Introduction
Lung cancer is the leading cause of cancer-related
death in many industrialized countries. Platinum-based combination
chemotherapy has been shown to improve survival and quality of life
in patients with advanced non-small cell lung cancer (NSCLC).
However, chemotherapy for advanced NSCLC has been of limited
benefit and appears to have reached a plateau, with response rates
of approximately 30% and a median survival period of 8 months
(1–4). Various molecular-targeted agents were
developed, a number of which are now standard treatment, with or
without conventional cytotoxic agents (5–7). Among
these agents, tyrosine kinase inhibitors (TKIs) of epidermal growth
factor receptor (EGFR) have produced a marked change in the
clinical practice of NSCLC.
At present, two different types of EGFR-TKIs are
widely used: gefitinib and erlotinib. In predicting the efficacy of
these agents, certain clinical factors, such as histology, gender,
smoking status and ethnicity, are regarded as significant (8). Somatic mutations of the tyrosine
kinase domain of EGFR were found and were shown to be the most
reliable predictive marker for the response to EGFR-TKIs (8–10).
Findings of a recent population-based study showed that EGFR
mutations significantly predict both a survival benefit of
gefitinib and a favorable prognosis in patients with advanced lung
adenocarcinoma (11). In the recent
version of the American Society of Clinical Oncology (ASCO)
guideline, gefitinib was accepted as the first-line chemotherapy
for patients with activating EGFR mutations (12). The survival benefit is substantial
and patients who are known to have EGFR mutations usually receive
EGFR-TKIs during the treatment period.
Consequently, the EGFR mutational status may need to
be incorporated as a stratification factor in randomized clinical
trials even when EGFR-TKIs are not included in the experimental
regimens as they appear to strongly affect survival when used in a
second-line setting or beyond. This study aimed to show the
significance of the EGFR mutational status as a stratification
factor for future randomized trials by clarifying the impact of the
EGFR mutational status on the survival of NSCLC patients receiving
cytotoxic agents, but not EGFR-TKIs, as first-line chemotherapy.
Additionally, patients with EGFR mutations were examined to
determine whether the timing of EGFR-TKI administration plays a
role in patient outcome.
Patients and methods
Patients
Between July 2003 and December 2009, 538 advanced
(stage IIIB/IV) NSCLC patients were admitted to our department, and
327 patients received chemotherapy alone. Among them, 116 patients
were examined for EGFR mutational status. Of the 116 patients, 83
received cytotoxic agents as their first-line treatment, and the
remaining patients received EGFR-TKIs. Of the 116 patients, 52 had
activating mutations of EGFR and also received EGFR-TKIs.
This study analyzed the correlation between clinical
factors, including EGFR mutational status, evaluated prior to
initial treatment, and overall survival (OS) in the 83 patients
whose EGFR mutational status was known and who received cytotoxic
agents as their first-line treatment (Cohort 1). Among the 52
patients who had EGFR mutations and received EGFR-TKIs (Cohort 2),
OS was compared between the patients who received EGFR-TKIs as
first-line treatment (first-line TKI group; n=24) and those who
received EGFR-TKIs following chemotherapy (second-line TKI group;
n=28).
Analysis of clinical factors
Analysis of factors such as age (<70/≥70 years),
gender (female/male), Eastern Cooperative Oncology Group
performance status (PS) (0–1/2–4), histology
(adenocarcinoma/non-adenocarcinoma), disease stage (IIIB/IV),
smoking status (+/−), EGFR mutational status (mutation/wild-type),
and administration of a first-line regimen
(platinum-based/single-agent) was carried out.
Mutational analysis of EGFR
Formalin-fixed paraffin-embedded tissue was cut into
6- to 8-mm sections and mounted on pretreated glass slides.
Non-cancer cells and necrotic parts were manually removed from the
slide under a microscope. The slides were deparaffinized, and DNA
was extracted with phenol-chloroform and ethanol precipitation. The
peptide nucleic acid/locked nucleic acid (PNALNA) polymerase chain
reaction (PCR) clamp method, designed to detect 11 different EGFR
mutations, was used for the determination of the EGFR gene mutation
status in this study (13–15).
Tumor evaluation and statistical
analysis
Tumor response was assessed according to the
Response Evaluation Criteria in Solid Tumors (RECIST) (16). OS was calculated from the
commencement of first-line chemotherapy to either the time of death
from any cause or the date when patients were last known to be
alive. The survival curve was estimated using the Kaplan-Meier
method and compared using the log-rank test. Individual clinical
factors were compared using the χ2 test. Multivariate
analysis was conducted according to the Cox proportional hazards
model. P<0.05 was considered to be statistically significant.
