Introduction
Histological subtypes of non-small-cell lung
carcinomas (NSCLC) include adenocarcinomas (ADC), squamous cell
carcinomas (SCC) and adenosquamous carcinomas (ADSC). NSCLCs
represent 80% of lung cancers and are further classified into ADC,
including bronchioloalveolar carcinoma (BAC), and SCC (1). Receptor tyrosine kinases (RTKs)
regulate various key processes in mammalian development, cell
function and tissue homeostasis. Alterations at the level of the
receptor and its ligand lead to the activation of a number of
signaling pathways, each of which may contribute to cancer
progression. Deregulation of RTKs by mutation, gene rearrangement,
gene amplification and overexpression of both receptor and ligand
play a role as causative factors in the development and progression
of various types of human cancer (2–4). The
tyrosine-kinase epidermal growth factor receptor (EGFR) pathway has
been shown to play a crucial role in the pathogenesis of NSCLC,
leading to the development of targeted therapeutic agents using
small molecule EGFR tyrosine kinase inhibitors (TKIs) such as
gefitinib or erlotinib (5–7).
Recent clinical evidence of EGFR-TKIs in refractory
and advanced NSCLCs potentially indicate that EGFR-TKIs target
other deregulated growth factor signaling pathways, such as the
hepatocyte growth factor (HGF)/MET pathway. A recent study showed
that MET amplification leads to gefinitib secondary
resistance and may also explain the resistance noted in certain
patients (8). The MET gene
is located on band 7q31, and encodes a transmembrane tyrosine
kinase receptor for HGF/scatter factor (SF), located on band 7q25.
The MET gene is the prototypic member of a subfamily of
RTKs. The MET RTK family is structurally distinct from other
RTK families and is the only known high-affinity receptor for HGF,
also known as SF (9,10). In addition to the proliferative and
antiapoptotic activities that are common to various growth factors,
MET elicits unique motogenic and morphogenic effects by
stimulating cell-cell detachment, migration, invasion, tubule
formation and branching (11,12).
These activities of the MET signaling pathway provided
examples of the mechanisms by which this pathway is involved in
tumor development and progression. MET is usually considered
to be an oncogene (13) and appears
to play a role in ADCs. Cigarette smoking induces overexpression of
HGF in type II alveolar pneumocytes and lung cancer cells (14). Overexpression of HGF in lung cancer
cells induces alveolar differentiation/proliferation, and
MET activation may play a crucial role in
well-differentiated lung ADCs (15–17).
MET amplification has already been described in gastric and
esophageal cancers (18,19).
The transcription factor SOX2, a member of
the SRY-high mobility box transcription factor family, is expressed
in epithelial cells of the foregut, including the pharynx,
esophagus, trachea, bronchi and bronchioles, but is excluded from
the peripheral and alveolar regions of the lung (20). SOX2 is also expressed in
developing respiratory epithelium, but is restricted to the
conducting airways of the mature lung (21). SOX2 is induced in the
bronchiolar epithelium during repair following toxicant-induced
injury (22). Overexpression of
SOX2 in lung epithelium during early development disrupted
branching morphogenesis, resulting in cystic lungs and neonatal
death (21). It has been suggested
that SOX2, which belongs to group B of the SOX
family, plays a critical role in cell fate determination,
differentiation and proliferation (23,24). A
recent study showed SOX2 amplification on band 3q26.3 and
intense SOX2 immunostaining in lung SCC, indicating
potential active transcriptional regulation by SOX2.
SOX2-overexpressing lung epithelial cells and embryonic stem
cells (ESCs) reveal that SOX2 contributes to the activation
of ESC-like phenotypes and provides clues pertaining to the
de-regulated genes involved in the malignant phenotype (25). Limited data are available with
regards to the MET and SOX2 copy number in NSCLC. Our
study aimed to simultaneously analyze the MET and
SOX2 status in a NSCLC cohort to elucidate the potential
role of the MET and SOX2 signaling pathway and to
demonstrate MET and SOX2 gene amplification to its
clinicopathological features.
Materials and methods
Samples
A total of 115 primary lung cancers were analyzed
from Chinese patients whose surgeries were performed in Beijing
Chest Hospital, China, between 2007 and 2009. Resected tumors were
formalin-fixed and paraffin-embedded until the DNA was extracted.
Corresponding non-malignant peripheral lung tissues were also
collected. The specimens were reviewed by two reference
pathologists (Hai-Qing Zhang and Yi-Ran Cai) to confirm the
diagnosis and predominance (>70%) of cancer tissue in the tumor
specimens. BAC was defined as previously described (26). The two observers were blind to the
patient outcomes. Clinicopathological characteristics such as age,
gender, histological subtype and feature of tumors were
obtained.
DNA extraction
Genomic DNA was derived from formalin-fixed
paraffin-embedded tumors in blocks. The tissues (50–100 mg) were
scraped off the block, de-paraffinized twice in xylene, rinsed
twice with absolute ethanol and washed with pure water. The tissues
were suspended in 500 μl of glutaraldehyde containing 100–200 μg of
proteinase K (Promega, Madison, WI, USA) and incubated overnight at
55°C. Finally, the DNA was purified through columns (DNeasy Tissue
Kit, Tiangen, Beijing, China) following the manufacturer’s
instructions. The lysis mixture was centrifuged for 1 min to remove
undigested tissue and then the eluant (DNA) was combined with 200
μl of GB buffer, vortexed and incubated at 70°C for 10 min.
Following the addition of 210 μl of 100% ethyl alcohol, the sample
was vortexed, added to a spin column and centrifuged at 9000 rpm
for 1 min. The filtrate was discarded, 500 μ1 of GD buffer was
added, and the column was centrifuged at 8000 rpm for 1 min. The
filtrate was discarded, and the preceding step was repeated twice,
with the column spin being rinsed by PW buffer. The DNA was then
suspended in 100 μl of TE [10 mM Tris (pH 8) and 1 mM EDTA (pH 8)].
The DNA concentration was measured by UV absorbance at 260 nm, and
the samples were stored at 4°C until further use.
Real-time quantitative PCR
The Taq Man PCR reaction was used to evaluate the
amplification of MET and SOX2 genes in lung cancer.
