Introduction
Early and specific diagnosis is crucial for the
successful treatment of prostate cancer (PCa). Novel biomarkers are
required to complement prostate-specific antigen (PSA) measurements
by enhancing its diagnostic and prognostic performance. It is
crucial to define additional clinical markers for the accurate
detection of PCa and differential diagnosis of benign prostatic
hyperplasia (BPH) (1,2).
One of the strategies for cancer serum biomarker
development has been the detection of autoantibodies produced
against tumor antigens in patient sera (2,3). This
approach measures the autoantibodies for a spectrum of tumor
antigens, with sensitivity and specificity exceeding those obtained
using the conventional circulating tumor antigen (4). A number of asymptomatic individuals
who develop cancer exhibit circulating autoantibodies preceding
clinical onset of the disease, indicating that immune response
against tumor antigens is an early event in oncogenesis (5). The expression of tumor-associated
antigens is the result of various events, including mutation,
overexpression or altered expression patterns. In addition,
autoantibody responses in cancer were correlated with patient
survival and patient response to treatment (6–8). The
identification of immunologically identified proteins that are
specific or associated with PCa may lead to the development of
specific targets for diagnosis, provide novel insights into the
biology of prostate carcinogenesis and describe novel potential
targets for immunotherapy-based vaccines.
Osteopontin (OPN) is a glycoprotein involved in a
range of physiological and pathological processes. Extensive
research has elucidated the pivotal role of OPN in regulating the
cell signaling that controls tumor progression and metastasis
(9–11). Circulating OPN has been positively
associated with PCa bone metastases and negatively correlated with
survival and other tumor burden characteristics (11–14).
Overexpression of OPN in PCa cell lines induced increased
proliferation, invasion and, most notably, enhanced the ability of
these cells to intravasate blood vessels (15).
It has been shown that OPN expression is already
deregulated in early neoplastic lesions (15). However, to the best of our
knowledge, the evaluation of circulating OPN levels in the early
stages of PCa and its potential use as a sensitive marker for PCa
diagnosis has yet to be described. In order to evaluate the early
expression of OPN in tumor conditions, anti-OPN antibodies in the
early stages of tumor development should be detected. Anti-OPN
antibodies in auto-immune diseases were previously described
(16), suggesting that OPN is
immunogenic and that loss of immune tolerance occurs in such
pathological conditions. Based on these data, it was hypothesized
that in early tumor development, circulating OPN triggers humoral
immune responses. Using anti-OPN antibody immune responses as
biomarkers may be noteworthy, since they represent a form of
biological amplification of signals that are otherwise weak,
especially in the early stages of various types of cancer.
The present study investigated whether OPN initiates
an immune response in PCa patients by evaluating the presence of
anti-OPN antibodies in plasma samples from PCa patients. We then
compared the frequency of reactivity of anti-OPN antibodies between
PCa, BPH and HD plasma samples. Furthermore, certain properties of
anti-OPN antibodies as potential serum markers for PCa were
evaluated.
Materials and methods
Patients
Plasma from biopsy-proven clinically-localized PCa
patients was collected prior to prostatectomy and stored in
aliquots at −80°C. Plasma samples were obtained from 29
biopsy-proven PCa patients (52–79 years of age, median 67), 18
patients with benign prostate hyperplasia (BPH) (62–87 years of
age, median 72) and 30 healthy male donors (HD) (18–36 years of
age, median 25) with no history of cancer or any other prostatic or
auto-immune diseases. The HD samples were collected from
non-hospitalized volunteers in Rio de Janeiro and used as control
samples. The BPH cases corresponded to patients that were submitted
to surgical treatment by open adenomectomy. The local Ethics
Committee approved this study and the participants provided
informed consent. Table I
summarizes the data from the PCa patients included in the
study.
 | Table IClinical and pathological data of the
PCa patients included in this study and their anti-OPN reactivity
levels. |
Table I
Clinical and pathological data of the
PCa patients included in this study and their anti-OPN reactivity
levels.
