Introduction
According to statistics from the 2005 Spanish Agency
of Cancer, pancreatic cancer ranked 7th and 5th in incidence among
cancers in males and females, respectively, and the rates are on
the increase (1). Smoking,
high-calorie diets, certain chemical exposures, chronic
pancreatitis, cystic fibrosis and diabetes are known to induce
pancreatic cancer. However, the exact cause has yet to be
elucidated.
Pancreatic cancer is associated with a poor
prognosis, even after curative resection, due to the low resection
rates and late diagnosis. Only 15–20% of patients undergo resection
and the 5-year survival rate is approximately 20% (2). In addition, adjuvant therapies have
yet to be standardized.
Carcinogenesis in the pancreas is a process that
involves various factors in DNA, RNA and protein synthesis.
Previously, mutation of the tumor suppressor gene and oncogenes
(Her-2/neu, COX-2, p16, p21 and p53) was reported as one of the
frequent genetic alterations in pancreatic carcinoma (3). An increased expression of the
epidermal growth factor receptor (EGFR) and vascular epidermal
growth factor receptors (VEGFs) in malignant pancreatic neoplasm
has also been noted (4). However,
the patterns of expression or co-expression of these markers and
their relationship with clinicopathological data remain
unclear.
In the present study, the expression of VEGF
receptors (R)-1 and -2, EGFR, Her-2/neu, COX-2, p16, p21 and p53
was compared in tumoral and normal tissue using tissue microarrays
(TMAs). The principal aim was to detect the effect of patterns of
expression in the prognostic survival of the patients included in
this study.
Materials and methods
Patients and tissue samples
A total of 50 patients were included in the study,
32 of whom were male (63%) and 18 female (37%). Histopathology,
tumor stage and survival time were based on the original
histopathology reports and patient clinical records. This study was
approved by the Ethics Committee of the University Hospital,
Santiago de Compostela, Spain. A Tissue Arrayer (Beecher
Instruments, Sun Prairie, WI, USA) was used to construct two
different TMA blocks, according to the manufacturer’s instructions
(4). The cases were histologically
reviewed and the most representative areas were marked in the
paraffin blocks.
Two 1-mm-diameter cylinders selected from two
different areas were included in each case from 50 pancreatic
ductal adenocarcinomas. The cases were obtained from the files of
the Department of Pathology, University Hospital, Santiago de
Compostela, Spain. A total of 6 tissue controls were included in
each TMA. The TMA blocks were sectioned to produce 4 μm
sections.
Immunohistochemistry
Immunohistochemical analysis was performed with a
universal second antibody kit that used a peroxidase-conjugated
labeled-dextran polymer (EnVision, Peroxidase/DAB; Dako, Glostrup,
Denmark). A commercially available panel of monoclonal and
polyclonal antibodies was used for the following markers: VEGF-R1
and -2, EGFR, Her2/neu, COX-2, p16, p21 and p53. Detailed data on
the antibodies are shown in Table
I. Only nuclear immunoreactivity was considered positive for
p16, p21, p53. Only cytoplasmatic staining showed immunoreactivity
for VEGF-R1 and -R2, EGFR, Her2/neu and COX-2. Equivocal staining
was considered to be negative. The immunohistochemical results were
recorded as positive when >5% of the neoplastic cells were
immunoreactive. The percentage of positive cells was scored on a
semi-quantitative scale as: 0, <5% of positive cells; 1, 5–33%
of positive cells; 2, 34–66% of positive cells; and 3, ≥67% of
positive cells. Staining intensity was graded as: 1+, weak; 2+,
moderate; and 3+, strong.
 | Table IData on immnunohistochemical
markers. |
Table I
Data on immnunohistochemical
markers.
| Biomarker | Clone | Company | Dilution |
|---|
| p16 | EGH4 | Mtm (molecular tools
in medicine) | 1:25 |
| p21 Waf/Cip1 | CP-74 Isotype mouse
IgG | Sigma | 1:100 |
| p53 | DO-7 | Novocastra | 1:20 |
| Her2/neu | K | Dako | 1:10 |
| COX-2 | COX-2 | Cayman Chemical | 1:50 |
| VEGF-R1 | SC-316 | Santa Cruz
Biotechnology | 1:1,000 |
| VEGF-R2 | SC-6251 | Santa Cruz
Biotechnology | 1:2,000 |
| EGFR | EGFR.113 | Novocastra | 1:10 |
Statistical analysis
Percentage scores of 1, 2 and 3 (≥5 cells stained)
were considered to be positive for the comparison between groups.