This PCR reaction included a dual-labeled fluorogenic probe (5_
reporter and 3_ quencher dyes) and the amount of fluorescence
detected was directly proportional to the amount of DNA synthesized
(27–29). The Taq Man probes and primers were
designed using Primer Express software (Applied Biosystems,
Warrington, UK) and were optimized according to the manufacturer’s
guidelines. Target gene probes (MET and SOX2) and reference gene
probes (GAPDH) contained a TAMRA dye at the 3_ end and FAM as a
reporter dye at the 5_ end. The primer and probe sequences were:
GAPDH primer (forward, 5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′;
reverse, 5′-ACC AAA TCC GTT GAC TCC GAC CTT-3′; and probe,
5′-6FAM-AGC CAC ATC GCT CAG ACA CCA TGG G-TAMRA-3′); MET primer
(forward, 5′-TGC AGC GCG TTG ACT TAT TCA TGG-3′; reverse 5′-GAA ACC
ACA ACC TGC ATG AAG CGA-3′; and 5′-6FAM-AGG AGA CCT CAC CAT AGC TAA
TCT TGG G-TAMRA-3′); SOX2 primer (forward, 5′-CAC ATG AAG GAG CAC
CCG GAT TAT-3′; reverse 5′-GTT CAT GTG CGC GTA ACT GTC CAT-3′; and
5′-6FAM-TGA AGA AGG ATA AGT ACA CGC TGC CC-TAMRA-3′). Monoplex
real-time quantitative PCR was performed using a 50 ng template
extracted from paraffin-embedded tissues. The reactions were
carried out in a total volume of 25 μl containing 1X SsoFast probes
supermix (BioRad, USA). The primer and probe concentrations were
optimized for each target according to the manufacturer’s
instructions. The PCR program consisted of 50°C for 2 min and 95°C
for 10 min followed by 40 cycles of 95°C for 15 sec and 56°C for 1
min. In each run, the templates were assayed in triplicate and for
the majority of samples each run was repeated at least twice.
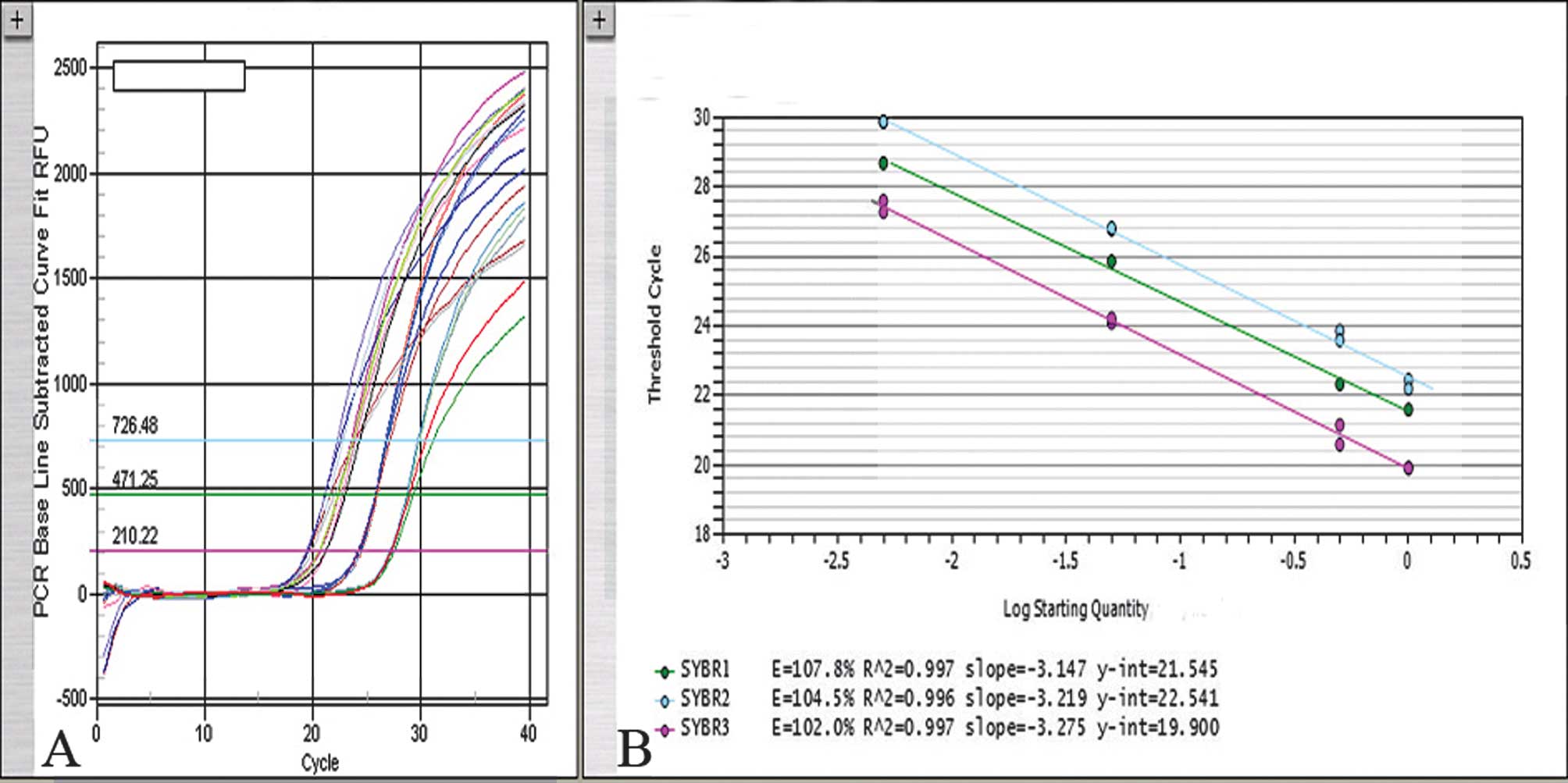
Standard curve
Real-time PCR technology is often used for the
relative quantification of nucleic acids (30). The slightest impurities in the
sample and uneven template fragmentation due to improper fixation
severely distort absolute quantification. Consequently, a relative
quantification algorithm is strongly recommended. In performing
relative calculations, the distortions of the amplification
efficiency due to fixation artifacts or sample impurities were
eliminated. Whether PCR would be quantitative when the DNA had been
extracted from fixed tissues under our experimental conditions was
defined. For fixed tissues, the 260 nm DNA concentration is not an
exact reflection of the DNA quantity to be amplified, since it is
altered by the presence of PCR inhibitory factors, as well as by
degradation or chemical modification of the DNA due to the
fixative. Consequently, a standard curve was constructed by
amplifying serial dilutions (0, 1:2, 1:20, 1:200) of normal DNA
from 500 to 2.5 ng (measured at 260 nm) (Fig. 1). A constant numerical ratio of
target and reference genes was measured in this concentration
range. The PCR efficiency in all of the cases was controlled (range
100–110%). The standard deviation (SD) of the values was in the
range of 0.5–1 cycle. Data analysis was carried out using iCycler
IQ5 optical system software (version 2.0, BioRad) which calculates
the threshold cycle numbers (Ct). The N cut-off value for gene
amplification was determined for the MET and SOX2
genes in paraffin wax-embedded tissues using cumulative frequencies
of the values. Individual measurements that fell outside the 97.5
percentile (the cut-off point) were considered to be outside the
normal values. Only standard curves with correlation coefficients
of ≥0.98 were used. The copy number change of the MET and
SOX2 genes in correlation to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was determined using the formula
(Ttarget
gene/TGAPDH)/(Ntarget
gene/NGAPDH), where Ttarget
gene and TGAPDH were determined from
sample DNA using MET, SOX2 and GAPDH; and the
normalized ratio of MET and SOX2 was determined from
a normal sample of 20 tuberculosis patients selected at random. The
results of samples for increased MET and SOX2
relative copy numbers were confirmed by repeating the experiments
≥3 times. An interval for the normalized ratio values was
calculated corresponding to the mean (M) ±2 SD. A lung tumor sample
was considered to be amplified if its ratio was >M +2 SD, or as
deleted if its ratio was <M -2 SD (31).