| Variable | No. of patients
(%) | Anti-OPN antibody
reactivity (%) |
|---|
| Total cases of
PCa | 29 | 18/29 (62) |
| Gleason score |
| ≤6 | 14 (48) | 8/14 (57) |
| 7 (3+4) | 5 (17) | 4/5 (80) |
| 7 (4+3) | 4 (14) | 4/5 (80) |
| ≥8 | 6 (21) | 3/6 (50) |
| PSA level before
surgerya |
| Mean (median,
range) |
| <10 ng/ml | 15 (68) | 10/15 (67) |
| ≥10 ng/ml | 7 (32) | 6/7 (86) |
| Pathology pT
stageb |
| pT2a - pT2b -
pT2c | 10 (45) | 7/10 (70) |
| pT3a - pT3b | 12 (55) | 8/12 (67) |
| Lymph node
statusa |
| Positive | 3 (14) | 3/3 (100) |
| Negative | 19 (86) | 0/19 (0) |
Recombinant osteopontin
A GST-OPN fusion construct containing the complete
OPN cDNA in the plasmid pGEX-6P2 (17) was used to generate the recombinant
OPN (rOPN-GST), as previously described (17,18).
GST-OPN and GST recombinant proteins were purified under
non-denaturing conditions using an in batch GST Purification Module
(Amersham Biosciences, Piscataway, NJ, USA).
Western blotting for anti-osteopontin
antibodies
Plasma IgGs against bacterial proteins were adsorbed
by incubating bacterial extracts and plasma samples at a proportion
of 1:1 vol/vol for 2 h at room temperature with gentle agitation.
For immunoblot assays, 50 μg of GST-OPN and GST-purified
recombinant proteins were subjected to electrophoresis via a 12%
SDS-polyacrylamide gel and then transferred onto nitrocellulose
membranes using the mini-PROTEAN 3 (Bio-Rad) system. Comparative
immunoblot assays were performed simultaneously, using the same OPN
recombinant protein batch. The membranes were probed with plasma
samples diluted 1:200 in 1X PBS containing 0.05% Tween-20
containing 5% non-fat dry milk (PBSTNF) and subsequently with
anti-human IgG alkaline phosphatase conjugate (Sigma, St. Louis,
MO, USA) diluted 1:30,000 in PBSTNF. Immunoreactive bands were then
visualized by the addition of nitroblue tetrazolium and
5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP; Sigma)
substrates.
Assays with OPN monoclonal antibody were performed
in identical conditions to those described above, using the OPN mAb
(Chemicon, Canada, USA) in a 1:2,500 dilution followed by
incubation in a 1:5,000 dilution of anti-rat IgG conjugated to
horseradish peroxidase (HRP). Immunoreactive complexes were
developed with the ECL Plus System (Amersham Biosciences) after
exposure to Kodak BioMax Light Film.
Enzyme linked immunosorbent assay
(ELISA)
Recombinant OPN fusion protein solution (50 μl) at a
concentration of 25 μg/ml in coating buffer carbonate/bicarbonate
0.1 M pH 9.6 was added to 96-well plates and incubated overnight at
40°C. The plasma samples were serially diluted from 1:100 to
1:1,000 in PBSTNF. The plates were incubated for 1 h at room
temperature on an orbital agitator and then washed with 1X PBS.
HRP-conjugated human IgG-specific secondary antibody (1:4,000 in
PBSTNF) was added and the plates were incubated for one more hour
at room temperature. After being washed five times in 1X PBS, 3,3′,
5,5′-tetramethyl-benzidine (TMB; Invitrogen) was added. The
reactions were stopped by the addition of 100 μl of 0.5 N
H2SO4 per well. Absorbance values were read
at 450 nm using a microplate reader SpectraMax 90 (Molecular
Devices, Sunnyvale, CA, USA).
Sensitivity, specificity and predictive
values
In order to evaluate the potential use of anti-OPN
antibodies as serum markers for PCa, the sensitivity, specificity,
positive predictive value (PPV) and negative predictive value (NPV)
were calculated, as previously described (12).