Correlations between the markers and individual clinicopathological
variables were evaluated using the Chi-square test. The survival
time was defined as the time since resection surgery to the last
contact or until the patient succumbed to the disease. The survival
curves were calculated using the Kaplan-Meier method, and the
Mantel-Cox log-rank test was used to determine the differences
between the curves. The effect of independent binary variables on
the incidence of expression markers was calculated using the
logistic regression modeling technique. The reported p-values
(p<0.05) were considered to be significant. The statistical
analysis was performed using the SPSS version 12 software (Chicago,
IL, USA).
Results
Patient characteristics
Table II summarizes
the clinical and histopathological findings in our patient
population. The cohort includes 50 patients, the majority of whom
(75%) were >60 years of age (range 44–78, median 61).
 | Table IIClinicopathological characteristics of
the 50 patients with pancreatic adenocarcinoma. |
Table II
Clinicopathological characteristics of
the 50 patients with pancreatic adenocarcinoma.
| Characteristics | No. (%) |
|---|
| Gender |
| Male | 32 (64.00) |
| Female | 18 (36.00) |
| Location |
| Head | 29 (76.30) |
| Body/tail | 9 (23.60) |
| Histological
degree |
| Well
differentiated (G1) | 17 (35.40) |
| Moderately
differentiated (G2) | 27 (56.20) |
| Poorly
differentiated (G3) | 4 (8.33) |
| Tumor size and
depth of invasiona |
| T1 | 9 (19.10) |
| T2 | 9 (19.10) |
| T3 | 28 (59.50) |
| T4 | 1 (2.13) |
| Lymph node
metastasis |
| Without | 20 (42.50) |
| With | 27 (57.40) |
| Stagea |
| IA | 5 (10.20) |
| IB | 5 (10.20) |
| IIA | 10 (20.40) |
| IIB | 28 (57.14) |
| III | 1 (2.04) |
| IVA | 0 (0.00) |
| IVB | 0 (0.00) |
| Chemotherapy |
| Without | 30 (60.00) |
| With | 20 (40.00) |
Molecular markers and patient
survival
Table III shows
the percentage of positive/negative staining in the patients and
controls for each marker. When univariate analysis was used,
positivity for VEGF-R1 (74%) was associated with tumoral
differentiation degree (p<0.05), size (p<0.02) and tumoral
location (head, p<0.03). EGFR immunopositivity (50%) was
associated with tumor differentiation degree (p<0.03), size
(p<0.02), perineural infiltration (p<0.02) and lymph node
metastasis (p<0.03). A significant correlation was observed in
p53 expression (50%) with a degree of differentiation (p<0.01).