Statistical analysis
The associations between the categorical variables
were examined using the χ2 or Fisher’s exact tests.
Statistical tests were two-sided. The significant level α=0.05 and
P<0.05 were considered to indicate statistical significance for
the cross table (Table I), but when
referred to co-comparison within the cross table, the significant
level was specified at α/n (n = combinations of comparison). The
correlation between MET and SOX2 amplifications was
evaluated by calculating the r value. The statistical analysis was
performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA)
for Windows.
 | Table ICorrelation of SOX2 and
MET gene amplifications with their pathological
features. |
Table I
Correlation of SOX2 and
MET gene amplifications with their pathological
features.
| SOX2 | MET |
|---|
|
|
|---|
| Amplification |
Non-amplification | Amplification |
Non-amplification |
|---|
|
|
|---|
| Variable | Patient no. | % | Patient no. | P | Patient no. | % | Patient no. | P-value |
|---|
| All 57 cases | 30 | 26.1 | 85 | | 13 | 11.3 | 102 | |
| Gender |
| Male | 24 | 29.6 | 57 | 0.18 | 10 | 12.4 | 71 | 0.75 |
| Female | 6 | 17.7 | 28 | | 3 | 8.8 | 31 | |
| Age, years |
| <64 | 20 | 27 | 54 | 0.75 | 9 | 12.2 | 65 | 0.77 |
| ≥64 | 10 | 24.4 | 31 | | 4 | 9.8 | 37 | |
| Histological
type |
|
Adenocarcinoma | 10 | 20 | 40 | 0.39 | 1 | 2 | 49 | 0.02 |
| Squamous cell
carcinoma | 18 | 31.6 | 39 | | 11 | 19.3 | 46 | |
| Adenosquamous
carcinoma | 2 | 25 | 6 | | 1 | 12.5 | 7 | |
| Differentiation of
tumor |
| Well | 2 | 20 | 8 | 0.94 | 0 | 0 | 10 | 0.6 |
| Moderate and
poor | 28 | 26.7 | 77 | | 13 | 12.4 | 92 | |
| Tumor size
(cm) |
| <5 | 18 | 23.7 | 58 | 0.5 | 8 | 10.5 | 68 | 0.76 |
| ≥5 | 12 | 30.8 | 27 | | 5 | 12.8 | 34 | |
| Lymph node
metastasis |
| Positive | 17 | 31.5 | 37 | 0.29 | 6 | 11.1 | 48 | 0.95 |
| Negative | 13 | 21.3 | 48 | | 7 | 11.5 | 54 | |
| Smoking status |
| No history | 12 | 25 | 36 | 0.82 | 1 | 2.1 | 47 | 0.008 |
| Former or
current | 18 | 26.9 | 49 | | 12 | 17.9 | 55 | |
Results
Patient characteristics and
histopathological features
The clinicopathological characteristics of 115
non-small cell carcinomas are listed in Table I. The patients were primarily male
(70.4%, n=81). A total of 67 patients (58.3%) had a former or
current history of smoking. The median age was 58 years (range
27–77). The patients were diagnosed with 57 SCCs (49.5%) and 50
ADCs (43.5%), including 8 BACs and 8 ADSCs (7%). ADSCs, as a
special substance in histological types, which exhibit ADC and SCC,
were used as connectors to assess the relationship between ADC and
SCC. A total of 54 patients (47%) presented with lymph node
metastasis. Of the tumors, 10 (8.7%) were well differentiated,
whereas 58 (50.4%) exhibited moderate and 47 (40.9%) exhibited poor
differentiation. From a total of 57 cases in the SCC subgroup, 28
cases (49.1%) had tumor dimensions of <5 cm, 49 cases (86%) had
a history of smoking and all 57 cases were moderately or poorly
differentiated. Additionally, in 24 cases (42.1%) a local lymph
node invasion was detected. Of 50 cases in the ADC subgroup, 38
(76%) had no history of smoking, 43 (86%) had tumor dimensions of
<5 cm, 40 (80%) were moderately or poorly differentiated, 28
(56%) were female and 35 (70%) were <64 years of age. The ADSCs
histologically comprised ADC and SCC patterns. In this subgroup, 6
cases (75%) were smokers, 5 cases had tumor dimensions of <5 cm,
4 cases were <64 years of age, and 4 cases exhibited lymph node
metastasis.
