Introduction
Breast cancer is the most commonly diagnosed cancer
in women all over the world and the leading cause of cancer-related
death (1). Age is the greatest risk
factor for the development of new cases of breast cancer, and the
incidence increases with age: 1 in 50 by age 50, 1 in 14 by age 70
and 1 in 9 by age 85 (2).
Approximately 50% of breast cancers occur in women of 65 years of
age or older and 35% occur after the age of 70 (3). Breast cancer has benefitted from
mortality-lowering earlier diagnosis and more effective treatments
in the last three decades, but studies regarding possible
differences in the biology and clinical outcomes of breast cancer
according to age are relatively limited, owing to the fact that
elderly women have been excluded from large randomized controlled
trials (4,5).
In spite of the paucity of data, physicians consider
age to be an important determinant of therapy. Median life
expectancy is calculated to be 14.8 years for women of 70 years,
while at 80 years the median survival remains 8.4 years, even
though it is often underestimated by clinicians (6). However, co-morbid diseases affect
choice of treatment, with a 20-fold higher rate of non-breast
cancer mortality in patients with three or more significant
co-morbidities (7). Therefore,
mobility, cognition, concurrent medications and social factors
should be taken into consideration when determining the proper
treatment for older patients (8).
The tumor features and outcome of elderly breast
cancer in the Chinese population have yet to be reported.
Furthermore, the sensitivity of systemic adjuvant therapies in
elderly patients has yet to be reported in this population. Taking
these factors into account, a retrospective analysis was performed
in order to better understand the nature of elderly breast cancer
and determine the prognostic factors and treatment sensitivity of
elderly patients.
Materials and methods
Patients
This study was based on the retrospective analysis
of our department's database. The selection criteria of the case
group were: female gender, ≥70 years of age, with pathologically
diagnosed breast cancer and surgery at our institute. A total of
594 patients meeting the selection criteria and treated from August
1991 to October 2006 were identified in this database. A control
group of 657 patients aged <70 years was selected and randomized
from the same database by age and year of diagnosis. Data recorded
on the database for each patient included age at diagnosis, date of
diagnosis, menarche age, body mass index (BMI), marriage status,
family history of cancer, diagnostic method, clinical stage,
treatment details (surgery and adjuvant therapy), histopathological
features [tumor size, lymph node (LN) status, estrogen/progesterone
receptor (ER/PR) and Her-2 expression] and follow-up information.
Routine follow-up was carried out every 3 months after diagnosis
during the first 2 years, every 6 months during the following 2
years and then once annually. Follow-up information was obtained
from hospital and office records and from the patients and their
families. The date of the last follow-up and date of recurrence or
death were recorded.
The stage was determined from pathological records
and classified according to the AJCC TNM guidelines. We defined
stages 0, I and II as ‘early stage’ and stages III and IV as
‘advanced stage’. Non-infiltrating carcinoma was defined as ductal
carcinoma in situ and lobular carcinoma in situ.
Infiltrating carcinoma comprised infiltrating ductal and lobular
carcinoma or other types of infiltrating carcinomas.
Immunohistochemical staining of ER, PR and Her-2/neu were carried
out in the Pathology department of our hospital. The scoring system
for ER and PR concluded the proportion and intensity scores.
Subsequently, staining results ranged from score 0 to 12, and
scores of 1-12 with the nucleic staining of carcinoma cells were
defined as positive. Her-2/neu was defined as negative for scores
of 0–8 (namely 0, 1+ and 2+ in the Dako scoring system) and
positive for strong membranous staining with scores of 9–12 (namely
Dako score 3+). To examine whether the proportion of
ER/PR+ tumors differed by age, the patients were divided
into 5 groups according to age: 23–34, 35–49, 50–64, 65–79 and
80–94 years. Patients treated with breast-conserving surgery (BCS)
or with locally advanced breast cancer received irradiation. The
dose to the whole breast was 50 Gy in 1.8–2.0 Gy per fraction, with
a boost to the tumor bed in breast-conserving patients, while the
dose to the chest wall and regional nodes was 50 Gy, as recommended
for mastectomy patients. Patients with ER+ and/or
PR+ diseases received adjuvant endocrine therapy for at
least 3 years. Patients recorded as ‘chemotherapy-given’ were
defined as patients who had received adjuvant chemotherapy for no
less than 2 cycles. The majority of the elderly patients received
cyclophosphamide, methotrexate and 5-fluorouracil (CMF) or
anthracycline-based chemotherapy, using the recommended dose and
schedule. Among them, ~86% of the patients received ≥4 cycles and
only 14% of the patients recieved a reduction in the dose owing to
the side effects. Trastuzumab was not used in any of the
patients.
Statistical methods
The date of surgery was used as the point of
commencement for survival analysis. Relapse-free survival (RFS) was
calculated from the date of surgery to the first evidence of
disease recurrence (any site); all other patients were censored for
progression at the date of the last visit without signs of
progression or death. Overall survival (OS) was measured from the
date of surgery until the patients succumbed to any cause or until
the last follow-up. The significance of differences in categorical
variables was evaluated using the Chi-square test and the
significance of differences in continuous variables was evaluated
using the Student's t-test. Cumulative RFS and OS were calculated
using the Kaplan-Meier method (9).
P<0.05 was considered to be statistically significant.
Statistical analyses were performed using SPSS version 15.0 (SPSS,
Chicago, IL, USA).