Statistical analysis
Statistical analysis was performed with SPSS 14.0
and Microsoft Excel. The Chi-square test was used for the
statistically significant differences in the humoral immune
response to OPN among patients with PCa, BPH and the HD control
groups. The associations between the humoral immune response
against OPN and the various clinical and pathological
characteristics were determined using the Spearman’s correlation
test. P≤0.05 was considered to be statistically significant.
Results
In order to evaluate the presence of autoantibodies
against OPN in PCa plasma samples, a recombinant human osteopontin
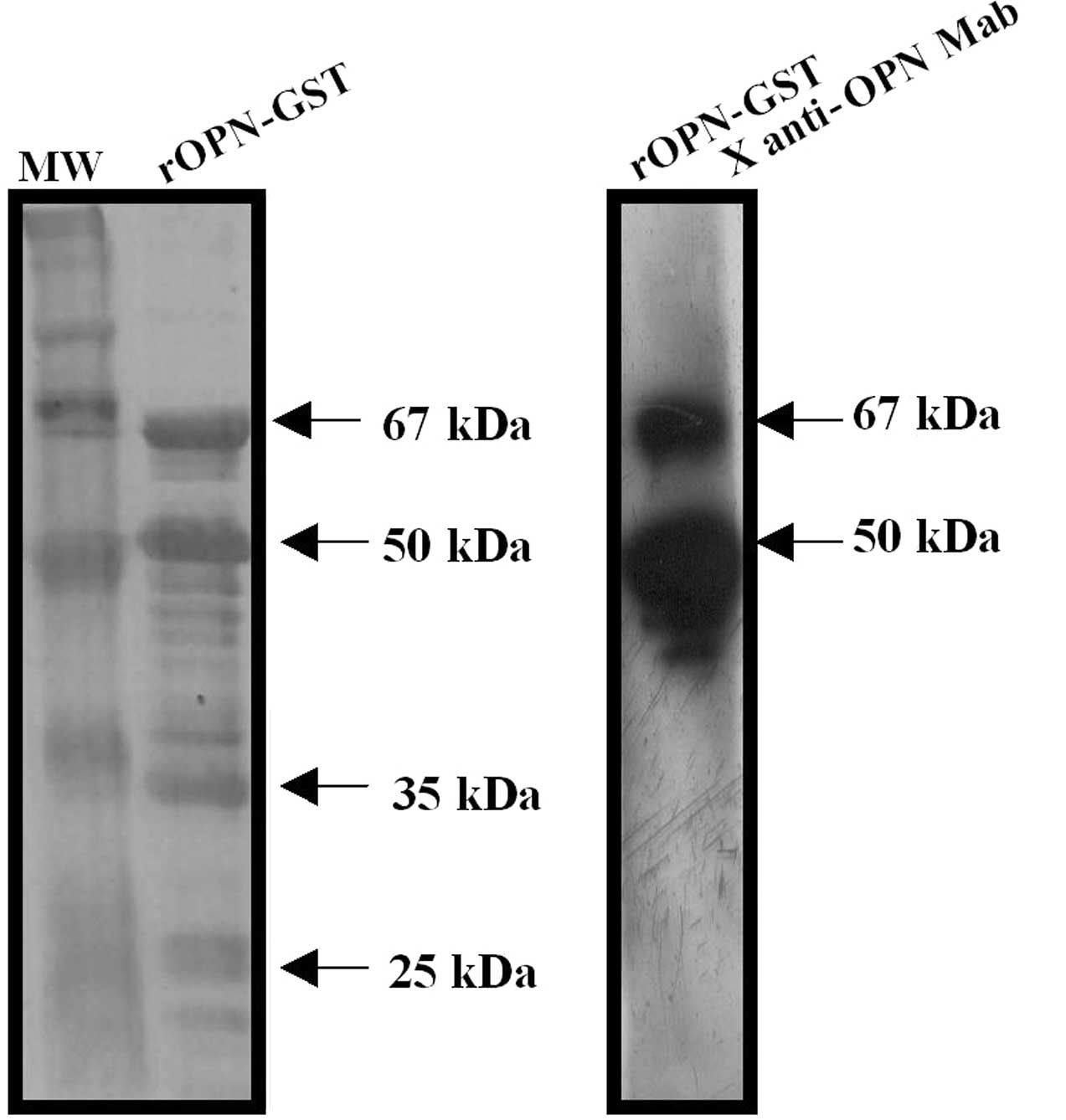
(rOPN) was used as an antigen in immunoblot assays. Purified
rOPN-GST presented the predicted molecular weight of 67 kDa as a
result of GST (28 kDa) plus the full-length human OPN protein (39
kDa) (Fig. 1, left panel).