SOX2 and MET gene amplifications in
non-small-cell lung carcinoma
The amplifications of the MET and SOX2
oncogenes were detected by relative quantitative real-time PCR
analysis in 115 NSCLC samples with paraffin-embedded tissues. The
normalized ratios of MET and SOX2 calculated from
non-cancer tissues were 0.942±0.09 and 0.96±0.08, respectively. Of
this cohort, 11.3% (13/115) and 26.1% (30/115) exhibited MET and
SOX2 amplifications, respectively. No deletion was found in either
of the genes. The patients were classified into amplified and
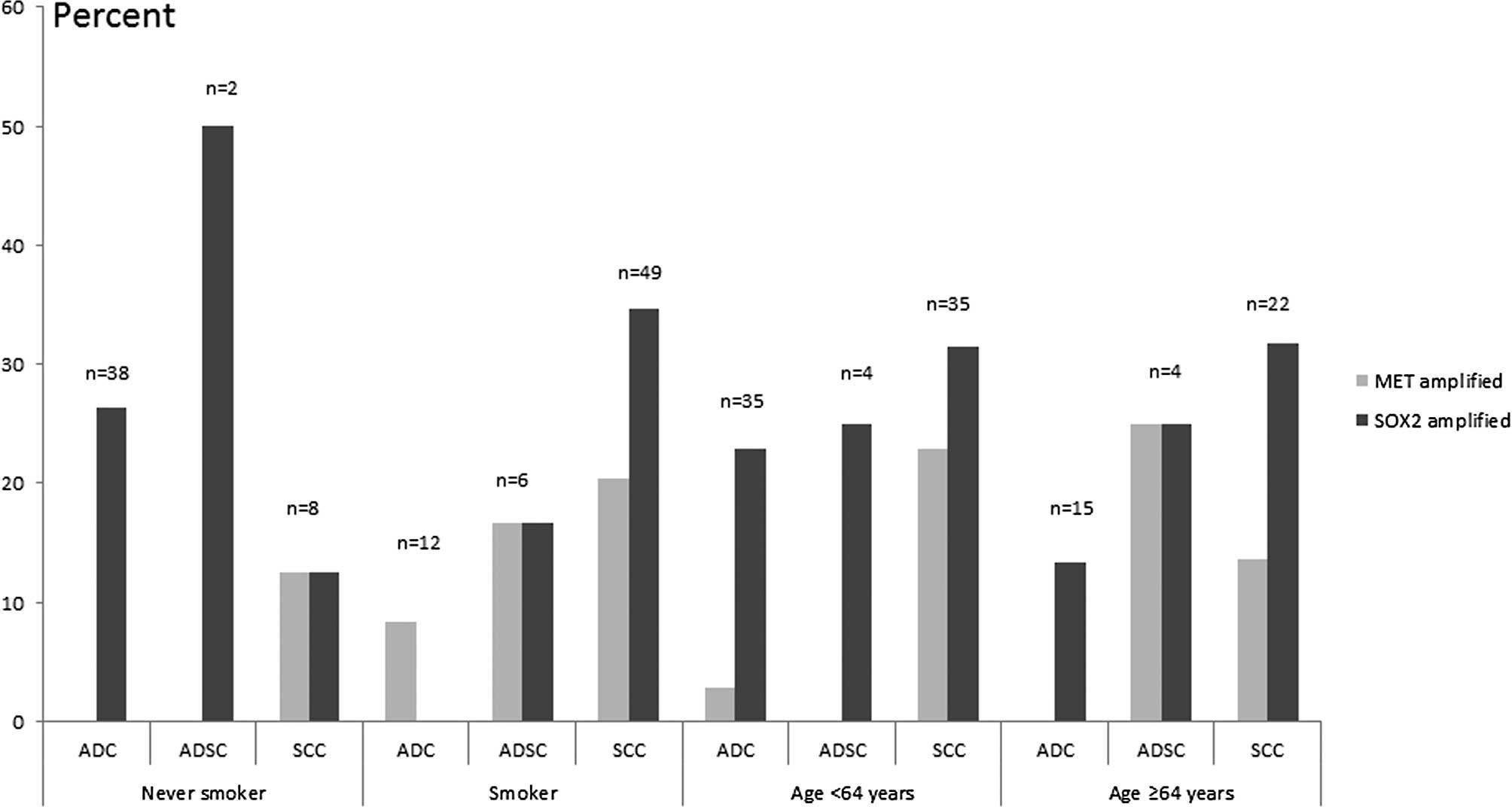
unamplified groups and stratified by other factors (Table I, Figs.
2 and 3).
In ADCs, the 38 cases with no history of smoking
were unamplified in the MET gene vs. 10 cases amplified in
SOX2. MET amplification indicated a low level in this group
despite clinicopathological characteristics such as tumor
dimension, lymph node invasion and age. Of the 12 smokers, 1 case
was found to be amplified in MET vs. no cases in
SOX2. Among 43 cases exhibiting a tumor dimension of <5
cm, 2.3% of the cases were found to be amplified in MET and
20.9% in SOX2. Of the ADCs, 26 cases were positive for lymph
node invasion and 6 cases (23.1%) indicated SOX2
amplification vs. no cases detected in MET.
In the SCC subpopulation, all 57 cases were
moderately or poorly differentiated, 18 cases (31.6%) had
SOX2 amplification and 11 cases (19.3%) had MET
amplification, including 10 cases (91%) with a history of smoking.
When subdivided by the smoking status of SCCs, the amplification
level of the SOX2 gene was higher than that in ADCs among
smokers (χ2=6.25, P=0.014; below the significant level
of 0.017). Of the 35 cases that were younger than 64 years of age
showed 1 case (2.86%) amplified in MET and 8 (22.9%) in
SOX2 gene. From the 35 cases, MET amplification was
noted in 7 cases (25%) with a tumor dimension <5 cm each, and 4
(13.8%) with a tumor dimension ≥5 cm. MET and SOX2
gene amplification were not significantly associated with tumor
dimension or lymph node invasion. In this study, we demonstrated
that MET amplification was negatively correlated with SOX2
amplification although the difference was not statistically
significant (r=−0.19, P>0.05).
In the ADSC group, the 8 cases were moderately or
poorly differentiated. Of these cases, 1 (12.5%) and 2 (25%)
exhibited MET and SOX2 amplification, respectively.
Only 1 of 2 cases among non-smokers exhibited SOX2
amplification, whereas no cases exhibited MET amplification.