Results
Clinical features
Table I shows the
clinical characteristics of patients in the two groups. A total of
1,251 breast cancer patients were selected, 594 patients aged ≥70
years were in the case group and 657 patients aged <70 years
were in the control group. The median age was 75.2±4.4 years (range
70–92) in the case group and 49.8±9.2 years (range 23–69) in the
control group. Age of menarche, parous status and BMI index were
similar in the two groups. No significant difference was found in
the proportion of breast cancer in first-degree relatives between
the two groups; however, elderly patients showed a relatively lower
frequency of other cancer history compared to young patients (9.9
vs. 15%, P=0.008). Only 11.8% (70/594) of the elderly patients
received a pre-operative pathological diagnosis, <25% (164/657)
of the patients in the control group. A cytology diagnosis and
fiberoptic ductoscopy were more commonly used in the elderly, while
a histology diagnosis, such as core needle biopsy (CNB), was
preferred in young patients (P<0.001). A total of 537 patients
in the case group and 657 in the control group had detailed surgery
records and the difference in surgery types was significant.
Approximately 10% of the elderly patients had no axillary treatment
(lumpectomy or mastectomy alone), a percentage that was higher as
compared to the control group (P<0.001). Furthermore, the
proportion of breast-conserving surgery performed (lumpectomy ±
axillary, 3.2%) was lower in the case group (P<0.001).
 | Table IThe clinical characteristics of the
patients in the case and control groups. |
Table I
The clinical characteristics of the
patients in the case and control groups.
| Case (%) | Control (%) | P-value |
|---|
| No. of
patients | 594 | 657 | |
| Mean age
(years) | 75.2±4.4 | 49.8±9.2 | |
| Menarche age
(years) | 15.2±1.7 | 14.8±1.6 | |
| BMI
(kg/m2) | 24.65 | 23.96 | |
| Parous | | | |
| No | 516 (97.9) | 640 (98.3) | 0.620 |
| Yes | 11 (2.1) | 11 (1.7) | |
| Family history of
breast cancer | | | |
| No | 573 (96.5) | 614 (94.5) | 0.100 |
| Yes | 21 (3.5) | 36 (5.5) | |