Statistical analyses were performed using SPSS 11.0 statistical
software (SPSS II for Windows, Standard version 11.0; SPSS Inc.,
Chicago, IL, USA).
Results
Cohort 1
Patient characteristics
Table I shows the
patient characteristics of Cohort 1. The median age of the patients
was 65 years (range 36–82). Of the 83 patients, 39 (47%) were
female, 66 (80%) had histologically confirmed adenocarcinoma and 38
(46%) were never smokers. Activated EGFR mutations were confirmed
in 28 (34%) patients. A total of 68 (82%) patients received
platinum-based regimens and 15 received a single-agent as their
first-line chemotherapy. A total of 52 patients (63%) received
EGFR-TKIs in a second-line setting or beyond. The EGFR mutant
patients received EGFR-TKIs (100%), whereas 24 of 55 wild-type
patients received EGFR-TKIs (44%).
 | Table IPatient characteristics (Cohort
1). |
Table I
Patient characteristics (Cohort
1).
| Characteristics | n=83 |
|---|
| Age (years) |
| Median | 65 |
| Range | 36–82 |
| Gender |
| Female | 39 |
| Male | 44 |
| Performance
status |
| 0–1 | 70 |
| 2–4 | 13 |
| Histology |
| Adenocarcinoma | 66 |
| Squamous cell
carcinoma | 3 |
| Large-cell
carcinoma | 14 |
| Disease stage |
| IIIB | 16 |
| IV | 67 |
| Smoking status |
| Current smoker | 20 |
| Former smoker | 25 |
| Never smoker | 38 |
| EGFR |
| Mutation | 28 |
| Wild-type | 55 |
| First-line
chemotherapy |
| Platinum-based | 68 |
| Single-agent | 15 |
| No. of regimens |
| Median | 3 |
| Range | 1–9 |
| EGFR-TKI
treatment |
| + | 52 |
| − | 31 |
Overall survival
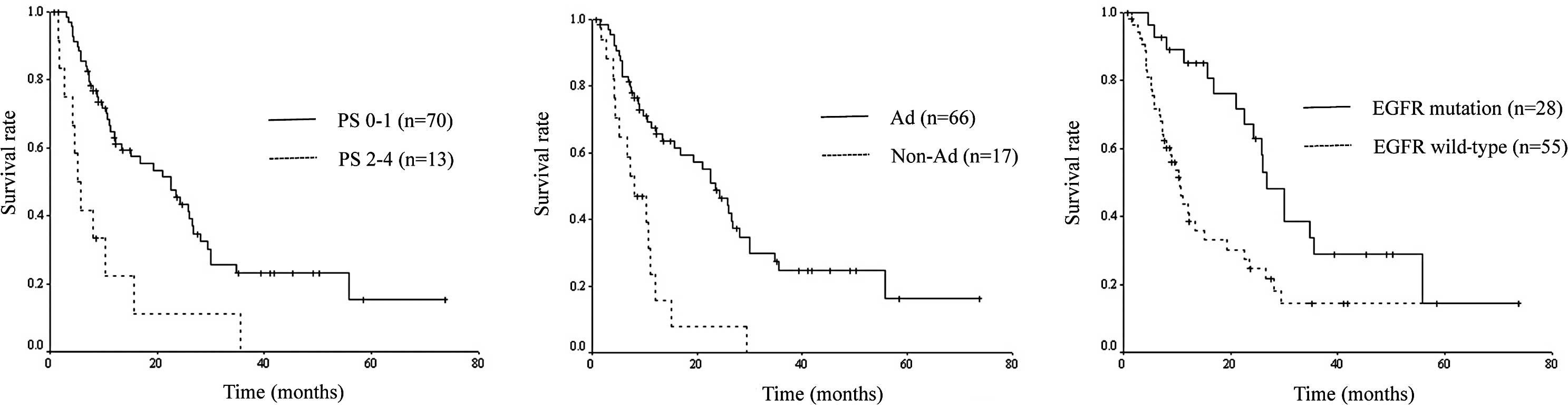
According to the results of the univariate analysis,
OS was significantly associated with gender (p=0.019), PS
(p≤0.001), histology (p<0.001), smoking status (p=0.007) and
EGFR mutational status (p=0.003). Multivariate analysis identified
PS (p<0.001), histology (p=0.039) and EGFR mutational status
(p=0.040) as independent prognostic factors for OS (Table II). Survival curves drawn according
to PS, histology and EGFR mutational status are shown in Fig. 1.