ADSC patients younger than 64 years of age were not amplified in
MET gene, and only 1 case (25%) was amplified in SOX2
gene. Of the cases with lymph node invasion, 1 (25%) and 2 (50%)
showed in MET and SOX2 amplification, respectively.
No amplification was noted in either MET and SOX2
without lymph node metastasis (Fig.
2).
A significant difference was found in the MET
amplification level among former or current smokers vs. non-smokers
(χ2=6.99, P<0.01). SOX2 amplification occurred
in 20% of ADCs, 19.3% of SCCs and 25% of ADSCs (P=0.39). MET
amplifications were detected in ADCs (2%), SCCs (19.3%) and ADSCs
(12.5%) (χ2=7.96, P=0.02). Further analysis showed that
the MET level was higher in SCCs than in ADCs
(χ2=8.0, P=0.005; compared under a significant level of
0.017). SOX2 amplification was correlated with patient
characteristics and tumor pathology. With the exception of a higher
frequency observed in SCCs with smoking status as compared to ADCs,
SOX2 amplification was not significantly associated with any
other clinical or pathological variable, including gender, age,
histological subtype, grade and lymph node invasion. MET
amplification did not correlate with gender. Only 2 (3.51%) SCCs
with a history of smoking presented co-amplification of MET
and SOX2. No co-amplification of the two genes was detected
in ADCs or ADSCs. A total of 8 (22.9%) SCCs younger than 64 years
of age (n=35) were correlated with amplified MET gene.
However, no correlation was noted with amplified MET in
ADSCs, and only 1 (2.9%) in ADCs (P=0.03) (Fig. 2). MET amplification was also
detected preferentially in patients with a former or current
history of smoking (17.9%), compared with non-smokers (2.1%). Among
the patients with no history of smoking, no cases of ADCs and ADSCs
had amplified MET with the exception of 1 SCC. No
significant difference was noted among smokers with MET
amplification in ADCs (8.3%, n=12), ADSCs (16.7%, n=6) or SCCs
(20.4%, n=49) (Fig. 2). In
addition, when stratified into subgroups by age and
differentiation, the frequency of MET amplification in SCCs
younger than 64 years of age and moderate differentiation was
marginally higher than those in ADCs and ADSCs (P=0.06, n=66).
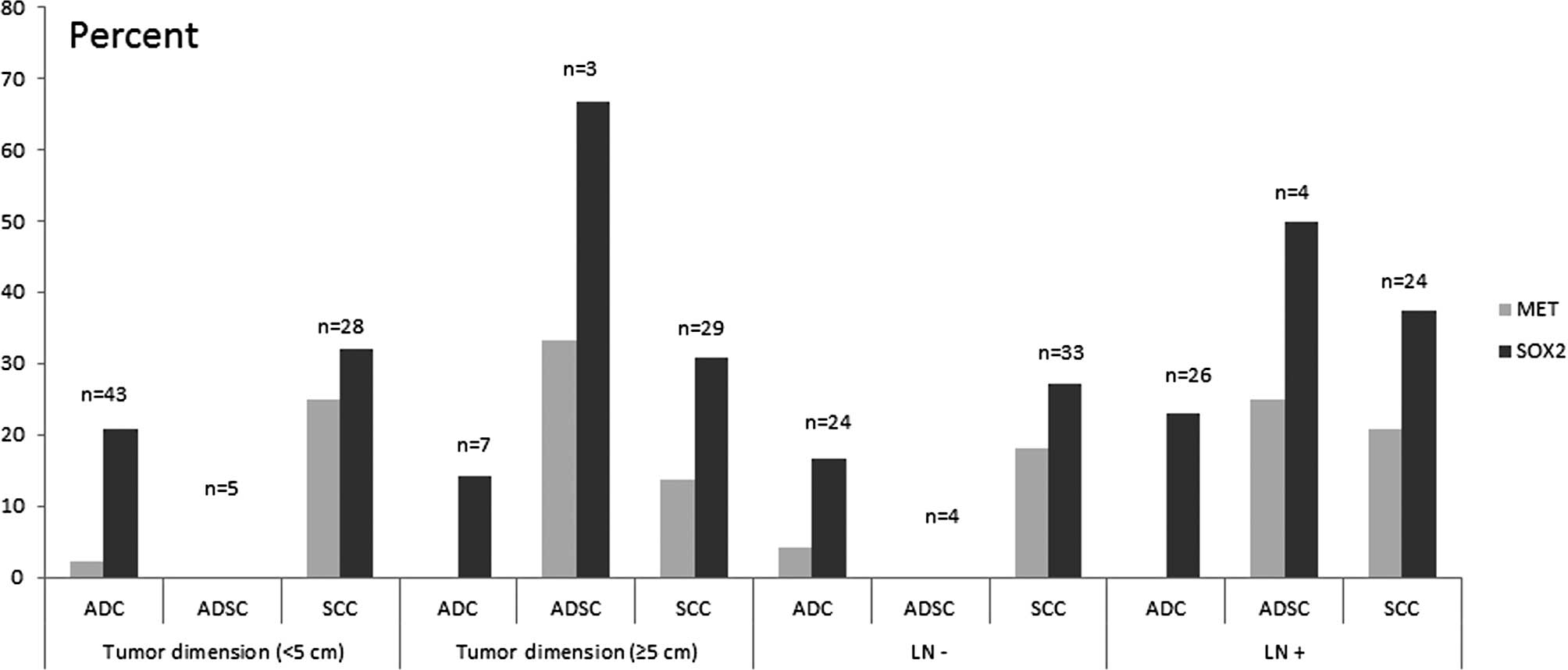
MET amplification in SCCs were more frequent than in ADCs
combined with clinicopathological characteristics such as younger
than 64 years of age, lymph node metastasis and a tumor dimension
of <5 cm (Figs. 2 and 3). A total of 2 cases (20%) of
well-differentiated ADCs were detected with SOX2 amplification. In
contrast, 8 cases (20%) of ADCs, 2 cases (25%) of ADSCs and 18
cases (31.6%) of SCCs amplified in SOX2 with moderate and
poor differentiation were detected (P=0.44).
