S and Iwamori M: High expression of uridine
diphosphate-galactose: Lc3Cer beta 1–3
galactosyltransferase in human uterine endometrial cancer-derived
cells as measured by enzyme-linked immunosorbent assay and
thin-layer chromatography-immunostaining. Jpn J Cancer Res.
88:669–677. 1997.PubMed/NCBI
|
|
18
|
Weston BW, Nair RP, Larsen RD and Lowe JB:
Isolation of a novel human alpha (1,3)fucosyltransferase gene and
molecular comparison to the human Lewis blood group alpha
(1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic
genes encoding enzymes with distinct acceptor substrate
specificities. J Biol Chem. 267:4152–4160. 1992.
|
|
19
|
Lo NW, Dennis JW and Lau JT:
Overexpression of the alpha2,6-sialyltransferase, ST6Gal I, in a
low metastatic variant of a murine lymphoblastoid cell line is
associated with appearance of a unique ST6Gal I mRNA. Biochem
Biophys Res Commun. 264:619–621. 1999. View Article : Google Scholar : PubMed/NCBI
|