The expression of the remaining markers did not correlate with the
pathological tumor characteristics. The median survival following
surgery was 15 months; 60% of patients survived to 12 months and
27% survived to 24 months. Patients who received chemotherapy
(n=20) showed better overall survival times and VEGF-R1 expression
(36 vs. 15% months in positive or negative staining). When using
multivariate analysis, the only immunohistochemical marker that
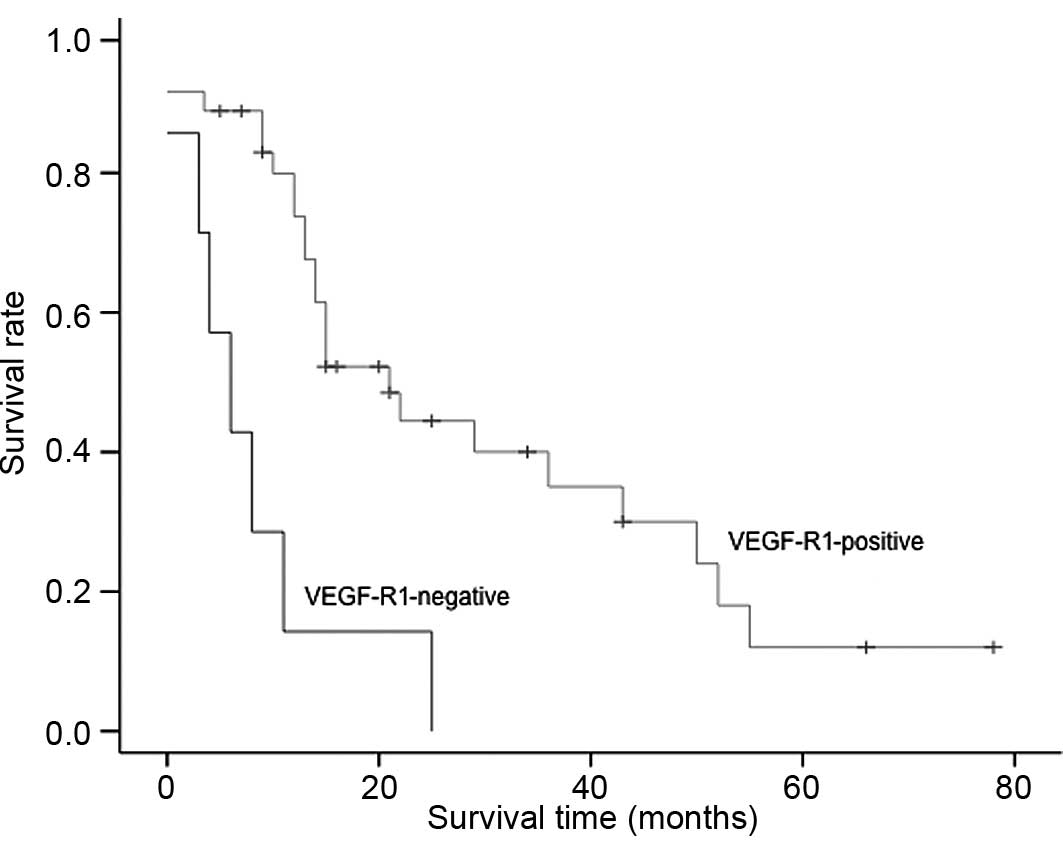
affected overall survival was VEGF-R1 (p<0.04) (Fig. 1). The risk of mortality was
5.86-fold greater (CI 95%) in patients without VEGF-R1
expression.
 | Table IIIPercentage of positive
immunohistochemical staining stratified by tumor and normal
tissue. |
Table III
Percentage of positive
immunohistochemical staining stratified by tumor and normal
tissue.
| Biomarker | Tumoral tissue,
n=50 (%) | Normal tissue, n=6
(%) | p-value |
|---|
| p16 |
| Positive | 15 (29.4) | 0 (0.0) | 0.121 |
| Negative | 36 (70.5) | 6 (100) | |
| p21 |
| Positive | 11 (21.7) | 0 (0.0) | 0.205 |
| Negative | 40 (78.4) | 6 (100) | |
| p53 |
| Positive | 25 (50.0) | 0 (0.0) | 0.019 |
| Negative | 25 (50.0) | 6 (100) | |
| Her2/neu |
| Positive | 4 (7.84) | 0 (0.0) | 0.476 |
| Negative | 46 (92.0) | 6 (100) | |
| COX-2 |
| Positive | 11 (21.5) | 0 (0.0) | 0.205 |
| Negative | 40 (78.0) | 6 (100) | |
| VEGF-R1 |
| Positive | 37 (74.0) | 0 (0.0) | 0.004 |
| Negative | 13 (26.0) | 6 (100) | |
| VEGF-R2 |
| Positive | 33 (65.0) | 0 (0.0) | 0.135 |
| Negative | 18 (35.0) | 6 (100) | |
| EGFR |
| Positive | 26 (52.0) | 0 (0.0) | 0.015 |
| Negative | 24 (48.0) | 6 (100) | |
Discussion
Pancreatic ductal adenocarcinoma remains a
challenging clinical problem. Resection is the most effective
treatment, but it is only applicable in a minority of patients,
whereas the remainder of patients present with an advanced stage of
the disease and few effective therapeutic options are available. In
this study, TMA technology was used to study growth factors,
oncogenes and the suppressor gene expression in pancreatic ductal
adenocarcinoma in order to establish a common predictor for
prognostic survival.