MET and SOX2 amplifications were not
significantly correlated whether or not they were distinguished by
histological type. In ADCs discriminated by smoking status,
however, it was observed that MET amplification occurred
preferentially in smokers, whereas SOX2 amplification was
noted in non-smokers (Fig. 2).
Discussion
The tyrosine kinase receptor EGFR pathway has
been extensively studied in NSCLC since gefitinib and erlotinib are
used in NSCLC, with a clinical response more commonly observed in
ADC/BAC histology arising in non-smokers, females and patients of
East Asian ethnicity (5). Recent
findings have shown that MET amplification is associated
with gefitinib resistance during therapy (8). On the other hand, it has been
demonstrated that, using high-throughput analysis both in cell
lines and in patients with lung cancer, subpopulations of cells
with MET amplification existed prior to drug exposure
(32). The MET gene
comprises 21 exons and 20 introns (33,34).
The role of MET in human tumors is enhanced by mutation or
amplification, leading to oncogenic changes such as cell
proliferation, reduced apoptosis, angiogenesis, altered
cytoskeletal function and metastasis. We found that in the subgroup
with local lymph node invasion, the MET copy level in SCCs
was marginally higher than in ADCs. Studies have shown that
MET and its ligand HGF are mis-and over-expressed in head
and neck SCC (HNSCC), resulting in the constitutive activation of
the RTK system. This aberrant activity probably induces the
mechanism of invasive growth by conferring an invasive potential to
these tumors. HGF and MET orchestrate invasive growth
during the progression of HNSCC by integrating a number of
independent biological responses using a specific set of signaling
pathways. Specifically, HGF/MET may facilitate the
detachment of neoplastic cells from primary HNSCCs through
MAPK signaling, resulting in the Snail-mediated
transcriptional down-regulation of E-cadherin (35). MET was also found to be
deleted in ADC/BACs and SCCs; MET deletion is not a
prognostic factor. However, MET appeared to be less
frequently deleted compared with MET amplification (5). We identified a population with
MET amplification (11.3%, 13/115) in our cohort including 11
cases of SCCs, with 10 cases being smokers. A previous study showed
that the MET gene copy status was not associated with
gender, smoking history, histology or stage. However, true MET
amplification occurred more frequently in patients with SCC than in
those with ADC. The incidence of MET amplification between
SCC and ADC was significantly different (36). The clinicopathologic factors of
MET amplification in NSCLC are conflicting in a number of
studies. Okuda et al (37)
reported that an increased MET gene copy number (GCN) was
observed in 5.6% of patients with NSCLC, who were male and smokers,
whereas no difference in the MET GCN status was noted with
regards to histological type. In contrast, other studies observed
that MET amplification (or high GCN) was not significantly
associated with gender, smoking history or histology (38,39).
However, Go et al (36)
showed that the majority of patients with true MET
amplification were male smokers with SCC. Partially consistent with
this study, we found that MET amplifications are not only
associated with smoking status, but are more prevalent in SCCs as
compared to ADCs, suggesting that MET amplification may be
more involved in the oncogenesis of SCCs resulting from smoking
than ADCs and may play a key role in lymph node invasion in SCC
compared to ADC.
SOX2 is a key transcription factor involved
in the stabilization of embryonic stem cells in a pluripotent state
(40). Advances of stem cell
biology have proven that both embryonic and cancer stem cells
exist. Functions of such cancer stem cells include self-renewal
which drives tumorigenesis, and aberrant differentiation that
contributes to cellular heterogeneity. It was suggested that tumors
contain a cellular population that retains key stem cell properties
(41) which, in turn, have gene
expression signatures closely related to embryonic stem cells
(42). A high SOX2
expression was found in breast cancer (43), testicular germ cell tumors (44) and gastric adenocarcinoma (23). Expression of SOX2 protein has
not been extensively studied in lung cancer. However, a recent
study showed that SOX2 is strongly and diffusely expressed
in approximately 90% of pulmonary SCC and 20% of ADC (45). To the best of our knowledge, this is
the first report concerning high SOX2 amplification in a
cohort of NSCLC. We showed that the amplification of SOX2 in
SCCs and ADCs was 31.6 and 20%, respectively. SOX2 is
considered to be a master pluripotency controller that was recently
identified as a major novel oncogene, recurrently amplified and
activated in SCC (46,47). These studies used a similar strategy
of chromosomal aberrations screening to identify the SOX2
locus as one of the most frequently amplified sites over the SCC
genome. They have further highlighted the recurrent SOX2
activation and its indispensable role for squamous cell survival.
The studies showed that SOX2 is involved in the early steps
of lung SCC since it participates in transforming human bronchial
epithelial cells. Furthermore, SOX2 overexpression induces
the expression of the squamous markers p63 and keratin 6,
indicating that SOX2 plays a role in SCC differentiation
(47). However, neither study
assessed the impact of the recurrent activation of SOX2 in
advanced primary tumors nor how SOX2 may mechanistically be
involved in tumor progression and aggressiveness. The
above-mentioned studies therefore elucidate and offer novel
perspectives on the multiple roles that SOX2 exerts on SCC
carcinogenesis.
In our study population, 89.1% (49/55) of SCC and
24% (12/50) of ADC had a history of smoking, and 34.7% (17/49) of
SCCs had SOX2 amplification, whereas no amplification was
found in ADCs. In contrast, 10 of 38 (26.3%) cases involving
patients with no history of smoking and with ADC presented
SOX2 amplification, indicating that SOX2
amplification may be an activating pathway to ADC. Additionally,
SCCs presented a higher proportion of SOX2 amplification
than in ADCs and ADSCs among smokers (Fig. 2). The evidence points to the
discrepancy in the oncogenesis of SCC and ADC. Comparative genomic
hybridization studies have demonstrated that more than 90% of SCCs
and approximately 20% of ADCs have copy number gain involving
3q26,4, i.e., the same proportions that have been shown to have a
high level of SOX2 expression at the protein level (45). Notably, these studies have found a
high level of amplification only in SCC (48). These observations warrant additional
studies to determine the molecular mechanisms involved in
SOX2 expression in ADC. In this study, the SOX2 gene
was more likely to be amplified in the subgroup of patients with no
history of smoking and with ADCs. This pilot study was biased to
include surgically resectable, early stage tumors. Consequently,
sufficient higher-stage tumors in order to perform a well-powered
statistical analysis were not available. However, we hypothesize
that the SOX2 and MET genes play different roles in
the carcinogenesis of SCCs and ADCs, respectively. Previous studies
suggest that overexpression of SOX2 is key in the activation of the
Nanog/Oct4/SOX2 pathway, which promotes tumor cell proliferation
and is associated with the short survival time of patients in stage
I ADC (49). SOX2 is also
activated in more advanced SCC tumors, as previously reported
(25,45). We conclude that SOX2 is activated
not only by overexpression but also by amplification as previously
mentioned (25,50). MET and SOX2 gene
amplifications are more common in the SCCs of smokers. Moreover,
MET amplification is intrinsic to SCCs, particularly among
smokers, with regards to tumor growth, local lymph node metastasis
and negative correlation with SOX2 amplification. The
incidence of amplifications of MET and SOX2 is in the
early stage of tumorigenesis in NSCLC. We speculate that the
SOX2 gene is not only activated by amplification but is also
affected by other regulators that promote its transcription,
affecting its downstream genes.