| Family history of
other cancer | | | |
| No | 535 (90.1) | 551 (85.0) | 0.008 |
| Yes | 59 (9.9) | 97 (15.0) | |
| Pre-operative
diagnosis | 70 | 164 | |
| Histology | 29 (41.4) | 110 (67.1) | 0.001 |
| Cytology | 24 (34.3) | 21 (12.8) | |
| Fiberoptic
ductoscopy | 12 (17.1) | 18 (11.0) | |
| Lymph node
biopsy | 5 (7.1) | 15 (9.1) | |
| Surgery | 537 | 657 | |
| Lumpectomy | 14 (2.6) | 8 (1.2) | 0.001 |
| Mastectomy | 41 (7.6) | 12 (1.8) | |
| MRM | 412 (76.7) | 503 (76.6) | |
| RM | 67 (12.5) | 37 (5.6) | |
| Lumpectomy +
axillary | 3 (0.6) | 74 (11.3) | |
|
Reconstruction | 0 (0.0) | 23 (3.5) | |
Tumor stage
Tumor stage distribution was generally different in
the two groups. We defined stages 0, I and II as ‘early stage’ and
stages III and IV as ‘advanced stage’. The percentage of
early-stage patients was significantly different between the two
groups: 89.4% in the case vs. 83.6% in the control group (P=0.004,
OR=1.70, 95% CI 1.18–2.33).
Pathological and biological
characteristics
Table II shows the
pathological and biological characteristics of the two groups. The
two groups had similar histological subtypes. Patients in the case
group had more LN-, Her-2-and p53-negative diseases (P<0.05).
The percentage of large tumor size (>5 cm) in the case group was
higher than that in the control group, 13.2 vs. 8.4% (P=0.023). No
difference was noted in ER and PR status (P=0.16 and 0.79,
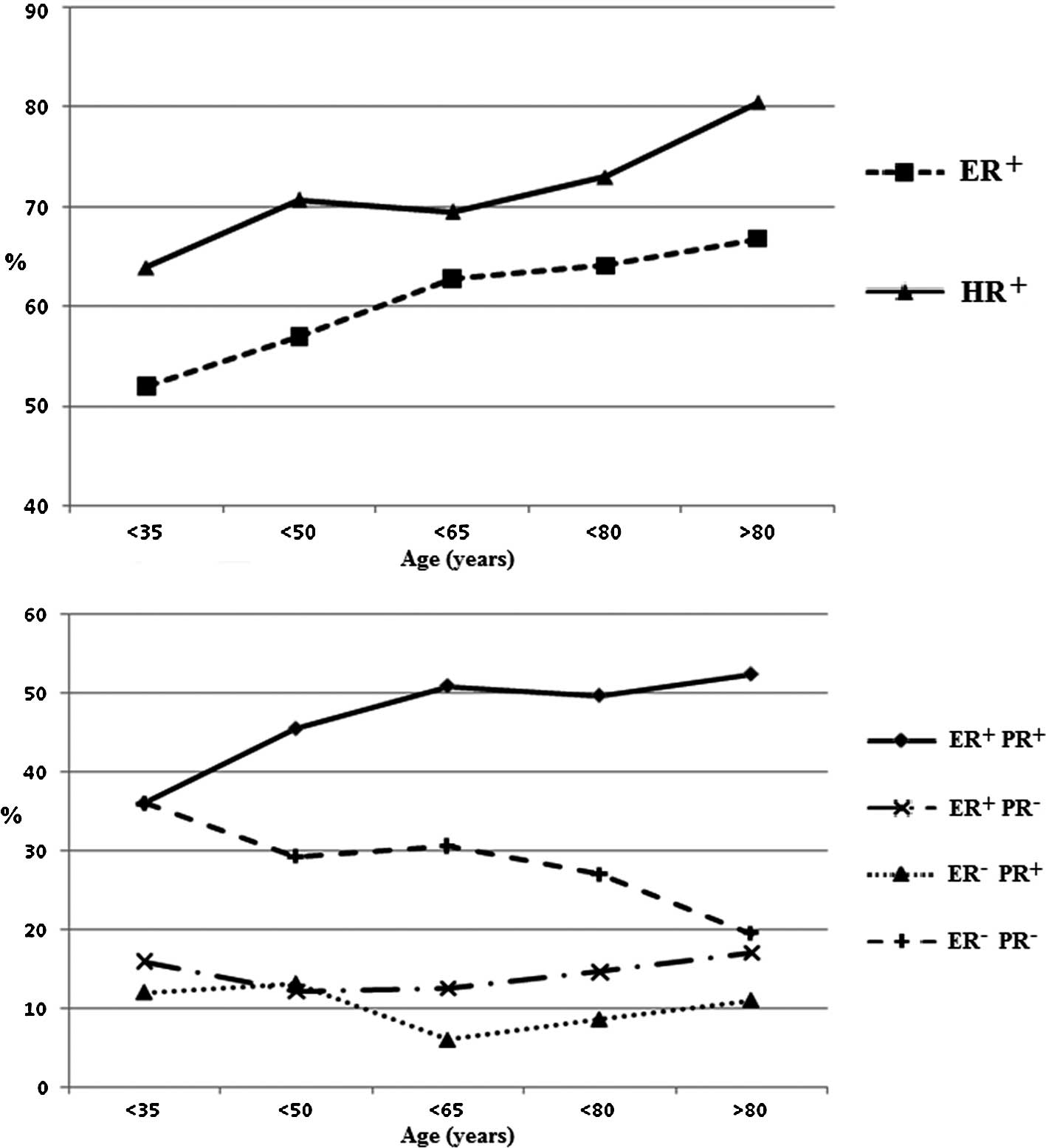
respectively). However, we found that the proportion of
ER+ and hormone receptor (HR)-positive tumors increased
with increasing age (ER+ or PR+ defined as
HR+). The overall ER+PR− or
ER−PR+ proportion did not vary with age,
while in the elderly patients we found a higher proportion of
ER+PR+ and a lower proportion of
ER−PR− diseases compared to patients aged
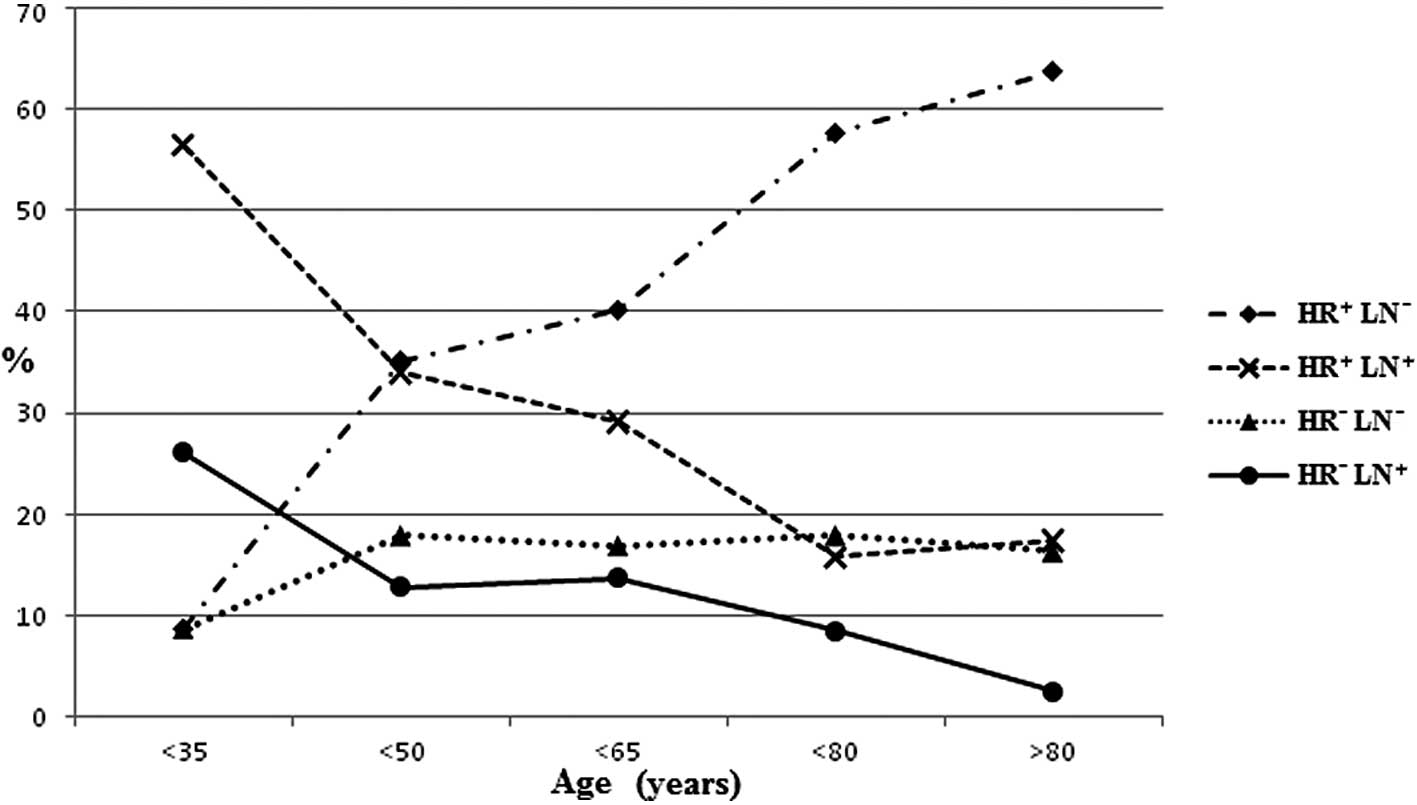
<70 years (Fig. 1). Fig. 2 shows the distribution of HR and LN
status according to age. Whatever the HR status proved to be, elder
patients had less LN+ disease. For these LN−
patients, only the HR+ proportion increased along with
age.