The incidence of p16 gene mutation and protein
expression in pancreatic cancer were reported to be between 30 and
87% (5,6), with the wide range possibly caused by
a difference in subjects or examination methods. Previous studies
showed that, compared to other tissues, pancreatic cancer cell
lines expressed p16 gene mutation more (6). However, discrepancies were noted in
the findings of these studies due to the mutation study methods and
immunohistochemical staining utilized. The results of our study, in
which p16 protein expression was detected in 29.4% of the patients,
were similar to those of previous studies. Numerous results have
been published regarding the association of the p16 gene with
clinicopathological characteristics and the survival rate. Bartsch
et al (7) noted that the
median survival time of cases with and without p16 expression was
8.5 and 17 months, respectively. However, other studies (8,9),
including our study, found no correlation between p16 expression
and the survival rate.
The tumor suppressor p53 and the cyclin kinase
inhibitor p21 are among the key gene products involved in cell
growth arrest, differentiation and senescence. Immunohistochemistry
has been used to examine p53 expression in pancreatic
adenocarcinoma (10,11). Half of our pancreatic adenocarcinoma
patients (50%) showed p53 expression in their tumors (inclusive of
scores 1, 2 and 3). Various studies have proposed contrasting
results in the association between p53 mutation and
clinicopathological characteristics (8,9,12–14).
On the other hand, Yokoyama et al (15) reported a correlation between p53
mutation and clinical stages. In this study, p53 protein expression
was correlated to the grade of histological differentiation
according to Jeong et al (16).
The p21 expression is compartmentalized in certain
post-replicative cell types, including colonic epithelial cells in
the upper halves of crypts, and this expression has been associated
with the earlier clinical stages of pancreatic cancer. Dergham
et al (17) reported p21
expression in 57% of pancreatic adenocarcinomas. Moreover, it was
found to correlate with stage I and II of the disease and poor
survival prediction (stage I: p21-negative, 5.9 months;
p21-positive, 13.5 months). Our results showed that 22% of patients
exhibited positive immunoreaction for this protein, but we did not
find any clinical variable correlation. Further studies are
required in order to evaluate whether p21 expression is correlated
to survival time differences among patients within each stage.
In pancreatic cancer, COX-2 is considered to be a
molecule that plays a key role in tumorigenesis. The COX-2
expression inhibits apoptosis and increases the invasiveness of
malignant cells. We found COX-2 expression in 11 out of 50 cancer
cases. This expresson is slightly lower than that reported in the
literature, which may be as high as 74% depending on the method
used, including Northern and Western blotting as well as
immunohistochemical analysis (18,19).
Okami et al (20) reported
weak staining in the majority of the investigated carcinoma
specimens. COX-2 staining in pancreatic carcinoma was extremely
heterogeneous and large variations between specimens were
noted.
Koshiba et al (21) suggested that for pancreatic
carcinoma, COX-2 is associated with the degree of malignancy
comparing intraductal papillary mucinous adenomas, intraductal
papillary carcinomas and intraductal carcinomas. In our study, a
positive or negative correlation between COX-2 expression and the
clinicopathological data was not shown.
The Her-2/neu (c-erb-b2) proto-oncogene encodes a
185-kDa transmembrane glycoprotein that is closely related in
structure to the EGFR and has been found to be amplified and
over-expressed in pancreatic tumors (22,23).