Additional protein fragments were observed in SDS-PAGE, likely
resulting from the degradation of rOPN-GST during antigen
preparation. Hence, purified rOPN-GST presented four major bands.
The upper band corresponds to the full-length OPN (67 kDa) and the
three additional lower protein fragments (50, 35 and 25 kDa) were
evidently degradation products. The 67 and 50 kDa major protein
bands were reactive to an anti-OPN monoclonal antibody, confirming
OPN identity (Fig. 1, right panel).
Conversely, the remaining protein fragments were not reactive,
suggesting that a loss of the OPN epitope identified by this
monoclonal antibody occurred via the lower degradation
products.
Using the rOPN-GST described above, immunoblot
assays were performed to evaluate autoantibody responses to rOPN in
plasma samples from 29 PCa patients with different clinical and
pathological staging, 18 from patients with BPH and 30 from HD
controls. Fig. 2 shows
representative immunoblot assays of these tested plasma
samples.
Antibodies against rOPN-GST were detected in 19/29
(66%), 6/18 (33%) and 3/30 (10%) plasma samples from PCa, BPH and
HD, respectively (Table II). The
immunoreactivity to rOPN-GST was specific for the OPN portion,
since no immunoreactivity to GST was observed using pre-adsorbed
plasma samples (Materials and methods) from patients with PCa, BPH
or from the HD controls (Fig. 2,
right panels of each plasma sample tested). In order to validate
the specificity of the humoral immune response to OPN observed in
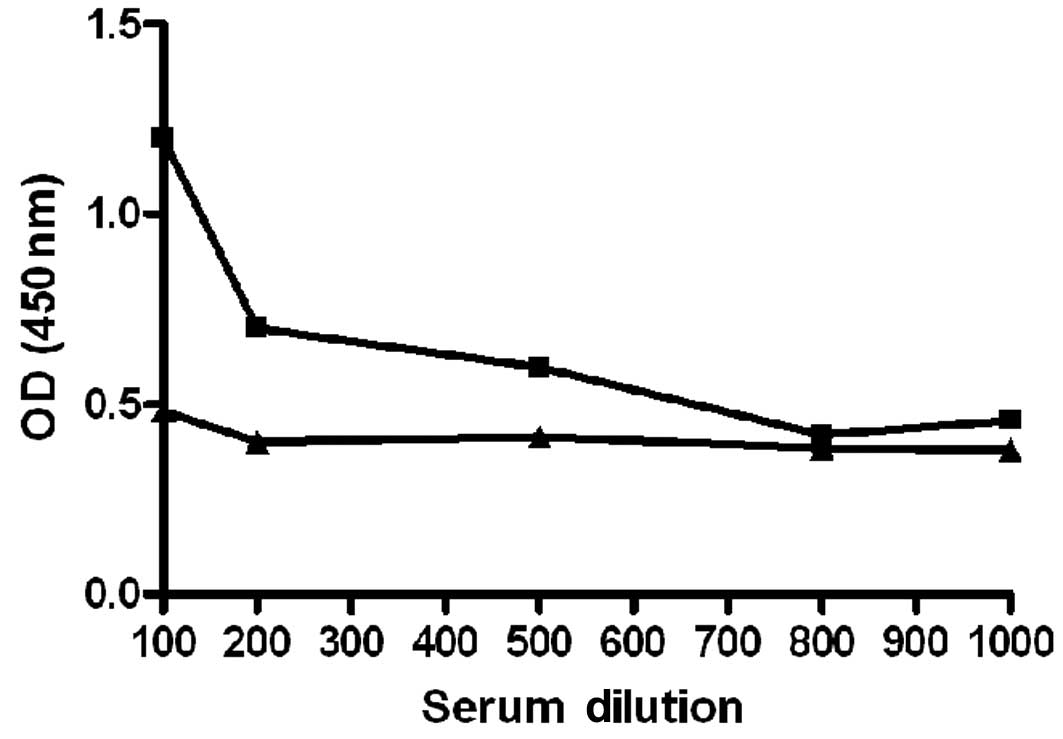
the immunoblot tests, ELISA titration curves of anti-OPN reactive
and non-reactive plasma samples (Fig.