ADSCs are morphologically mixed tumors comprising
the two cell components ADC and SCC. To determine whether these
types of tumors are a ‘simple’ mix of ADC and SCC or whether they
present molecular specificities, as compared with the molecular
characterization of the two components, Bastide et al found
that genes were differentially expressed when comparing ADCs SCCs,
ADSCs SCCs and ADCs ADSCs (51).
Partially consistent with their findings, we observed that, when
classifying the three histological subtypes, using MET and
SOX2 gene amplifications that distinguished ADCs and SCCs,
all ADSCs were identified as intermediate between ADCs and SCCs,
with some being similar to ADCs, but others to SCCs. The results
indicate that ADSCs are considered a mix of ADCs and SCCs, in
various proportions. Moreover, molecular specificities were
observed since we found MET and SOX2 gene
amplifications among smokers in the three histological types. In
conclusion, the ADSC mixed lung tumors are more complex than a
‘simple’ mix of ADC and SCC components. A recent study showed that
neuroendocrine differentiation and ERK proliferation pathways
appeared to be preferentially deregulated in ADSCs compared to ADCs
and SCCs, which warrants further investigation since these pathways
may partially explain the high clinical aggressiveness of ADSCs
(51). Amplification of SOX2
was also found to be more preferential in ADSCs than the MET
gene (Figs. 2 and 3). One limitation of our study is the
insufficient number of cases. Thus, more studies should be
conducted to determine the molecular specificities of ADSCs.
Acknowledgements
We thank Xue-Jing Chen, Li Zhang and Chen Zhang for
their support in sample collection. Supported by the Beijing
Foundation for Distinguished Scientists grant 2009D003013000001,
awards from the Beijing Board of Health, China.
References
|
1
|
Travis WD, Brambilla E and
Muller-Hermelink HK: World Health Organization Classification of
Tumours, Pathology and Genetics: Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon: 2004
|
|
2
|
Laird AD and Cherrington JM: Small
molecule tyrosine kinase inhibitors: clinical development of
anticancer agents. Expert Opin Investig Drugs. 12:51–64. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maulik G, Kijima T and Salgia R: Role of
receptor tyrosine kinases in lung cancer. Methods Mol Med.
74:113–125. 2003.PubMed/NCBI
|
|
4
|
Lamorte L and Park M: The receptor
tyrosine kinases: role in cancer progression. Surg Oncol Clin N Am.
10:271–288. 2001.PubMed/NCBI
|
|
5
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 21:2237–2246.
2003.
|
|
6
|
Kris MG, Natale RB, Herbst RS, et al:
Efficacy of gefitinib, an inhibitor of the epidermal growth factor
receptor tyrosine kinase, in symptomatic patients with non-small
cell lung cancer: a randomized trial. JAMA. 290:2149–2158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al: Erlotinib in previously treated non-small-cell lung
cancer. N Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplification leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naldini L, Weidner KM, Vigna E, et al:
Scatter factor and hepatocyte growth factor are indistinguishable
ligands for the MET receptor. EMBO J. 10:2867–2878. 1991.PubMed/NCBI
|
|
10
|
Bottaro DP, Rubin JS, Faletto DL, et al:
Identification of the hepatocyte growth factor receptor as the
c-met proto-oncogene product. Science. 251:802–804. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maulik G, Shrikhande A, Kijima T, Ma PC,
Morrison PT and Salgia R: Role of the hepatocyte growth factor
receptor, c-Met, in oncogenesis and potential for therapeutic
inhibition. Cytokine Growth Factor Rev. 13:41–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooper CS, Park M, Blair DG, et al:
Molecular cloning of a new transforming gene from a chemically
transformed human cell line. Nature. 311:29–33. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JT, Lin TS, Chow KC, et al: Cigarette
smoking induces overexpression of hepatocyte growth factor in type
II pneumocytes and lung cancer cells. Am J Respir Cell Mol Biol.
34:264–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura Y, Niki T, Goto A, et al: c-Met
activation in lung adenocarcinoma tissues: an immunohistochemical
analysis. Cancer Sci. 98:1006–1013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsao MS, Liu N, Chen JR, et al:
Differential expression of Met/hepatocyte growth factor receptor in
subtypes of non-small cell lung cancers. Lung Cancer. 20:1–16.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beau-Faller M, Gaub MP, Schneider A, et
al: Allelic imbalance at loci containing FGFR, FGF, c-Met and HGF
candidate genes in non-small cell lung cancer sub-types,
implication for progression. Eur J Cancer. 39:2538–2547. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smolen GA, Sordella R, Muir B, et al:
Amplification of MET may identify a subset of cancers with extreme
sensitivity to the selective tyrosine kinase inhibitor PHA-665752.
Proc Natl Acad Sci USA. 103:2316–2321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller CT, Lin L, Casper AM, et al:
Genomic amplification of MET with boundaries within fragile site
FRA7G and upregulation of MET pathways in esophageal
adenocarcinoma. Oncogene. 25:409–418. 2006.PubMed/NCBI
|
|
20
|
Que J, Luo X, Schwartz RJ and Hogan BL:
Multiple roles for Sox2 in the developing and adult mouse trachea.