Hall et al (24) reported
that Her-2/neu was detected in the cell cytoplasm of chronic
pancreatitis and ductal pancreatic adenocarcinoma. The study by
Yamanaka et al (25) also
showed the expression of Her-2/neu in normal acinar and ductal
cells. Our study showed a low level of staining in tumor samples (4
out of 50 cases) and no staining in the normal pancreatic tissue.
Certain authors have reported that the c-erb-b2 oncoprotein is
potentially involved in the proliferative responses of pancreatic
epithelium. Dugan et al (26) reported that Her-2/neu expression is
rare in tumors that lack glandular differentiation and suggested
that the pattern of the Her-2/neu expression is related to
glandular differentiation and early oncogenesis.
EGFR belongs to a family of closely related
transmembrane proteins, including Her-2/neu (27). Its expression has been reported in
30–50% of pancreatic carcinomas (28–30).
Our results are consistent with these reports. Various data are
available regarding the possibility of synergy or other
interactions between the products of the c-erb-b2 gene and other
growth factor receptors, including EGFR (31). It has been suggested that the
co-expression of Her-2/neu and EGFR, at levels that are
individually insufficient to transform cells, converts fibroblasts
to a completely malignant state (32). It is possible that Her-2/neu and
EGFR have a synergistic effect in the carcinogenesis of pancreatic
tumors. However, no statistical significance was found regarding
this correlation.
The angiogenic system of VEGF and its receptors
VEGF-R1 and -2 is linked to the formation of new blood vessels in
malignant and non-malignant conditions (33–35).
Four VEGF binding receptors have been identified (36–38).
Among them, VEGF-R2 (KDR, flk-1) appears to be the most significant
since it mediates the mitogenic effects of VEGF on endothelial
cells, whereas VEGF-R1 mediates endothelial cell migrations
(36,39).
In the present study, VEGF and its receptors were
found to be markedly expressed in the human pancreatic cancer
specimens (R1 74%; R2 65%) and were associated with tumor size,
tumoral differentiation degree and survival time. In pancreatic
cancer, the functional and prognostic significance of VEGF and
VEGF-R1 and -2 expression has been investigated.
Immunohistochemical analysis if frequently used to show that VEGF
was expressed in the majority of types of cancer and that
expression, in general, was correlated with a worse outcome and was
therefore a marker of a poor prognosis, such as advanced stage
(40,41). For example, Itakura et al
showed that VEGF mRNA and protein expression in the pancreatic cell
lines tested and used immunohistochemical analysis to demonstrate a
predominant membrane/cytoplasm localization. The staining results
of these authors were confirmed with in situ hybridization
studies. However, other studies have produced conflicting results
with no correlation with outcome (42,43).
Follow-up studies from Itakura et al also confirmed the
co-expression of VEGF-R1 and -2 in cultured pancreatic cancer cells
lines. Buchler et al (44)
demonstrated similar findings. It is noteworthy that their results
showed that R2, but not R1, abrogated the in vitro
stimulatory effects of VEGF on pancreatic cancer cell
proliferations, suggesting that the growth-promoting effect of VEGF
in pancreatic cancer may be dependent predominantly on R2 rather
than R1.
R1 has shown a both a positive and negative
divergent effect on tumor growth potential. The conflicting
literature may reflect the fact that R1 probably has different
roles, depending on the physiological and pathological conditions
and the tissue type in which it is expressed. In the present study,
a low tumor R1 expression was associated with decreased survival
times and was correlated with advanced disease. This suggests that
it has a protective effect and acts as a negative regulator of
angiogenesis. Although, in our study, the R1 antibody presumably
only detected full-length R1 and not the soluble form, since it
identifies the intracellular domain, potential decoy roles for this
receptor have been attributed to both forms. Similar results were
noted by Chung et al (45).
