Introduction
The prognosis of patients with esophageal cancer
remains poor, prompting the search for novel treatment strategies.
Given the high malignant potential of this type of cancer, many
patients developed local recurrence of the tumor or distant
metastasis within a short period of time. Molecular biological
studies have shown that esophageal squamous cell carcinoma (ESCC)
is caused by the accumulation of multiple genetic changes in
oncogenes and tumor suppressor genes (1,2).
Thymidylate synthetase (TYMS) plays a role in catalyzing the
methylation of deoxyuridine monophosphate (dUMP) to deoxythymidine
monophosphate (dTMP), a crucial synthetic step in nucleotide
metabolism. Dihydropyrimidine dehydrogenase (DPYD) is also a
key enzyme in the metabolic pathway involved in the degradation of
the pyrimidine bases uracil and thymine.
TYMS and DPYD are significant enzymes
in de novo DNA synthesis and the salvage pathway in cancer
cells, respectively. This study investigated the TYMS and
DPYD mRNA expression in ESCC by real-time RT-PCR using a
LightCycler system. The results were analyzed with reference to the
clinico- pathological characteristics and prognosis of the ESCC
patients.
Materials and methods
Tissue samples
Tissue samples were obtained from 56 patients with
primary ESCC who underwent radical esophagectomy at the Department
of Surgery, Nagoya City University Medical School, between 1996 and
2000. The study design was approved by the Institutional Review
Board of the university hospital and written consent was obtained
from each patient. The tumors were classified according to the
Guidelines for the Clinical and Pathological Studies on Carcinoma
of the Esophagus (3). The patient
population comprised 44 males and 12 females (mean age 63.2±8.4
years; range 46–80). The samples were immediately frozen in liquid
nitrogen and stored at −80°C until use. None of the patients
received chemotherapy or radiation therapy prior to or following
surgery.
RT-PCR assays for thymidylate synthetase
and dihydropyrimidine dehydrogenase
The RNA concentration was determined using a
spectrophotometer and adjusted to a concentration of 200 ng/ml. RNA
(1 μg) was reverse transcribed by the Superscript II enzyme (Gibco
BRL, Gaitherburg, MD, USA) with 0.5 mg oligo(dT) (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). The reaction mixture was
incubated at 42°C for 50 min followed by incubation at 72°C for 15
min. To ensure the quality of mRNA extraction and reverse
transcription, the samples were subjected to PCR amplification with
oligonucleotide primers specific for the constitutively expressed
gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and
normalized to it. PCR was performed using LightCycler-Fast start
DNA Master SYBR-Green I kit (Roche Molecular Biochemicals,
Mannheim, Germany). The primer sequences for the TYMS gene
were: forward primer, 5′-TTACCTGAATCACATCGAGC-3′ and reverse
primer, 5′-ATATCCTTCGAGCTCCTTTG-3′. The cycling conditions were:
initial denaturation at 95°C for 10 min, followed by 60 cycles at
94°C for 15 sec, 55°C for 5 sec and 72°C for 8 sec. The primer
sequences for the DPYD gene were: forward primer,
5′-GTTCTGGCTACCAGGCTAT-3′ and reverse primer,
5′-CATAAGGTGTTGTCCTGGAA-3′. The cycling conditions were: initial
denaturation at 95°C for 10 min, followed by 60 cycles at 94°C for
15 sec, 56°C for 5 sec and 72°C for 6 sec. Amplified cDNAs were
separated on 1% agarose gels and the bands were visualized by
ethidium bromide.
Statistical methods
Data are expressed as the means ± standard deviation
(SD). Statistical analysis was performed using the Stat-View
software package (Abacus Concepts, Berkeley, CA, USA). The
Mann-Whitney U test was used to evaluate the significance of the
expression in paired groups. The survival of patients with ESCC was
examined using the Kaplan-Meier method, and the survival times were
compared using the log-rank test. Survival was measured from the
day of surgery. Multivariate analysis was performed using Cox’s
regression model and the logistic multivariate regression model.
P<0.05 was considered to be statistically significant.