3) were performed. Imunoblot-positive PCa plasma samples
exhibited a decrease of anti-OPN reactivity according to successive
plasma dilution. The same was not observed for the HD plasma
sample, which exhibited negative immunoblot results. These results
confirm the specificity of the humoral immune response against OPN
detected by these immunoblot assays.
 | Table IIDistribution of positive and negative
reactive immunoblot results for anti-OPN antibodies between PCa and
non-PCa control groups (BPH and HD). |
Table II
Distribution of positive and negative
reactive immunoblot results for anti-OPN antibodies between PCa and
non-PCa control groups (BPH and HD).
| Immunoblot results
for anti-OPN antibodies | PCa (n=29) (%) | BPH (n=18) (%) | HD controls (n=30)
(%) |
|---|
| Positive | 19/29 (62)a | 6/19 (32)b | 3/30 (10) |
| Negative | 10/29 (38) | 13/19 (68) | 27/30 (90) |
| Total | 29 | 18 | 30 |
The majority of the plasma samples were reactive to
both the 67- and 50-kDa rOPN-GST products. However, certain samples
were reactive for only one of the major protein molecular weights
(e.g., PCa plasma samples nos. 12 and 20 in Fig. 2, which were reactive for only the
50- and 67-kDa rOPN-GST molecular weights, respectively). This
differential reactivity may be the result of an individual humoral
immune response to different osteopontin antigenic domains.
Notably, anti-OPN reactive plasma samples were significantly more
frequent in PCa (66%), as compared to BPH patients (33%) and the HD
(10%) control group (Table
II).
Certain diagnostic properties of anti-OPN antibodies
were then evaluated as potential serum markers to detect PCa and to
discriminate PCa from BPH and HD controls. The results from the
screening were used to calculate sensitivity, specificity and
predictive values. These characteristics were based on the premise
that control patients (true negative group) are those with BPH or
the HD controls. Our analysis resulted in a sensitivity of 66%,
specificity of 81%, PPV of 68% (positive test) and NPV of 80%
(negative test).
To evaluate whether the presence of anti-OPN
antibodies is an early event in PCa progression, anti-OPN
reactivity levels were investigated based on certain clinical data
from PCa patients whose plasma samples were tested (Table I). Of the patients presenting a pT2
stage (45% of the samples), 70% were positive for anti-OPN
antibodies. A total of 57% of patients presenting tumors with a
Gleason score of less than 6 were positive for anti-OPN antibodies.
Additionally, the data showed that 67% of patients included in this
study, with a pre-surgery PSA value of less than 10 ng/ml, were
also positive for anti-OPN antibodies. The results showed that even
PCa patients with organ-confined disease (pT2 pathological
staging), PSA plasma levels lower than 10 ng/ml and with a Gleason
score of less than 6, exhibited humoral immune responses against
OPN.
The association between the presence of anti-OPN
antibodies and clinical and pathological characteristics, including
PSA level, Gleason score and TNM staging, was evaluated. No
statistically significant association was found between humoral
immune response against OPN or any of these characteristics
(Table III).
 | Table IIIAssociation between OPN humoral
response and clinical and histopathological characteristicsa. |
Table III
Association between OPN humoral
response and clinical and histopathological characteristicsa.
| Variable | Analysis | PSA | Gleason score | Pathological
stage |
|---|
| OPN humoral
response | Spearman’s
correlation test (P-value) | 0.279 | 0.719 | 0.805 |
Discussion
The identification and molecular characterization of
self-antigens expressed by human malignancies are an active field
in tumor immunology. The approach employed to measure
autoantibodies for a spectrum of tumor antigens results in
sensitivity and specificity results exceeding those obtained using
the measurement of tumor antigen levels (19,20). A
number of asymptomatic individuals who develop cancer exhibit
circulating autoantibodies preceding the clinical onset of the
disease, suggesting that an immune response against tumor antigens
is an early event in oncogenesis (5). In addition, autoantibody responses in
cancer have been correlated with patient survival and response to
treatment (6–8).