 | Table IIThe pathological and biological
characteristics of the patients in the case and control groups. |
Table II
The pathological and biological
characteristics of the patients in the case and control groups.
| Case (%) | Control (%) | P-value | OR (95% CI) |
|---|
| Histological
subtype | 573 | 647 | | |
| Carcinoma in
situ | 53 (9.2) | 74 (11.4) | 0.327 | |
| Microinvasive | 18 (3.1) | 15 (2.3) | | |
| Invasive | 502 (86.7) | 557 (86.1) | | |
| Lymph node | 552 | 600 | | |
| Negative | 428 (77.5) | 347 (57.8) | <0.001 | 0.40
(0.31–0.51) |
| Positive | 124 (22.5) | 253 (42.2) | | |
| Tumor size | 575 | 616 | | |
| ≤2 cm | 162 (28.2) | 194 (31.5) | 0.023 | - |
| 2–5 cm | 337 (58.6) | 370 (0.1) | | |
| >5 cm | 76 (13.2) | 52 (8.4) | | |
| Estrogen
receptor | 453 | 487 | | |
| Negative | 160 (35.3) | 194 (39.8) | 0.160 | - |
| Positive | 293 (64.7) | 293 (60.2) | | |
| Unknown | 141 | 170 | | |
| Progesterone
receptor | 438 | 484 | | |
| Negative | 184 (42.0) | 208 (43.0) | 0.790 | - |
| Positive | 254 (58.0) | 276 (57.0) | | |
| Unknown | 156 | 173 | | |
| Her-2 | 434 | 482 | | |
| Negative | 389 (89.6) | 410 (85.1) | 0.047 | 1.52
(1.02–2.26) |
| Positive | 45 (10.4) | 72 (14.9) | | |
| Unknown | 160 | 175 | | |
| p53 | 411 | 466 | | |
| Negative | 179 (43.6) | 159 (34.1) | 0.004 | 1.50
(1.13–1.96) |
| Positive | 232 (56.4) | 307 (65.9) | | |
| Unknown | 183 | 191 | | |
Relapse-free and overall survival
The median follow-up time was 45.45 months in the
whole cohort of 1,154 patients (case group 543 and control group
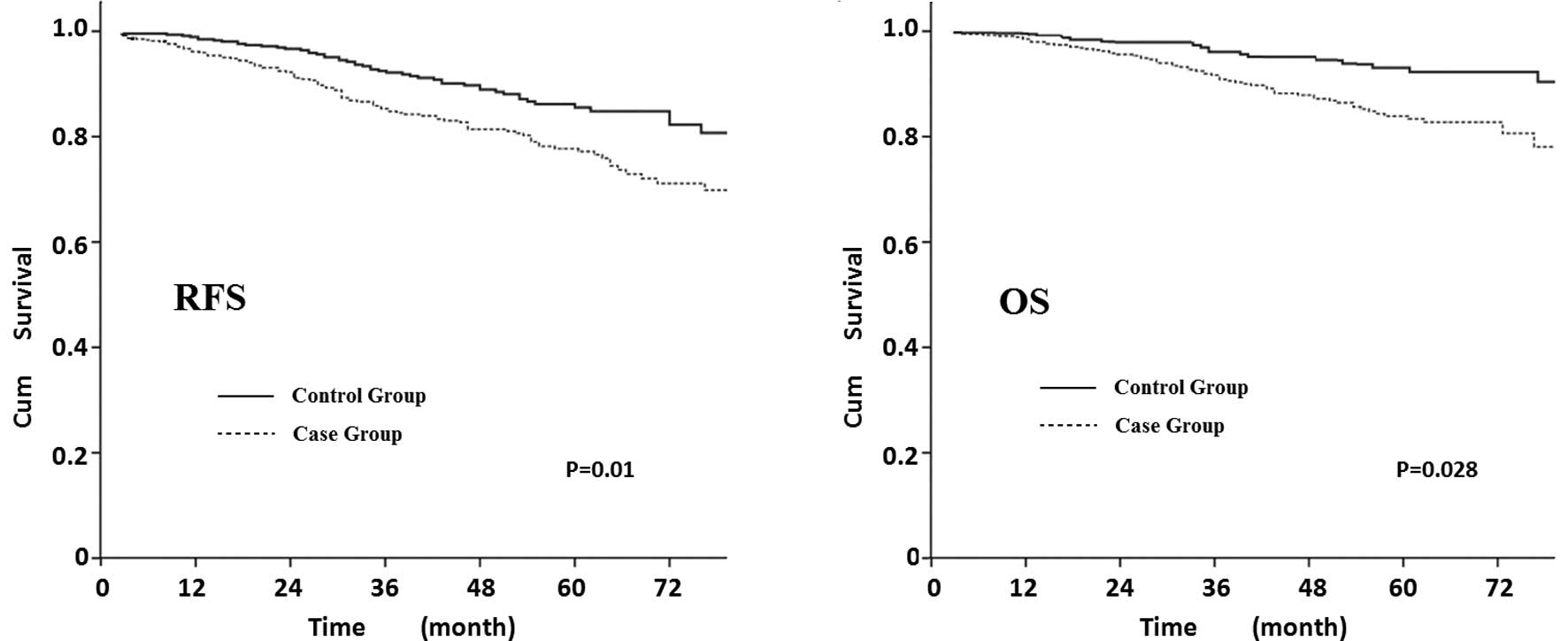
611). Fig. 3 shows that elderly
patients exhibited a decreased survival curve compared to younger
patients, the 5-year RFS was 77% in the elderly patients and 86% in
the young patients (P=0.01) and the 5-year OS was 82 and 93%,
respectively (P=0.028; the RFS and OS were adjusted by tumor size,
LN, HR, Her-2 and adjuvant therapy).