In conclusion, our study indicates that growth
factor receptors, oncogenes and tumor suppressor genes are
frequently expressed in pancreatic cancer tissue. The expression of
VEGF-R1, EGFR and p53 is associated with poor tissue
differentiation and perineural and lymph node infiltration. VEGF-R1
immunopositive staining is associated with longer survival times,
related to a more favorable response to adjuvant chemotherapy.
References
|
1
|
Mortalidad por cáncer en Espana. 2005,
www.aecc.esurisimplewww.aecc.es.
|
|
2
|
Crist DW and Cameron JL: The current
status of the Whipple operation for periampullary carcinoma. Adv
Surg. 36:59–152. 1992.
|
|
3
|
Redston MS, Caldas C, Seymour AB, Hruban
RH, da Costa L and Yeo CJ: p53 mutations in pancreatic carcinoma
and evidence of common involvement of homocopolymer tracts in DNA
microdeletions. Cancer Res. 54:3025–3033. 1996.PubMed/NCBI
|
|
4
|
Garcia JF, Camacho FI, Morente M, Fraga M,
Montalban C, Alvaro T, Bellas C, Castano A, Diez A, Flores T,
Martin C, Martinez MA, Mazorra F, Menarguez J, Mestre Mj, Mollejo
M, Saez AI, Sanchez L and Piris MA; Spanish Hodgkin’s Lymphoma
Study Group. Hodgkin’s and Reed-Stenberg cells harbor alterations
in the major tumor suppressor pathways and cell-cycle checkpoints:
analyses using tissue microarrays. Blood. 101:681–689. 2003.
|
|
5
|
Googins M, Achutte M, Lu J, Moskaluk CA,
Weinstein CL and Petersen GM: Germline BRCA2 gene mutations in
patients with apparently sporadic pancreatic carcinomas. Cancer
Res. 56:5360–5364. 1996.PubMed/NCBI
|
|
6
|
Huang L, Goodrow TL, Zhang S, Klein-Szanto
AJP, Chang H and Ruggeri BA: Deletion and mutation analyses of the
p16/MTS1 tumour suppressor gene in human ductal pancreatic cancer
reveals a higher frequency of abnormalities in tumor-derived cell
lines than in primary ductal adenocarcinomas. Cancer Res.
56:1137–1141. 1996.
|
|
7
|
Moskaluk CA, Hruban RH and Kern SE: P16
and K-ras gene mutations in the intraductal precursors of human
pancreatic adenocarcinoma. Cancer Res. 57:2140–2143.
1997.PubMed/NCBI
|
|
8
|
Rozenblum E, Schutte M, Goggins M, Hahn
SA, Panzer S and Zaurak M: Tumor-suppressive pathways in pancreatic
carcinoma. Cancer Res. 57:1731–1734. 1997.PubMed/NCBI
|
|
9
|
Kawesha A, Chaneh P, Andren-Sandberg A,
Ograed D, Skar R and Dawiskiba S: K-ras oncogene subtype mutations
are associated with survival but not expression of p53, p16INK4A,
p21WAF-1, cyclin D1, erbB-2 and erbB-3 in resected pancreatic
ductal adenocarcinoma. Int J Cancer. 89:469–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki T and Takano Y: Comparative
immunohistochemical studies of p53 and proliferating cell nuclear
antigen expression and argyrophilic nucleoral organizer regions in
pancreatic duct cell carcinomas. Jpn J Cancer Res. 84:1072–1077.
1993. View Article : Google Scholar
|
|
11
|
Zhang SY, Ruggeri B, Agarwal P, Sorling
AF, Obara T and Ura H: Immunohistochemical analysis of p53
expression in human pancreatic carcinomas. Arch Pathol Lab Med.
118:150–154. 1994.PubMed/NCBI
|
|
12
|
Dergham ST, Dugan MC, Kucway R, Du W,
Kamarauskiene DS and Vaitkevicius VK: Prevalence and clinical
significance of combined K-ras mutation and p53 aberration in
pancreatic adenocarcinoma. Int J Pancreat. 21:127–143.