Results
Analysis of thymidylate synthetase and
dihydropyrimidine dehydrogenase mRNA levels by real-time RT-PCR
assay using LightCycler
TYMS/GAPDH mRNA levels of the 56
esophageal cancer tissue samples were 1.553±0.275. The relationship
between TYMS/GAPDH mRNA and the patient
clinicopathological characteristics were examined (Table I). No significant differences were
noted in TYMS/GAPDH mRNA with respect to age, gender,
pathological differentiation, tumor status, lymph node status,
stage or vessel invasion. The TYMS/GAPDH mRNA
expression levels in patients positive with lymphatic invasion were
significantly higher compared to those in patients who exhibited
negative lymphatic invasion (P=0.0127).
 | Table ICorrelation of TYMS mRNA
expression in esophageal cancer with clinicopathological
characteristics. |
Table I
Correlation of TYMS mRNA
expression in esophageal cancer with clinicopathological
characteristics.
| Characteristics | No. of patients
(n=56) | TYMS
expression relative to GAPDH | P-value |
|---|
| Age at surgery | | | 0.9638 |
| ≤65 years | 33 | 1.542 | |
| >65 years | 23 | 1.568 | |
| Gender | | | 0.7396 |
| Male | 44 | 1.637 | |
| Female | 12 | 1.896 | |
| Pathological
subtype | | | 0.3309 |
| Well | 16 | 1.98 | |
| Non-well | 40 | 1.382 | |
| Tumor status | | | 0.6742 |
| T1/2 | 21 | 1.402 | |
| T3/4 | 35 | 1.644 | |
| Lymph node
status | | | 0.5005 |
| n+ | 41 | 1.666 | |
| n− | 15 | 1.243 | |
| Pathological
stage | | | 0.4452 |
| 0/I/II | 20 | 1.268 | |
| III/IV | 36 | 1.711 | |
| Lymphatic
invasion | | | 0.0126 |
| Negative | 11 | 0.775 | |
| Positive | 42 | 1.746 | |
| Blood vessel
invasion | | | 0.9554 |
| Negative | 19 | 1.566 | |
| Positive | 34 | 1.532 | |
The DPYD/GAPDH mRNA expression levels
were 5.463±1.807. By contrast, the DPYD/GAPDH mRNA
expression levels in patients positive with lymphatic invasion were
significantly lower compared to those in patients who exhibited
negative lymphatic invasion (P=0.0417). Moreover, no significant
differences were noted with respect to other factors (Table II).
 | Table IICorrelation of DPYD mRNA
expression in esophageal cancer with clinicopathological
characteristics. |
Table II
Correlation of DPYD mRNA
expression in esophageal cancer with clinicopathological
characteristics.
|
Characteristics | No. of patients
(n=56) | DPYD
expression relative to GAPDH | P-value |
|---|
| Age at surgery | | | 0.8581 |
| ≤65 years | 33 | 5.190 | |
| >65 years | 23 | 5.855 | |
| Gender | | | 0.1240 |
| Male | 44 | 6.265 | |
| Female | 12 | 2.525 | |
| Pathological
subtype | | | 0.2431 |
| Well | 16 | 3.217 | |
| Non-well | 40 | 6.515 | |
| Tumor status | | | 0.3333 |
| T1/2 | 21 | 7.741 | |
| T3/4 | 35 | 4.096 | |
| Lymph node
status | | | 0.4785 |
| n+ | 41 | 4.680 | |
| n− | 15 | 7.605 | |
| Pathological
stage | | | 0.5420 |
| 0/I/II | 20 | 6.959 | |
| III/IV | 36 | 4.632 | |
| Lymphatic
invasion | | | 0.0418 |
| Negative | 11 | 13.225 | |
| Positive | 42 | 3.717 | |
| Blood vessel
invasion | | | 0.3017 |
| Negative | 19 | 8.347 | |
| Positive | 34 | 4.206 | |
No significant clinicopathological differences were
noted in patients classified into groups with TYMS levels
higher (n=27) and lower (n=29) than 0.855. However, a significantly
higher risk of lymph node metastasis was noted in patients with
higher levels of DPYD (n=28, DPYD/GAPDH mRNA
levels >1.70).