Prognostic factors
The elderly patients had more ER+ and
LN− diseases; thus the prognostic factors were analysed
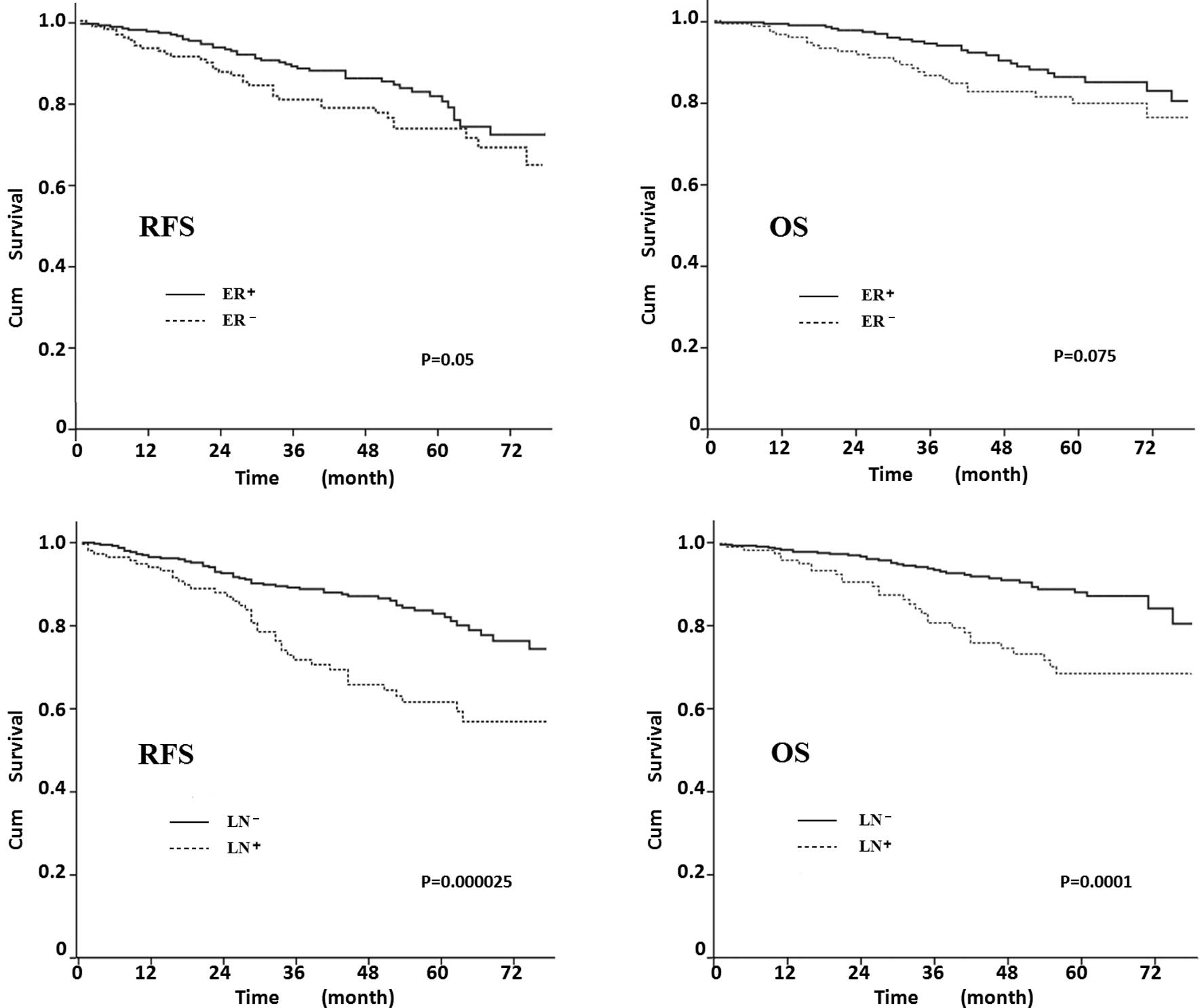
to determine whether the factors affected RFS and OS (Fig. 4). The 5-year RFS was 81% in
ER+ patients, 73% in ER− (P=0.05), 83% in
LN− and 63% in LN+ patients (P=0.000025). The
OS of ER− patients was lower than that of ER+
patients, although the differences were not statistically
significant (P=0.075; Fig. 4B).
LN− patients had a higher OS, as shown in Fig. 4D (P=0.0001).