1997.PubMed/NCBI
|
|
13
|
Ruggeri BA, Huang L, Berger D, Chang H,
Klein-Szanto AJ and Goodrow T: Molecular pathology of primary and
metastatic ductal pancreatic lesions. Cancer. 79:700–716. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gansauge F, Gansauge S, Schmidt E, Muller
J and Beger HG: Prognostic significance of molecular alterations in
human pancreatic carcinoma – an immunohistological study.
Langenbecks Arch Surg. 383:152–155. 1998.
|
|
15
|
Yokoyama M, Yamanaka Y, Friess H, Buchler
M and Murray K: P53 expression in human pancreatic cancer
correlates with enhanced biological aggressiveness. Anticancer Res.
14:2477–2483. 1997.PubMed/NCBI
|
|
16
|
Jeong J, Park YN, Park JS, Yoon DS, Chi HS
and Kim BR: Clinical significance p16 protein expression loss and
aberrant p53 protein expression in pancreatic cancer. Yonsei Med J.
46:519–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dergham S, Dugan M, Joshi U, Chen Y, Du W,
Smith D, Arlauskas P, Crissman J, Vaitkevicius V and Sarkar F: The
clinical significance of p21 WAF1/CIP-1 and p53 expression in
pancreatic adenocarcinoma. Cancer. 80:372–381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Molina MA, Sitja-Arnau M, Lemoine MG,
Frazier ML and Sinicrope FA: Increased cyclooxygenase-2 expression
in human pancreatic carcinomas and cell lines: growth inhibition by
nonsteroidal anti-inflammatory drugs. Cancer Res. 59:4356–4362.
1999.PubMed/NCBI
|
|
19
|
Kokawa A, Kondo H, Gotoda T, Ono H, Saito
D, Nakadaira S, Kosuge T and Yoshida S: Increased expression of
cyclooxigenase-2 in human pancreatic neoplasms and potential for
chemoprevention by cyclooxygenase inhibitors. Cancer. 91:333–338.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okami J, Yamamoto H, Fujiwara Y, Tsujie M,
Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K, Ishikawa
O, Sakon M, Matsuura N, Nakamori S and Monden M: Overexpression of
cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res.
5:2018–2024. 1999.PubMed/NCBI
|
|
21
|
Koshiba T, Hosotani R, Miyamoto Y, Wada M,
Lee JU, Fujimoto K, Tsuji S, Nakajima S, Doi R and Imamura M:
Immunohistochemical analysis of cyclooxygenase-2 expression in
pancreatic tumors. Int J Pancreatol. 26:69–76. 1999. View Article : Google Scholar
|
|
22
|
Williams TM, Weiner DB, Greene MI and
Maguire HC: Expression of erbB-2 in human pancreatic
adenocarcinoma. Pathobiology. 59:46–52. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Day DJ, Di Giuseppe JA and Yeo C:
Immunohistochemical evaluation of Her-2/neu expression in
pancreatic adenocarcinoma and pancreatic intraepithelial neoplams.
Hum Pathol. 27:119–124. 1996. View Article : Google Scholar
|
|
24
|
Hall PA, Hughes CM, Staddon SL, Richman
PI, Gullick WJ and Lemoine NR: The c-erbB-2 proto-oncogene in human
pancreatic cancer. J Pathol. 161:195–200. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamanaka Y, Friess H and Kobrin MS:
Overexpression of Her2/neu oncogene in human pancreatic carcinoma.
Human Pathol. 24:1127–1134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dugan MC, Dergham ST and Kucway R:
Her-2/neu expression in pancreatic adenocarcinoma: relation to
tumour differentiation and survival. Pancreas. 14:229–236. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sirivatanauksorn V, Sirivatanauksorn Y and
Lemoine NR: Molecular pattern of ductal pancreatic cancer.