Relationship between TYMS and DPYD and
survival
The correlation between the TYMS and
DPYD mRNA expression levels and the survival of ESCC
patients following surgery (median follow-up 19.7 months) was
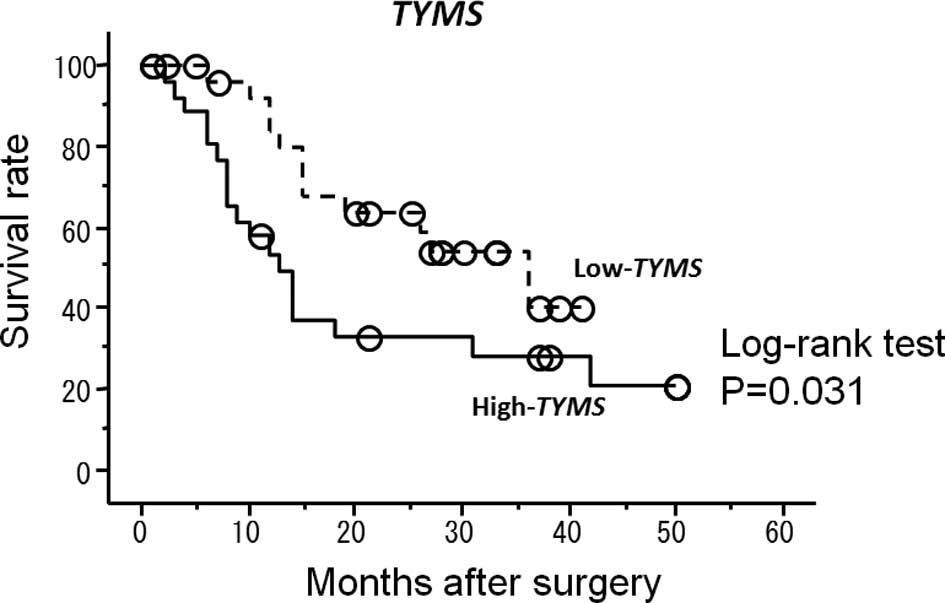
investigated. Patients with high TYMS mRNA expression levels
had a significantly shorter survival after surgery compared to
patients with low TYMS mRNA expression levels (P=0.031)
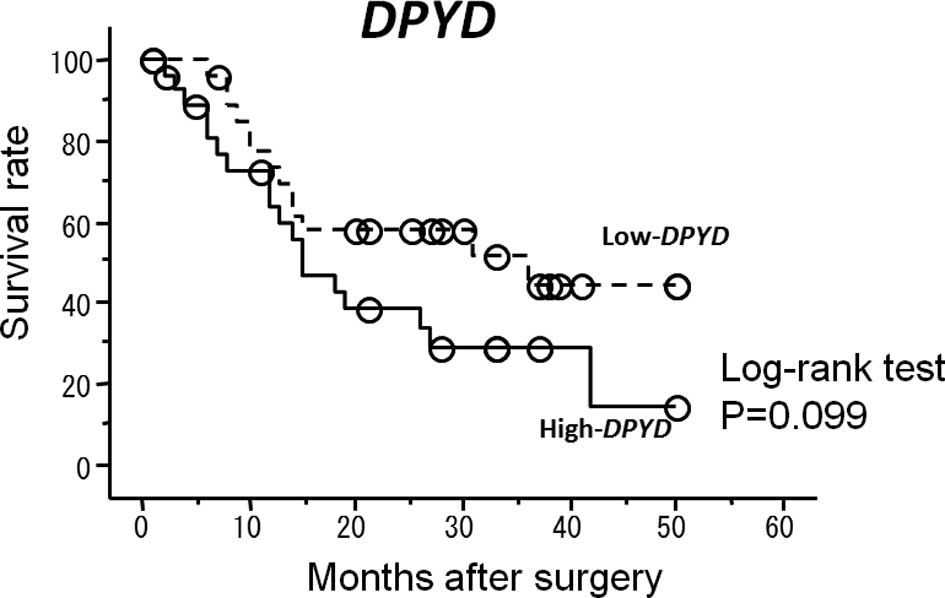
(Fig. 1). Patients with high
DPYD mRNA expression levels had a shorter survival, but no
significant difference was found (P=0.099) (Fig. 2).