 | Table IIUnivariate and multivariate analysis
for overall survival (Cohort 1). |
Table II
Univariate and multivariate analysis
for overall survival (Cohort 1).
| | | Univariate
analysis | Multivariate
analysis |
|---|
| | |
|
|
|---|
| Characteristics | No. of patients | MST (months) | p-value | Risk ratio | 95% CI | p-value |
|---|
| Age |
| <70 | 53 | 23.4 | 0.082 | | | |
| ≥70 | 30 | 10.7 | | | | |
| Gender |
| Female | 39 | 25.9 | 0.019 | 1.734 | 0.878–3.427 | 0.113 |
| Male | 44 | 15.1 | | | | |
| Performance
status |
| 0–1 | 70 | 22.5 | <0.001 | 3.674 | 1.780–7.581 | <0.001 |
| 2–4 | 13 | 5.2 | | | | |
| Histology |
| Adenocarcinoma | 66 | 23.4 | <0.001 | 2.113 | 1.040–4.294 | 0.039 |
|
Non-adenocarcinoma | 17 | 8.1 | | | | |
| Disease stage |
| IIIB | 16 | 19.3 | 0.722 | | | |
| IV | 67 | 15.1 | | | | |
| Smoking status |
| Current/former | 45 | 13.4 | 0.007 | 0.829 | 0.403–1.706 | 0.610 |
| Never | 38 | 26.5 | | | | |
| EGFR |
| Mutation | 28 | 26.8 | 0.003 | 2.053 | 1.033–4.080 | 0.040 |
| Wild-type | 55 | 10.6 | | | | |
| First-line
regimen |
| Platinum-based | 68 | 20.1 | 0.082 | | | |
| Single-agent | 15 | 10.3 | | | | |
Cohort 2
Patient characteristics
Table III shows
the patient characteristics of Cohort 2. Compared to the
second-line TKI group, the first-line TKI group comprised more
elderly and more PS 2–4 patients, whereas the proportion of females
was higher in the first-line TKI group. All patients had
histologicaly confirmed adenocarcinoma.
 | Table IIIPatient characteristics (Cohort
2). |
Table III
Patient characteristics (Cohort
2).
| Characteristics | First-line TKI group
(n=24) | Second-line TKI group
(n=28) | p-value |
|---|
| Age (years) |
| Median | 74 | 61 | 0.001 |
| Range | 34–86 | 39–74 | |
| Gender |
| Female | 20 | 15 | 0.023 |
| Male | 4 | 13 | |
| Performance
status |
| 0–1 | 16 | 24 | 0.104 |
| 2–4 | 8 | 4 | |
| Histology |
|
Adenocarcinoma | 24 | 28 | - |
|
Non-adenocarcinoma | 0 | 0 | |
| Disease stage |
| IIIB | 4 | 5 | 0.910 |
| IV | 20 | 23 | |
Overall survival
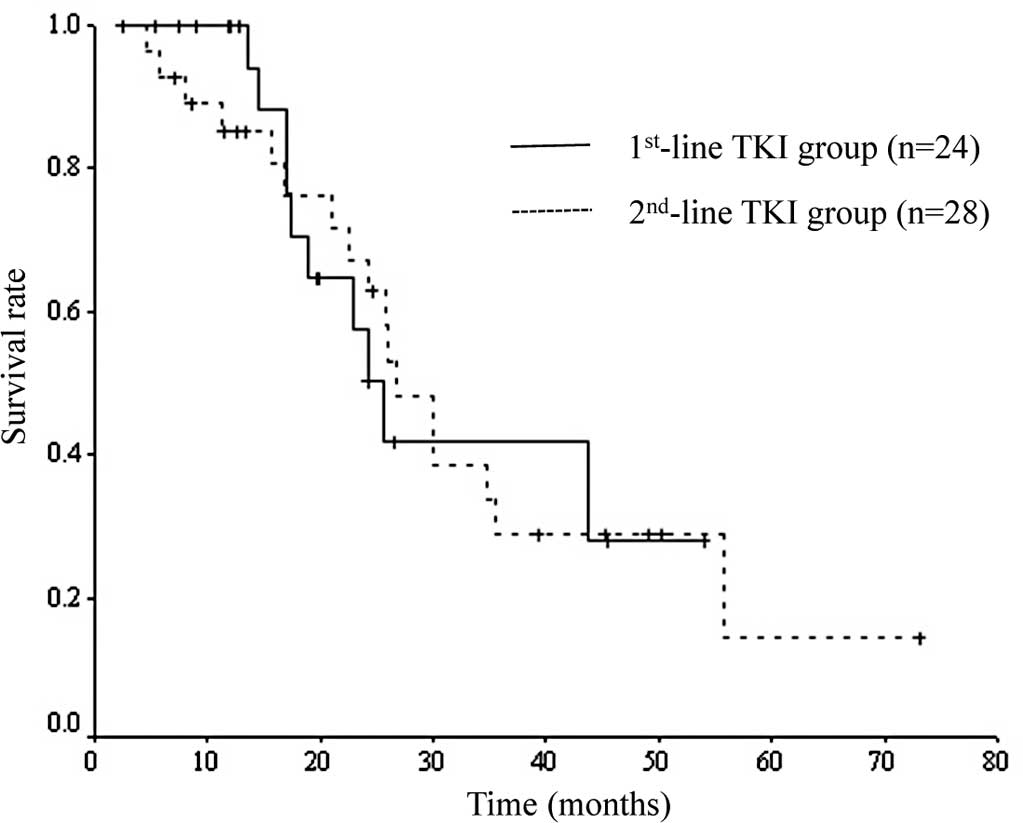
OS of the first-line TKI group was 25.6 months and
that of the second-line TKI group was 26.8 months. No significant
difference was noted between the two groups (p=0.914). Survival
curves of the two groups are shown in Fig. 2.
Discussion
Recently, effective second-line chemotherapies have
been developed using agents, such as docetaxel, pemetrexed,
gefitinb and erlotinib, for the treatment of NSCLC (12). Therefore, OS is not necessarily the
most favorable primary endpoint in randomized trials since the
exact difference of effectiveness between the investigated regimens
does not often translate into OS due to effective second-line