Langenbecks Arch Surg. 383:105–115. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemoine NR, Hughes CM and Barton CM: The
epidermal growth factor receptor in human pancreatic cancer. J
Pathol. 166:7–12. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kioppel G, Maillet B and Schewere K:
Immunocytochemical detection of epidermal growth factor receptor
(EGFr) and transferrin receptor (TR) on normal, inflamed and
neoplastic pancreatic tissue. Pancreas. 4:6231989.
|
|
30
|
Uegaki K, Nio Y and Inoue Y:
Clinicopathological significance of epidermal growth factor and its
receptor in human pancreatic cancer. Anticancer Res. 17:3841–3848.
1997.PubMed/NCBI
|
|
31
|
Sterm DF and Kamps MP: EGF-stimulated
tyrosine phos-phorylation of p185neu a potential model of receptor
interactions. EMBO J. 7:995–1001. 1988.PubMed/NCBI
|
|
32
|
Kokai Y, Myers JN and Wada T: Synergistic
interaction of p185neu and EGF receptor leads to transformation of
rodent fibroblasts. Cell. 58:287–292. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Millauer B, Wizigmann-Voss S and Schunürch
H: High affinity VEGF binding and developmental expression suggest
Flk-1 as a major regulator of vasculogenesis and angiogenesis.
Cell. 72:835–846. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrara N and Henzel WJ: Pituitary
follicular cells secrete a novel heparin-binding growth factor
specific for vascular endothelial cells. Biochem Biophys Res
Commun. 161:851–858. 1989. View Article : Google Scholar
|
|
35
|
Gospodarowicz D and Lau K: Pituitary
follicular cells secrete both vascular endothelial growth factor
and follistatin. Biochem Biophys Res Commun. 165:292–298. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Vries C, Escobedo JA and Ueno H: The
fms-like tyrosine kinase, a receptor for vascular endothelial
growth factor. Science. 255:989–991. 1992.
|
|
37
|
Terman BI, Dougher-Vermazen M and Carrion
ME: Identification of KDR tyrosine kinase as a receptor for
vascular endothelial cell growth factor. Biochem Biophys Res
Commun. 187:1579–1586. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Chedotal A and He Z: Neuropilin-2
a novel member of the neuropilin family, is a high affinity
receptor for the semaphorins Sema E and Sema IV but not Sema III.
Neuron. 19:547–559. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brown JM and Giaccia AJ: The unique
physiology of solid tumors. Opportunities (and problems) for cancer
therapy. Cancer Res. 58:1408–1416. 1998.PubMed/NCBI
|
|
40
|
Itakura J, Ishiwata T and Friess H:
Enhanced expression of vascular endothelial growth factor in human
pancreatic cancer correlates with local disease progression. Clin
Cancer Res. 3:1309–1316. 1997.PubMed/NCBI
|
|
41
|
Seo Y, Baba H, Fukuda T, Takashima M and
Sugimachi K: High expression of vascular endothelial growth factor
is associated with liver metastasis and poor prognosis for patients
with ductal pancreatic adenocarcinoma. Cancer. 88:2239–2245. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ellis LM, Takahashi Y, Fenoglio CJ, Cleary
KR, Bucana CD and Evans DB: Vessel counts and vascular endothelial
growth factor expression in pancreatic adenocarcinoma. Eur J
Cancer. 34:337–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujimoto K, Hosotani R and Wada M:
Expression of two angiogenic factors, vascular endothelial growth
factor and platelet-derived endothelial cell growth factor in human
pancreatic cancer, and its relationship to angiogenesis. Eur J
Cancer. 34:1439–1447. 1998. View Article : Google Scholar
|
|
44
|
Buchler P, Peber HA, Buchler MW, Friess H
and Hines OJ: VEGF-RII influences the prognosis of pancreatic
cancer. Ann Surg. 236:738–749. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chung G, Yoon H, Zerkowsi M, Ghosh S,
Thomas L, Harigopal M, Charette L, Salem R, Camp R, Rimm D and
Burtness B: Vascular endothelial growth factor, FLT-1, and FLK-1
analysis in pancreatic cancer tissue microarray. Cancer.
106:1677–1684. 2006. View Article : Google Scholar : PubMed/NCBI
|