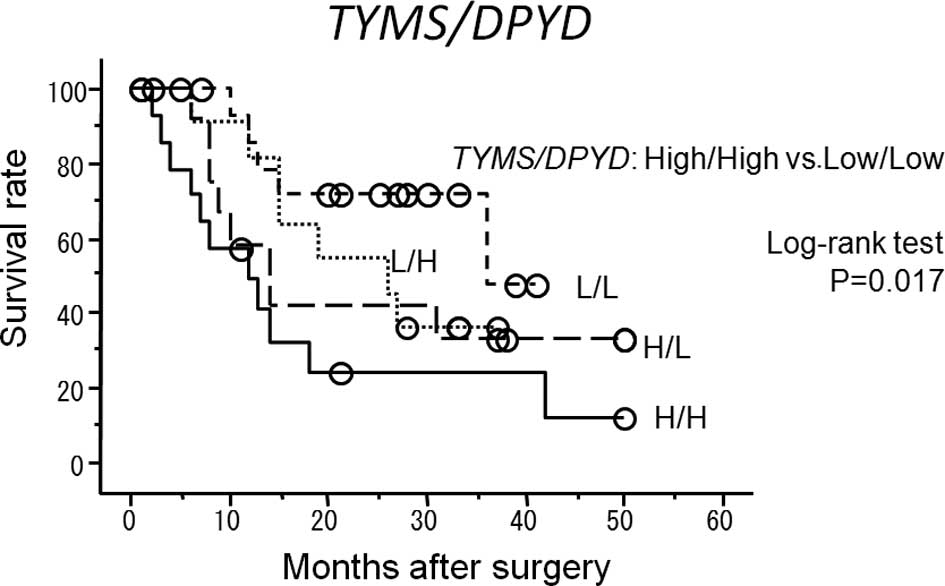
Of the four groups of patients (high
TYMS/DPYD, high TYMS but low DPYD, low
TYMS but high DPYD and low TYMS/DPYD),
the best survival rate was found in the group with low
TYMS/DPYD and the worst survival rate was observed in
the group with high TYMS/DPYD (P=0.017) (Fig. 3). The univariate analysis showed
that among the clinicopathological characteristics, local
invasiveness (tumor status) (risk ratio 7.38; P=0.0003), lymph node
metastasis (node status) (risk ratio 6.07; P=0.0032), lymphatic
invasion (risk ratio 5.48; P=0.021), blood vessel invasion (risk
ratio 3.56; P=0.0069) and TYMS mRNA expression (risk ratio
2.19; P=0.038) were statistically significant prognostic factors.
The multivariate analysis showed that local invasiveness (P=0.045)
and TYMS mRNA expression (P=0.041) were independent
prognostic factors (Table
III).
 | Table IIIUnivariate and multivariate analysis
of the expression levels of thymidylate synthetase and
dihydropyrimidine dehydrogenase and various clinical
characteristics. |
Table III
Univariate and multivariate analysis
of the expression levels of thymidylate synthetase and
dihydropyrimidine dehydrogenase and various clinical
characteristics.
| A, Univariate | | |
|---|
|
|---|
|
Characteristics | HR (95% CI) | P-value |
|---|
| Tumor status | 7.38
(2.52–21.66) | 0.0003 |
| Lymph node
status | 6.07
(1.83–20.14) | 0.0032 |
| Pathological
stage | 1.48
(0.71–3.11) | 0.3000 |
| Lymphatic
invasion | 5.48
(1.29–23.39) | 0.0210 |
| Blood vessel
invasion | 3.56
(1.42–8.96) | 0.0069 |
| TYMS | 2.19
(1.05–4.57) | 0.0380 |
| DPYD | 1.79
(0.88–3.67) | 0.1100 |
|
| B,
Multivariate |
|
| Tumor status | 4.67
(1.03–21.08) | 0.0450 |
| Lymph node
status | 2.37
(0.62–9.10) | 0.2100 |
| Lymphatic
invasion | 1.08
(0.13–9.17) | 0.9500 |
| Blood vessel
invasion | 1.42
(0.47–4.32) | 0.5400 |
| TYMS | 2.33
(1.03–5.24) | 0.0410 |
Discussion
Esophageal cancer is a digestive cancer with poor
prognosis and the mortality rate is steadily increasing. Three
types of treatment are currently available, i.e., operation,
chemotherapy and radiation therapy. Frequently, chemotherapy and
radiation therapy are combined, both before and after surgery. In
esophageal cancer, tumor growth is extremely rapid. Consequently,
prompt and correct diagnosis and staging, including identification
of remote metastases, and individual treatment are required. The
prediction of sensitivity to chemotherapeutic agents prior to
therapy is relevant. Chemotherapy for esophageal cancer relies
heavily on 5-fluorouracil (5-FU) and cisplatin. However, individual
variations in responsiveness to these chemotherapies exist.