Data indicate OPN to be a significant immunogen in
various pathological conditions. Dysregulated OPN expression in
certain autoimmune diseases is possibly correlated to the break of
immune tolerance to this self protein. Anti-OPN antibodies are
produced in such pathological conditions, but OPN is a weak
immunogen (0.95–15%) (16,21). Healthy individuals presented very
low or absent anti-OPN reactivity levels as compared to autoimmune
diseases (16,22). Similar mechanisms mediate tolerance
to tumor antigens and self-antigens in order to avoid autoimmunity,
and numerous autoimmune phenomena were reported in malignancies
(23,24). Based on this assumption, we
evaluated whether the overexpression of OPN in tumor conditions was
able to break immune tolerance against this protein, as was
observed in certain autoimmune diseases, thereby triggering humoral
immunity in cancer patients. Additionally, using PCa serum samples
as models to achieve this objective, we investigated whether
anti-OPN antibodies were potential serum markers for this disease.
The search for autoantibodies against tumor antigens as serological
markers for cancer diagnosis has been a long-term undertaking of
PCa investigators (2,25). A number of overexpressed antigens
were reported to initiate spontaneous humoral and cellular immune
responses in PCa (25). We proposed
that OPN may be one such example, in that occasionally it is one of
the antigens triggering humoral immune response in PCa
patients.
The present study showed that the frequency of
patients presenting autoantibody to OPN was significantly higher in
PCa (66%) plasma samples than in BPH (33%) and the HD (10%) control
group. BPH is a frequent cause of lower urinary symptoms, with a
prevalence of 50% by the sixth decade of life. It is anticipated
that in the next few years the male population aged 65 years and
older is due to increase, and it may become crucial to distinguish
male individuals with BPH from those with PCa warranting clinical
intervention. To achieve this aim, a definition of the additional
clinical markers for accurate detection of PCa and differential
diagnosis of BPH is required (1,2).
According to our data, anti-OPN antibodies may be appropriate serum
markers that, together with other markers, may aid to better
distinguish PCa from BPH and HD controls. BPH patients presenting
anti-OPN antibodies should be followed up in order to evaluate
whether or not they present a non-diagnosed PCa. The observed
difference in frequency of anti-OPN antibodies in the three groups
of individuals evaluated in this study may be the result of a
higher OPN expression level in PCa in comparison to BPH and normal
prostate tissues, as previously described (15,26).
To the best of our knowledge, no previously
published study exists regarding the direct identification of
autoantibodies against OPN in PCa or other types of cancer. Thus,
our data showed for the first time that OPN triggers antibody
immunity in cancer and that OPN is one of the most reactive
individual PCa tumor-associated antigens described thus far,
presenting a prevalence of anti-OPN antibody frequency of 66% among
PCa plasma samples. Currently, the most frequent anti-tumor
antibodies against single antigens in PCa are for α-methyl-acyl coA
racemase (AMACR) (70%), huntingtin (46%), p90 (30.8%) and FLJ23438
(37%) (27–30). In combination with these higher
reactive antigens and others yet to be described, antigen panels or
arrays may be optimized, including OPN, in order to obtain high
sensitivity and specificity levels, as previously described
(19,20,29,30).
Additionally, we observed that the frequency of PCa patients
presenting anti-OPN antibodies (66%) is higher than that previously
observed for patients presenting with autoimmune conditions
(0.95–15%) (16,21). It is likely that this phenomenon is
related to the higher level of OPN expression in tumors as compared
to autoimmune conditions.