chemotherapy or beyond. Consequently, progression-free survival has
been selected as a primary endpoint in a number of recent
randomized trials. However, progression-free survival is less
reliable than OS due to its arbitrariness, and it remains debatable
which is more adequate as a primary endpoint (17–19).
Should a definite biomarker be found that predicts the response of
second-line chemotherapy and patients are stratified based on such
a biomarker, then OS is likely to be the most favorable
endpoint.
The development of EGFR-TKIs and the finding of EGFR
mutations is a significant event in chemotherapy for NSCLC, since
individualized therapy is now feasible (20). Currently, the EGFR mutation is the
most powerful and widespread biomarker in NSCLC. A number of
biomarkers, such as excision repair cross-complementation group 1
(ERCC1)21 for cisplatin, ribonucleotide reductase subunit M1
(RRM1)22 for gemcitabine and thymidylate synthase (TS)23 for
pemetrexed, have been identified. However, no other biomarker apart
from the EGFR mutation has been of clinical use.
In the present study, the correlation between
clinical factors, including the EGFR mutational status evaluated
prior to the initial treatment, and OS was analyzed. We found that
the EGFR mutational status was an independent prognostic factor for
OS as well as PS and histology in NSCLC patients who received
cytotoxic agents, but not EGFR-TKIs, as their first-line treatment.
In addition, the efficacy of EGFR-TKIs was similar regardless of
the timing of the administration when the patients had EGFR
mutations, as previously reported (24). Patients who are known to have EGFR
mutations are generally treated with EGFR-TKIs; in the present
study, any patients who had EGFR mutations were treated with
EGFR-TKIs.
The prevalence of EGFR mutations is much higher in
Asia than in Western populations (8). Therefore, the importance of EGFR
mutations as a stratification factor is prominent in Asian-oriented
trials, although the same may not be the case of Western-oriented
trials. Our results should be confirmed in non-Asian regions as
well. More globalized clinical trials are currently underway and
the role of EGFR mutations as a stratification factor appears to be
of significance in such global trials.
In conclusion, the EGFR mutational status was an
independent prognostic factor for survival in NSCLC patients who
received cytotoxic agents, but not EGFR-TKIs, as their first-line
chemotherapy. In future randomized trials, particularly in Asia,
even when EGFR-TKIs are not included in the experimental regimens,
patients may need to be stratified by EGFR mutational status to for
study results to be evaluated appropriately.