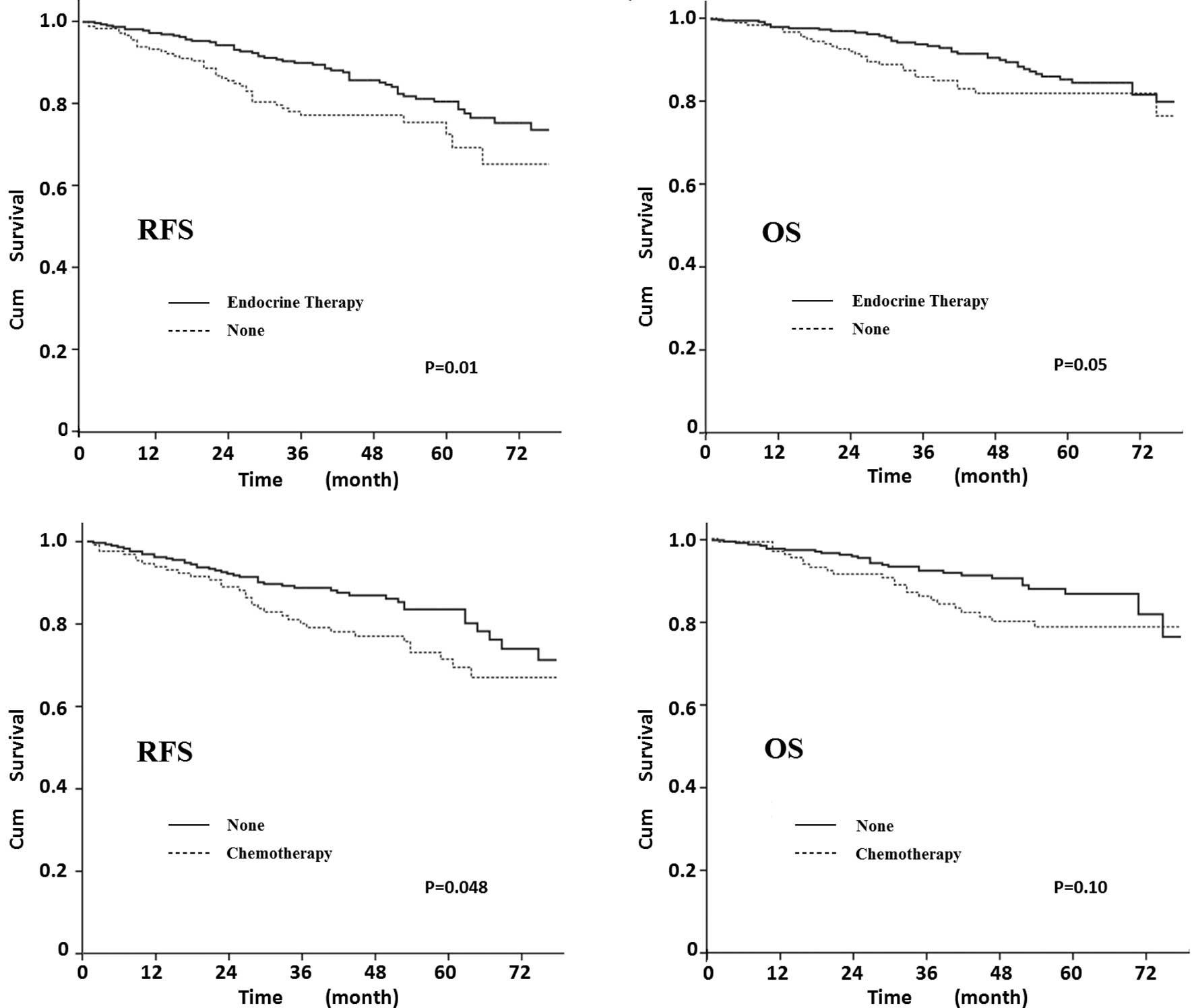
Adjuvant therapy
We investigated whether endocrine therapy and
chemotherapy were beneficial for elderly patients. Fig. 5A and B shows that RFS and OS were
significantly better in elderly patients that underwent endocrine
therapy (P=0.01 and 0.05, respectively). Patients undergoing
chemotherapy had a decreased 5-year RFS compared to patients
undergoing omitted chemotherapy (70 vs. 83%, P=0.048). Patients
without chemotherapy may therefore have a better survival, although
a statistically significant impact on OS was not demonstrated
(P=0.10).
Discussion
The WHO Health Report shows that the average life
expectancy is 76 years in developed countries and 67 years in
developing countries, and the median life expectancy of Chinese
women is 74 years (10). Therefore,
in modern society, clinicians are likely to increasingly treat
cancers in the elderly. However, the definition of ‘elderly’
remains controversial. Gennari and Audisio suggested that the term
‘elderly’ involves patients who are 70 years of age or older since
this age limit represents a milestone beyond which older people are
found (11). A survey by the Breast
International Group (BIG) comprising 277 oncologists from 28
countries indicated that 70 years is the cut-off age commonly used
to define a patient as elderly (12). Accordingly, this retrospective
analysis assumed 70 years to be the cut-off age.
No differences were detected in menarche age, parous
status, BMI index or family history of breast cancer between the
case and control groups. However, we found a significantly lower
proportion of family cancer history among the elderly patients,
which is in agreement with Pappo et al (13). The differences in pre-operative
pathological diagnostic methods were also confirmed by our study.
As older patients were less healthy, a histology biopsy, such as
CNB, was often substituted by a cytology exam prior to surgery.
Therefore, when treating the elderly, patient evaluation should be
closely assessed. A detailed baseline assessment, such as
Comprehensive Geriatric Assessment (CGA) and Multidimensional
Geriatric Assessment (MGA), may help to evaluate the performance
status and guide the decision-making process (14–16).