Therefore, the susceptibility testing of the anti-cancer drug
treatment in esophageal cancer was reported. We also examined the
relationship between the expression of TYMS, DPYD,
thymidylate synthetase (TYMP) and orotate phosphoribosyl
transferase (OPRT) and 5-FU sensitivity in 25 ESCC cell
lines. Our findings showed that the TYMS and DPYD
mRNA expression levels may aid in predicting the anti-tumor
activity of 5-FU in ESCC (4). In
colorectal cancer, Salon et al and Nishimura et al
reported a correlation between the clinical effect of 5-FU and the
expression of those genes (5,6). Oguri
et al reported that the degradation of 5-FU via DPYD
is a significant determination of 5-FU sensitivity, while the
induction of TYMS contributes to acquired resistance against
5-FU in lung cancer (7).
On the other hand, certain authors have reported
that TYMS and DPYD exhibit the malignant potential of
gastric and colon cancers. Terashima et al reported that in
a group of patients who did not receive adjuvant chemotherapy,
survival was poor in patients with high TYMS activity
(8). Shirota et al
investigated the correlation between DPYD and malignant
potential in colon cancer, reporting that higher DPYD levels
were associated with higher pathological classification,
micro-scopic lymph node metastasis and liver metastasis (9). Suda et al found that the
expression of TYMS in gastric cancer correlated with
recurrence and survival rate (10).
Therefore, TYMS and DPYD affect the
clinical outcome of esophageal cancer in two ways. One possibility
is that TYMS and DPYD affect the malignancy of
cancer, the other is that they affect the outcome of the
anti-cancer drug treatment. Therefore, in the present study cases
in which anti-cancer drug treatments were used pre- and
post-surgery were excluded. Additionally, Tanaka et al
reported that the expression of TYMS and DPYD was
altered by chemoradiation therapy (CRT) in residual tumor cells of
esophageal cancer, when comparing mRNA levels in pre-CRT biopsies
and post-CRT specimens (11).
Brucher et al found no significant correlation between
clinical or histological factors and the relative gene expression
of TYMS, TYMP, DPYD or Her-2/neu.
However, patients exhibiting these factors underwent pre-operative,
combined radiochemotherapy (12).
Therefore, not only were the cases with anti-cancer treatment
excluded, but also those cases with radiation therapy. As a result,
we examined the correlation between the malignant potential of
esophageal cancer and the expression of TYMS and
DPYD.
In this study, TYMS mRNA expression was
significantly correlated with lymphatic invasion. However, no other
clinicopathological characteristics correlated with TYMS
mRNA levels. With regard to post-surgical survival, a high
expression of TYMS was associated with a poor prognosis.
Only the parameter and tumor status were noted in the multivariate
analysis. Comparable results were reported by Suda et al in
gastric cancer. These authors reported that the survival curve for
the TYMS-positive group was significantly lower compared to
that of the TYMS-negative group in the immunohistochemical
study (10). In addition to
TYMS, DPYD mRNA expression was statistically
correlated with lymphatic invasion. Nevertheless, no other factors,
including prognosis, correlated with DPYD. Certain studies
reported the usefulness of the combination analysis. Suda et
al reported that the TYMS-positive TYMP-positive
group was more inhibited than in other groups (8). Beck et al reported that in
cultured cells from colorectal cancers, those with low DPYD
and TYMS expression were experimentally more sensitive,
while the patients were clinically more sensitive to 5-FU (13). Danenberg et al reported that
in colorectal cancer, patients with low TYMS, DPYD
and TYMP levels exhibited the best survival curves (14). Ichikawa et al reported that
the combined expression of TYMS and DPYD predicted
the efficacy of chemotherapy (15).
In the present study, combination analysis was useful. The low
TYMS/DPYD group showed the best survival curves
statistically. A combined evaluation of the expression of other
genes, such as TYMP, is required for a more accurate
prediction of the response.
In conclusion, the present study showed that there
was a significant correlation between TYMS and DPYD
mRNA levels in esophageal cancer and the survival of patients
presenting with type of cancer. Based on the present data and the
relationship between gene expression and 5-FU sensitivity in
esophageal carcinoma cell lines, more effective treatment should be
established for individual patients.