References
|
1
|
Spiro SG and Porter JC: Lung cancer –
where are we today? Current advances in staging and nonsurgical
treatment. Am J Respir Crit Care Med. 166:1166–1196. 2002.
|
|
2
|
Kelly K, Crowley J, Bunn PA Jr, et al:
Randomized phase III trial of paclitaxel plus carboplatin versus
vinorelbine plus cisplatin in the treatment of patients with
advanced non-small cell lung cancer: a Southwest Oncology Group
trial. J Clin Oncol. 19:3210–3218. 2001.PubMed/NCBI
|
|
3
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced non-small
cell lung cancer. N Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
4
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shepherd FA, Rodrigues-Pereira J, Ciuleanu
T, et al: Erlotinib in previously treated non-small-cell lung
cancer. N Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 372:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takano T, Fukui T, Ohe Y, et al: EGFR
mutations predict survival benefit from gefitinib in patients with
advanced lung adenocarcinoma: a historical comparison of patients
treated before and after gefitinib approval in Japan. J Clin Oncol.
26:5589–5595. 2008. View Article : Google Scholar
|
|
12
|
Azzoli CG, Baker S Jr, Temin S, et al:
American Society of Clinical Oncology Clinical Practice Guideline
update on chemotherapy for stage IV non-small-cell lung cancer. J
Clin Oncol. 27:6251–6266. 2009. View Article : Google Scholar
|
|
13
|
Nagai Y, Miyazawa H, Huqun, et al: Genetic
heterogeneity of the epidermal growth factor receptor in non-small
cell lung cancer cell lines revealed by a rapid and sensitive
detection system, the peptide nucleic acid-locked nucleic acid PCR
clamp. Cancer Res. 65:7276–7282. 2005. View Article : Google Scholar
|
|
14
|
Sutani A, Nagai Y, Udagawa K, et al:
Gefitinib for non-small-cell lung cancer patients with epidermal
growth factor receptor gene mutations screened by peptide nucleic
acid-locked nucleic acid PCR clamp. Br J Cancer. 95:1483–1489.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka T, Nagai Y, Miyazawa H, et al:
Reliability of the peptide nucleic acid-locked nucleic acid
polymerase chain reaction clamp-based test for epidermal growth
factor receptor mutations integrated into the clinical practice for
non-small cell lung cancers. Cancer Sci. 98:246–252. 2007.
View Article : Google Scholar
|
|
16
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
17
|
Buyse ME, Squifflet P, Laporte S, et al:
Prediction of survival benefits from progression-free survival in
patients with advanced non-small cell lung cancer: evidence from a
pooled analysis of 2,838 patients randomized in 7 trials. ASCO
Meeting Abstracts. 26(Suppl 15): abs. 8019. 2008.
|
|
18
|
Hotta K, Kiura K, Takigawa N, et al:
Progression-free survival (PFS) and overall survival (OS) in phase
III trials of systemic chemotherapy in advanced non-small cell lung
cancer (NSCLC). ASCO Meeting Abstracts. 28(Suppl 15): abs. 7624.
2010.
|
|
19
|
Malik SM, Ibrahim A, Sridhara R, et al:
Use of progression-free survival (PFS) as an endpoint in advanced
non-small cell lung cancer (NSCLC) trials: FDA perspective. ASCO
Meeting Abstracts. 28(Suppl 15): e180012010.
|
|
20
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olaussen KA, Dunant A, Fouret P, et al:
DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Souglakos J, Boukovinas I, Taron M, et al:
Ribonucleotide reductase subunits M1 and M2 mRNA expression levels
and clinical outcome of lung adenocarcinoma patients treated with
docetaxel/gemcitabine. Br J Cancer. 98:1710–1715. 2008. View Article : Google Scholar
|
|
23
|
Ceppi P, Volante M, Saviozzi S, et al:
Squamous cell carcinoma of the lung compared with other histotypes
shows higher messenger RNA and protein levels for thymidylate
synthase. Cancer. 107:1589–1596. 2006. View Article : Google Scholar
|
|
24
|
Wu JY, Yu CJ, Yang CH, et al: First- or
second-line therapy with gefitinib produces equal survival in
non-small cell lung cancer. Am J Respir Crit Care Med. 178:847–853.
2008. View Article : Google Scholar : PubMed/NCBI
|