Development. 136:1899–1907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gontan C, de Munck A, Vermeij M, Grosveld
F, Tibboel D and Rottier R: Sox2 is important for two crucial
processes in lung development: branching morphogenesis and
epithelial cell differentiation. Dev Biol. 317:296–309. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park KS, Wells JM, Zorn AM, et al:
Transdifferentiation of ciliated cells during repair of the
respiratory epithelium. Am J Respir Cell Mol Biol. 34:151–157.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukamoto T, Mizoshita T, Mihara M, et al:
Sox2 expression in human stomach adenocarcinomas with gastric and
gastric-and-intestinal-mixed phenotypes. Histopathology.
46:649–658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XL, Eishi Y, Bai YQ, et al: Expression
of the SRY-related HMG box protein SOX2 in human gastric carcinoma.
Int J Oncol. 24:257–263. 2004.PubMed/NCBI
|
|
25
|
Hussenet T, Dali S, Exinger J, et al: SOX2
is an oncogene activated by recurrent 3q26.3 amplifications in
human lung squamous cell carcinomas. PLoS One. 5:e89602010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebright MI, Zakowski MF, Martin J, et al:
Clinical pattern and pathologic stage but not histologic features
predict outcome for bronchioloalveolar carcinoma. Ann Thorac Surg.
74:1640–1647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ, Flood SJ, Marmaro J, Giusti W
and Deetz K: Oligonucleotides with fluorescent dyes at opposite
ends provide a quenched probe system useful for detecting PCR
product and nucleic acid hybridization. PCR Methods Appl.
4:357–362. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higuchi R, Fockler C, Dollinger G and
Watson R: Kinetic PCR analysis: real-time monitoring of DNA
amplification reactions. Biotechnology (NY). 11:1026–1030. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holland PM, Abramson RD, Watson R and
Gelfand DH: Detection of specific polymerase chain reaction product
by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus
DNA polymerase. Proc Natl Acad Sci USA. 88:7276–7280. 1991.
|
|
30
|
Bieche I, Olivi M, Champeme MH, Vidaud D,
Lidereau R and Vidaud M: Novel approach to quantitative polymerase
chain reaction using real-time detection: application to the
detection of gene amplification in breast cancer. Int J Cancer.
78:661–666. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beau-Faller M, Ruppert AM, Voegeli AC, et
al: MET gene copy number in non-small cell lung cancer: molecular
analysis in a targeted tyrosine kinase inhibitor naive cohort. J
Thorac Oncol. 3:331–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turke AB, Zejnullahu K, Wu YL, et al:
Preexistence and clonal selection of MET amplification in EGFR
mutant NSCLC. Cancer Cell. 17:77–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maestrini E, Tamagnone L, Longati P, et
al: A family of transmembrane proteins with homology to the
MET-hepatocyte growth factor receptor. Proc Natl Acad Sci USA.
93:674–678. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Comoglio PM and Boccaccio C: The HGF
receptor family: unconventional signal transducers for invasive
cell growth. Genes Cells. 1:347–354. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Herdt MJ and Baatenburg de Jong RJ: HGF
and c-MET as potential orchestrators of invasive growth in head and
neck squamous cell carcinoma. Front Biosci. 13:2516–2526.
2008.PubMed/NCBI
|
|
36
|
Go H, Jeon YK, Park HJ, Sung SW, Seo JW
and Chung DH: High MET gene copy number leads to shorter survival
in patients with non-small cell lung cancer. J Thorac Oncol.
5:305–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okuda K, Sasaki H, Yukiue H, Yano M and
Fujii Y: Met gene copy number predicts the prognosis for completely
resected non-small cell lung cancer. Cancer Sci. 99:2280–2285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bean J, Brennan C, Shih JY, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cappuzzo F, Marchetti A, Skokan M, et al:
Increased MET gene copy number negatively affects survival of
surgically resected non-small-cell lung cancer patients. J Clin
Oncol. 27:1667–1674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masui S, Nakatake Y, Toyooka Y, et al:
Pluripotency governed by Sox2 via regulation of Oct3/4 expression
in mouse embryonic stem cells. Nat Cell Biol. 9:625–635. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: an old idea--a paradigm shift. Cancer Res. 66:1883–1890.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ben-Porath I, Thomson MW, Carey VJ, et al:
An embryonic stem cell-like gene expression signature in poorly
differentiated aggressive human tumors. Nat Genet. 40:499–507.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Shi L, Zhang L, et al: The
molecular mechanism governing the oncogenic potential of SOX2 in
breast cancer. J Biol Chem. 283:17969–17978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Biermann K, Heukamp LC, Steger K, et al:
Genome-wide expression profiling reveals new insights into
pathogenesis and progression of testicular germ cell tumors. Cancer
Genomics Proteomics. 4:359–367. 2007.PubMed/NCBI
|
|
45
|
Sholl LM, Long KB and Hornick JL: Sox2
expression in pulmonary non-small cell and neuroendocrine
carcinomas. Applied immunohistochemistry & molecular
morphology: AIMM/official publication of the Society for Applied
Immunohistochemistry. 18:55–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hussenet T and du Manoir S: SOX2 in
squamous cell carcinoma: amplifying a pleiotropic oncogene along
carcinogenesis. Cell Cycle. 9:1480–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bass AJ, Watanabe H, Mermel CH, et al:
SOX2 is an amplified lineage-survival oncogene in lung and
esophageal squamous cell carcinomas. Nat Genet. 41:1238–1242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bjorkqvist AM, Husgafvel-Pursiainen K,
Anttila S, et al: DNA gains in 3q occur frequently in squamous cell
carcinoma of the lung, but not in adenocarcinoma. Genes Chromosomes
Cancer. 22:79–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sholl LM, Barletta JA, Yeap BY, Chirieac
LR and Hornick JL: Sox2 protein expression is an independent poor
prognostic indicator in stage I lung adenocarcinoma. Am J Surg
Pathol. 34:1193–1198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McCaughan F, Pole JC, Bankier AT, et al:
Progressive 3q amplification consistently targets SOX2 in
preinvasive squamous lung cancer. Am J Respir Crit Care Med.
182:83–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bastide K, Ugolin N, Levalois C, Bernaudin
JF and Chevillard S: Are adenosquamous lung carcinomas a simple mix
of adenocarcinomas and squamous cell carcinomas, or more complex at
the molecular level? Lung Cancer. 68:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|