The evaluation of anti-OPN antibodies as potential
serum markers for PCa resulted in a sensitivity of 66%, specificity
of 81%, PPV of 68% and NPV of 80%, values considered reasonable for
a humoral immune response against an individual antigen. Compared
to the measurement of total PSA properties as a serum marker, which
has been described in the range of 55–75% for specificity and
70–95% for sensitivity (31),
anti-OPN antibodies may complement these properties to better
define PCa diagnosis. Our data provides similar sensitivity and
specificity results and higher PPV and NPV values when compared to
circulating OPN as a marker for PCa (12). However, as opposed to our data, this
study (12) focused on plasma
samples from metastatic patients. Patients presenting early
developing tumors have not been included, thereby hampering
appropriate comparisons.
Our findings support the potential use of anti-OPN
antibodies as additional serum markers to better aid in the early
detection of PCa. The fact that anti-OPN antibodies were also
detected in a high proportion of PCa patients presenting a low
Gleason score, PSA values less than 10 ng/ml and PCa-confined
disease, suggests that an early anti-OPN immune response occurs in
PCa development.
A correlation between the humoral immune response to
OPN and clinical and pathological characteristics, such as PSA
level, Gleason score and TNM staging, was not found in this study,
similar to results reported for other tumor antigens, including
FLJ23438 and AMACR (28,30). This suggests that the humoral immune
response to certain tumor antigens is an independent factor for the
evaluation of PCa progression.
In conclusion, this study describes for the first
time that OPN triggers humoral immunity in cancer. We provide
evidence that anti-OPN antibodies are potential serum markers for
early PCa stage and for the differential diagnosis of PCa and BPH.
As a strong immunogenic autoantigen in PCa, OPN potentially
initiates autoantibodies in other malignancies overexpressing this
protein, justifying further investigation in other systems. OPN, in
association with other tumor proteins, may be applied to antigen
panels in order to achieve more favorable sensitivity and
specificity results in the diagnosis of PCa.
Acknowledgements
This study was supported by the Swiss Bridge
Foundation, PRONEX-Rio, FAPERJ, CNPq, CAPES, FAF and INCa-MS.
References
|
1
|
Miller J and Tarter TH: Update on the use
of dutasteride in the management of benign prostatic hypertrophy.
Clin Interv Aging. 2:99–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor BS, Pal M, Yu J, et al: Humoral
response profiling reveals pathways to prostate cancer progression.
Mol Cell Proteomics. 7:600–611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu H, Goodell V and Disis ML: Humoral
immunity directed against tumor-associated antigens as potential
biomarkers for the early diagnosis of cancer. J Proteome Res.
7:1388–1394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho-Chung YS: Autoantibody biomarkers in
the detection of cancer. Biochim Biophys Acta. 1762:587–591. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lubin R, Schlichtholz B, Teillaud JL, et
al: p53 antibodies in patients with various types of cancer: assay,
identification and characterization. Clin Cancer Res. 1:1463–1469.
1995.PubMed/NCBI
|
|
6
|
Storr SJ, Chakrabarti J, Barnes A, et al:
Use of autoantibodies in breast cancer screening and diagnosis.
Expert Rev Anticancer Ther. 6:1215–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bachelot T, Ratel D, Menetrier-Caux C, et
al: Autoantibodies to endostatin in patients with breast cancer:
correlation to endostatin levels and clinical outcome. Br J Cancer.
94:1066–1070. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vural B, Chen LC, Saip P, et al: Frequency
of SOX Group B (SOX1, 2, 3) and ZIC2 antibodies in Turkish patients
with small cell lung carcinoma and their correlation with clinical
parameters. Cancer. 103:2575–2583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jacobs JP, Pettit AR, Shinohara ML, et al:
Lack of requirement of osteopontin for inflammation, bone erosion,
and cartilage damage in the K/BxN model of autoantibody-mediated
arthritis. Arthritis Rheum. 50:2685–2694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stromnes IM and Goverman JM:
Osteopontin-induced survival of T cells. Nat Immunol. 8:19–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shevde LA, Das S, Clark DW, et al:
Osteopontin: an effector and an effect of tumor metastasis. Curr
Mol Med. 10:71–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fedarko NS, Jain A, Karadag A, et al:
Elevated serum bone sialoprotein and osteopontin in colon, breast,
prostate, and lung cancer. Clin Cancer Res. 7:4060–4066.