Recent advances in anesthesia resulted in a
significant decrease in operative mortality proportions for fit
older women with breast cancer. Additionlly, extremely elderly
patients are safely treated by breast cancer surgery with a low
incidence of perioperative complications (17). We performed different types of
surgery for elderly patients (Table
I). However, the case group received less axillary treatment or
BCS than the control group, as was the case in the study by Pierga
et al (18). The axillary
dissection rate decreases with age (19); a rate which was half in the over
70-year age group when compared to younger patients (20). Whether older patients require LN
evaluation is often debated (21).
Studies have suggested that older patients with HR+
breast cancer or tumors smaller than 3 cm and who are clinically
node-negative omit node dissection or may be offered a sentinel LN
biopsy (22,23).
Another reason for omitting axillary dissection is
that breast cancer in older women often presents indolent features:
a higher frequency of ER/PR+, lower expression of HER-2
and p53 proteins, low proliferation rate, low S-phase or low Ki67
and a well-differentiated tumor (24–26).
The present study showed a favorable tumor profile in the elderly
patients, as well as a lower expression of Her-2 and p53 and an
increased proportion of ER+ and PR+ tumors
with age increase. In our series, the ER+PR−
and ER−PR+ the proportion of tumor profiles
was stable in all age groups, although the elder patients exhibited
more ER+PR+ and fewer
ER−PR− tumors. The distribution of HR and LN
status also varied with age. In patients less than 35 years of age
with LN− diseases, the HR+ and HR−
proportions were similar, but were lower than those in
LN+ diseases. However, the favorable prognosis subtype
HR+LN− proportion increased markedly with
age, while the LN− proportion markedly decreased.
Although the case group had a high proportion of
HR+, LN− and early-stage disease, RFS and OS
decreased. We hypothesized that i) the higher rate of large tumor
size in the case group may lead to an early relapse, as suggested
by Truong et al who found that older patients had similar or
higher local recurrence risks, particularly patients with tumors
larger than 5 cm or with more than 4 positive nodes (27); ii) more co-morbid diseases of older
women may result in more deaths, as reported by Louwman et
al who noted that 14% of the newly diagnosed patients at age
70–79 and 22% of patients older than age 80 suffered from more than
two concomitant conditions (28);
iii) more elderly patients received less than ‘optimal’ treatments
(29,30) and under-treatment may strongly
decrease outcome (31).
The present study focused on whether ER and LN
status had an impact on survival in the case group. Fig. 4 shows that LN status was the most
significant prognostic predictor in elderly patients, and markedly
affected RFS and OS (P<0.001). The significant decrease in the
survival of LN+ patients suggested the importance of
axillary surgery in elderly patients. Inadequate axillary treatment
may lead to an increased rate of regional failure (32) and LN status is reported to be a
predictor of distant metastasis in elderly breast cancer patients
(33). ER status also predicts
recurrence in elderly patients, and although OS was not
statistically different, ER+ had a better survival
curve.
The efficacy and utility of adjuvant systemic
therapy in elderly patients remains uncertain, particularly
chemotherapy (34,35). Thus, it remains to be determined
whether elderly patients exhibited the same sensitivity to systemic
adjuvant therapy. In our data, elderly patients with suitable
functional status received chemotherapy. However, these patients
exhibited a lower RFS and OS. It is likely that elderly patients
were less sensitive to chemotherapy, and, as Livi et al
reported, chemotherapy was correlated with a lower survival rate
(36). Nevertheless, this result
may be due to the fact that only patients with worse prognostic
factors received chemotherapy and that the majority of elderly
patients in our study received CMF or anthracycline-based
chemotherapy, which had a low effect and high cardiac toxicity
(37,38). Tamoxifen (TAM) is as effective for
reducing the risk of recurrence and cancer-related mortality in
women over 70 years of age as it is for younger women (39). The majority of the ER+
patients in the case group received TAM as adjuvant endocrine
therapy. We found that TAM significantly improved RFS and OS in the
elderly group with good tolerance.
In conclusion, elderly breast cancer patients had
favorable features. However, the co-morbid diseases and
under-treatment of elderly patients resulted in lower RFS and OS in
the elderly patients. ER+ and LN− were
favorable prognostic predictors. Careful evaluation should be
considered when recommending adjuvant system therapy.
References
|
1
|
Stewart BW and Kleihues P: World Cancer
Report. IARC Press; Lyon: 2003
|
|
2
|
Rao VS, Garimella V and Hwang M:
Management of early breast cancer in the elderly. Int J Cancer.
120:1155–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimmick GG and Balducci L: Breast cancer
and aging: clinical interactions. Hematol Oncol Clin North Am.
14:213–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van den Brandt PA, Spiegelman D and Yaun
SS: Pooled analysis of prospective cohort studies on height,
weight, and breast cancer risk. Am J Epidemiol. 152:514–527.
2000.PubMed/NCBI
|
|
5
|
Mitka M: Too few older patients in cancer
trials: experts say disparity affects research results and care.
JAMA. 290:27–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Extermann M, Balducci L and Lyman GH: What
threshold for adjuvant therapy in older breast cancer patients? J
Clin Oncol. 18:1709–1717. 2000.PubMed/NCBI
|
|
7
|
Satariano WA and Ragland DR: The effect of
comorbidity on 3-year survival of women with primary breast cancer.
Ann Intern Med. 120:104–110. 1994.PubMed/NCBI
|
|
8
|
Macaskill EJ, Renshaw L and Dixon JM:
Neoadjuvant use of hormonal therapy in elderly patients with early
or locally advanced hormone receptor-positive breast cancer.