References
|
1
|
Naganawa Y, Ishiguro H, Kuwabara Y, et al:
Decreased expression of FBXW7 is correlated with poor prognosis in
patients with esophageal squamous cell carcinoma. Exp Ther Med.
1:841–846. 2010.PubMed/NCBI
|
|
2
|
Ando T, Ishiguro H, Kuwabara Y, et al:
Expression of ACP6 is an independent prognostic factor for poor
survival in patients with esophageal squamous cell carcinoma. Oncol
Rep. 15:1551–1555. 2006.PubMed/NCBI
|
|
3
|
Japanese Society for Esophageal Disease.
Guidelines for the Clinical and Pathologic Studies on Carcinoma of
the Esophagus. 9th edition. Kanehara Publ. Co.; Tokyo: 1999
|
|
4
|
Ando T, Ishiguro H, Kuwabara Y, et al:
Relationship between expression of 5-fluorouracil metabolic enzymes
and 5-fluorouracil sensitivity in esophageal carcinoma cell lines.
Dis Eso. 21:15–20. 2007.
|
|
5
|
Salonga D, Danenberg KD, Johnson M, et al:
Colorectal tumors responding to 5-fluorouracil have low gene
expression levels of dihydropyrimidine dehydrogenase, thymidylate
synthase, and thymidine phosphorylase. Clin Cancer Res.
6:1322–1327. 2000.
|
|
6
|
Nishimura G, Terada I, Kobayashi T, et al:
Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels
in primary colorectal cancer show a relationship to clinical
effects of 5′-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol
Rep. 9:479–482. 2002.PubMed/NCBI
|
|
7
|
Oguri T, Achiwa H, Bessho Y, et al: The
role of thymidylate synthase and dihydropyrimidine dehydrogenase in
resistance to 5-fluorouracil in human lung cancer cells. Lung
Cancer. 49:345–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terashima M, Irunoda T, Fujiwara H, et al:
Roles of thymidylate synthase and dihydropyrimidine dehydrogenase
in tumor progression and sensitivity to 5-fluorouracil in human
gastric cancer. Anticancer Res. 22:761–768. 2002.PubMed/NCBI
|
|
9
|
Shirota Y, Ichikawa W, Uetake H, Yamada H,
Nihei Z and Sugihara K: Intratumoral dihydropyrimidine
dehydrogenase messenger RNA level reflects tumor progression in
human colorectal cancer. Ann Sur Oncol. 9:599–603. 2002. View Article : Google Scholar
|
|
10
|
Suda Y, Kuwashima Y, Tanaka Y, Uchida K,
Sakamoto H, Hashiguchi Y and Sekine T: Expression of thymidylate
synthase and thymidine phosphorylase in recurrence and survival
rates of advanced gastric cancer. Gastric Cancer. 2:165–172. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka K, Otake K, Mohri Y, et al:
Clinical significance of the gene expression profile in residual
tumor cells after neoadjuvant chemoradiotherapy for esophageal
cancer. Oncol Rep. 21:1489–1494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brücher BLDM, Keller G, Werner M, et al:
Using Q-RT-PCR to measure cyclin D1, TS, TP, DPD, and Her-2/neu as
predictors for response, survival, and recurrence in patients with
esophageal squamous cell carcinoma following radiochemotherapy. Int
J Colorectal Dis. 24:69–77. 2009.PubMed/NCBI
|
|
13
|
Beck A, Etienne MC, Cheradame S, Fischel
JL, Formento P, Renee N and Milano G: A role for dihydropyrimidine
dehydrogenase and thymidylate synthase in tumour sensitivity to
fluorouracil. Eur J Cancer. 30A:1517–1522. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Danenberg KD, Salonga D, Park JM, et al:
Dihydropyrimidine dehydrogenase and thymidylate synthase gene
expression identify a high percentage of colorectal tumors
responding to 5-fluorouracil. Proc Am Soc Clin Oncol. 17:258–262.
1998.
|
|
15
|
Ichikawa W, Takahasi T, Suto K, et al:
Thymidylate synthase and dihydropyrimidine dehydrogenase gene
expression in relation to differentiation of gastric cancer. Int J
Cancer. 112:967–973. 2004. View Article : Google Scholar : PubMed/NCBI
|