2001.PubMed/NCBI
|
|
13
|
Hotte SJ, Winquist EW, Stitt L, et al:
Plasma osteopontin: associations with survival and metastasis to
bone in men with hormone-refractory prostate carcinoma. Cancer.
95:506–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wai PY and Kuo PC: Osteopontin: regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forootan SS, Foster CS, Aachi VR, et al:
Prognostic significance of osteopontin expression in human prostate
cancer. Int J Cancer. 118:2255–2261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakata M, Tsuruha JI, Masuko-Hongo K, et
al: Autoantibodies to osteopontin in patients with osteoarthritis
and rheumatoid arthritis. J Rheumatol. 28:1492–1495.
2001.PubMed/NCBI
|
|
17
|
Yokosaki Y, Matsuura N, Sasaki T, et al:
The integrin alpha(9) beta(1) binds to a novel recognition sequence
(SVVYGLR) in the thrombin-cleaved amino-terminal fragment of
osteopontin. J Biol Chem. 274:36328–36334. 1999. View Article : Google Scholar
|
|
18
|
Chung CT, Niemela SL and Miller RH:
One-step preparation of competent Escherichia coli:
trasformation and storage of bacterial cells in the same solution.
Proc Natl Acad Sci USA. 86:2172–2175. 1989.PubMed/NCBI
|
|
19
|
Sardana G, Dowell B and Diamandis EP:
Emerging biomarkers for the diagnosis and prognosis of prostate
cancer. Clin Chem. 54:1951–1960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bensalah K, Lotan Y, Karam JA, et al: New
circulating biomarkers for prostate cancer. Prostate Cancer
Prostatic Dis. 11:112–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du H, Masuko-Hongo K, Nakamura H, et al:
The prevalence of autoantibodies against cartilage intermediate
layer protein, YKL-39, osteopontin, and cyclic citrullinated
peptide in patients with early-stage knee osteoarthritis: evidence
of a variety of autoimmune processes. Rheumatol Int. 26:35–41.
2005. View Article : Google Scholar
|
|
22
|
Watanabe M, Uchida K, Nakagaki K, et al:
Anti-cytokine autoantibodies are ubiquitous in healthy individuals.
FEBS Lett. 581:2017–2021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomer Y, Sherer Y and Shoenfeld Y:
Autoantibodies, autoimmunity and cancer. Oncol Rep. 5:753–761.
1998.PubMed/NCBI
|
|
24
|
Adler AJ: Mechanisms of T cell tolerance
and suppression in cancer mediated by tumor-associated antigens and
hormones. Curr Cancer Drug Targets. 7:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casiano CA, Mediavilla-Varela M and Tan
EM: Tumor-associated antigen arrays for the serological diagnosis
of cancer. Mol Cell Proteomics. 5:1745–1759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thalmann GN, Sikes RA, Devoll RE, et al:
Osteopontin: possible role in prostate cancer progression. Cancer
Res. 5:2271–2277. 1999.PubMed/NCBI
|
|
27
|
Bradley SV, Oravecz-Wilson KI, Bougeard G,
et al: Serum antibodies to huntingtin interacting protein-1: a new
blood test for prostate cancer. Cancer Res. 65:4126–4133. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pontes ER, Matos LC, da Silva EA, et al:
Auto-antibodies in prostate cancer: humoral immune response to
antigenic determinants coded by the differentially expressed
transcripts FLJ23438 and VAMP3. Prostate. 66:1463–1473. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi F, Zhang J, Liu D, et al: Preferential
humoral immune response in prostate cancer to cellular proteins p90
and p62 in a panel of tumor-associated antigens. Prostate.
63:252–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sreekumar A, Laxman B, Rhodes DR, et al:
Humoral immune response to alpha-methylacyl-CoA racemase and
prostate cancer. J Natl Cancer Inst. 96:834–843. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leman ES and Getzenberg RH: Biomarkers for
prostate cancer. J Cell Biochem. 108:3–9. 2009. View Article : Google Scholar : PubMed/NCBI
|