Oncologist. 11:1081–1088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Statist Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
10
|
The world health report 2007 – a safer
future: global public health security in the 21st century.
http://www.who.int/whr/2007/en/index.html.
|
|
11
|
Gennari R and Audisio RA: Breast cancer in
elderly women. Optimizing the treatment. Breast Cancer Res Treat.
110:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biganzoli L, Goldhirsch A and Straehle C:
Adjuvant chemotherapy in elderly patients with breast cancer: a
survey of the Breast International Group (BIG). Ann Oncol.
15:207–210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pappo I, Karni T and Sandbank J: Breast
cancer in the elderly: histological, hormonal and surgical
characteristics. Breast. 16:60–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Repetto L, Fratino L and Audisio RA:
Comprehensive geriatric assessment adds information to Eastern
Cooperative Oncology Group performance status in elderly cancer
patients: an Italian Group for Geriatric Oncology Study. J Clin
Oncol. 20:494–502. 2002. View Article : Google Scholar
|
|
15
|
Massa E, Madeddu C and Astara G: An
attempt to correlate a ‘Multidimensional Geriatric Assessment’
(MGA), treatment assignment and clinical outcome in elderly cancer
patients: results of a phase II open study. Crit Rev Oncol Hematol.
66:75–83. 2008.
|
|
16
|
Hurria A, Gupta S and Zauderer M:
Developing a cancer-specific geriatric assessment: a feasibility
study. Cancer. 104:1998–2005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samain E, Schauvliège F and Deval B:
Anesthesia for breast cancer surgery in the elderly. Crit Rev Oncol
Hematol. 46:115–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pierga JY, Girre V and Laurence V:
Characteristics and outcome of 1755 operable breast cancers in
women over 70 years of age. Breast J. 13:369–375. 2004.PubMed/NCBI
|
|
19
|
Al-Hilaly M, Willsher PC and Robertson JF:
Audit of a conservative management policy of the axilla in elderly
patients with operable breast cancer. Eur J Surg Oncol. 23:339–340.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bland KI, Scott-Conner CE and Menck H:
Axillary dissection in breast-conserving surgery for stage I and II
breast cancer: a National Cancer Data Base study of patterns of
omission and implications for survival. J Am Coll Surg.
188:586–595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martelli G, Miceli R and De Palo G: Is
axillary lymph node dissection necessary in elderly patients with
breast carcinoma who have a clinically uninvolved axilla? Cancer.
97:1156–1163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
International Breast Cancer Study Group.
Randomized trial comparing axillary clearance versus no axillary
clearance in older patients with breast cancer: first results of
International Breast Cancer Study Group Trial 10-93. J Clin Oncol.
24:337–344. 2006. View Article : Google Scholar
|
|
23
|
Gennari R, Rotmensz N and Perego E:
Sentinel node biopsy in elderly breast cancer patients. Surg Oncol.
13:193–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diab SG, Elledge RM and Clark GM: Tumor
characteristics and clinical outcome of elderly women with breast
cancer. J Natl Cancer Inst. 92:550–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eppenberger-Castori S, Moore DH Jr and
Thor AD: Age-associated biomarker profiles of human breast cancer.
Int J Biochem Cell Biol. 34:1318–1330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daidone MG, Coradini D and Martelli G:
Primary breast cancer in elderly women: biological profile and
relation with clinical outcome. Crit Rev Oncol Hematol. 45:313–325.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Truong PT, Lee J and Kader HA:
Locoregional recurrence risks in elderly breast cancer patients
treated with mastectomy without adjuvant radiotherapy. Eur J
Cancer. 41:1267–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Louwman WJ, Vulto JC and Verhoeven RH:
Clinical epidemiology of breast cancer in the elderly. Eur J
Cancer. 43:2242–2252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Enger SM, Thwin SS and Buist DS: Breast
cancer treatment of older women in integrated health care settings.
J Clin Oncol. 24:4377–4383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bouchardy C, Rapiti E and Blagojevic S:
Older female cancer patients: importance, causes, and consequences
of undertreatment. J Clin Oncol. 25:1858–1869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bouchardy C, Rapiti E and Fioretta G:
Undertreatment strongly decreases prognosis of breast cancer in
elderly women. J Clin Oncol. 21:3580–3587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wyld L and Reed M: The role of surgery in
the management of older women with breast cancer. Eur J Cancer.
43:2253–2263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chagpar AB, McMasters KM and Martin RC:
Determinants of early distant metastatic disease in elderly
patients with breast cancer. Am J Surg. 192:317–321. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hurria A, Leung D and Trainor K: Factors
influencing treatment patterns of breast cancer patients age 75 and
older. Crit Rev Oncol Hematol. 46:121–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du XL, Osborne C and Goodwin JS:
Population-based assessment of hospitalizations for toxicity from
chemotherapy in older women with breast cancer. J Clin Oncol.
20:4636–4642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livi L, Paiar F and Saieva C: Breast
cancer in the elderly: treatment of 1500 patients. Breast J.
12:353–359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doyle JJ, Neugut AI and Jacobson JS:
Chemotherapy and cardiotoxicity in older breast cancer patients: a
population-based study. J Clin Oncol. 23:8597–8605. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Crivellari D, Bonetti M and
Castiglione-Gertsch M: Burdens and benefits of adjuvant
cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for
elderly patients with breast cancer: the International Breast
Cancer Study Group Trial VII. J Clin Oncol. 18:1412–1422.
2000.PubMed/NCBI
|
|
39
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI
|