Introduction
Gastric cancer (GC) is a common type of cancer in
East Asia and the second leading cause of cancer death worldwide.
Advances in medicine resulted in this entity being detected and
treated much earlier than previously in Japan and Korea. However,
the disease has often achieved an advanced stage prior to
detection; subsequently, curative resection may no longer be an
option. Systemic chemotherapy is an essential treatment modality
for advanced GC, yielding improvements in quality of life and
prolonged survival. A number of phase 3 trials and one
meta-analysis showed that a 5-fluorouracil (FU)-based regimen
improves both survival and symptoms compared with best supportive
care (BSC) alone (1–3). Although results of certain randomized
phase 3 trials showed no survival benefit for FU plus cisplatin
(CDDP) as compared to FU alone (4),
an FU-based regimen in combination with CDDP is the most commonly
used first-line treatment for advanced GC (5–7). In
the phase 3 SPIRITS trial conducted in Japan (8), advanced GC patients treated with
combination chemotherapy consisting of S-1 plus CDDP in a
first-line setting had an overall survival (OS) of 13 months as
compared with 11 months in patients treated with S-1 alone [hazard
ratio (HR) for death 0.77; 95% CI, 0.61–0.98; P=0.04]. Although a
median OS of more than 1 year was achieved with the S-1 plus CDDP
regimen, the median progression-free survival (PFS) of the
first-line regimen was 6 months, and approximately 75% of the
patients received second-line treatment. However, the impact of the
second-line chemotherapy, which may have contributed to the
favorable survival benefit, remains to be determined, and there is
no standard regimen following the failure of first-line
fluoropyrimidine-based treatment.
Irinotecan (CPT-11) and its active metabolite,
SN-38, bind reversibly to the topoisomerase I-DNA complex and
induce cancer cell death by preventing religation of single-strand
DNA breaks. Irinotecan has shown anti-tumoral activity in
gastrointestinal cancers and is commonly used in a second-line
setting for metastatic colorectal cancer, either alone or in
combination with other agents (9,10). The
activity of CPT-11 as a single agent at a dosage of 100
mg/m2 weekly or 150 mg/m2 bi-weekly in
advanced GC was reported in 45 patients who had received previous
chemotherapy, and the overall response rate (RR) was 16.1%
(11). Kanat et al reported
that 350 mg/m2 tri-weekly CPT-11 in 16 patients in whom
FU-based therapy was unsuccessful resulted in an RR of 12.5% and a
median OS of 5 months (12). On the
other hand, a number of small phase 2 trials investigated
combination chemotherapy with CPT-11 and CDDP following the failure
of prior chemotherapy. In a Japanese trial, CPT-11 (70
mg/m2 on days 1 and 15) plus CDDP (80 mg/m2
on day 1) every 4 weeks in 15 patients previously treated with
chemotherapy yielded a response rate of 27% and significant
toxicity (13). A Korean trial
using the same combination regimen showed a median PFS and OS of 2
and 7.5 months, respectively, for the second-line group of 20
patients with metastatic or recurrent GC (14). Ajani et al administered
CPT-11 50 mg/m2 plus CDDP 30 mg/m2 weekly for
4 weeks over a 6-week cycle and achieved an RR of 31% and PFS of 7
weeks (15). However, severe toxic
effects developed in the majority of patients, and a modification
in dose was suggested. The aim of this retrospective study was to
evaluate the efficacy and safety of CPT-11 in a second-line setting
for recurrent or unresectable GC following the failure of
fluoropyrimidine-based regimens.
Patients and methods
Patients
Second-line chemotherapy with a CPT-11-based regimen
was administered in a total of 134 patients at the Cancer Institute
Hospital of the Japanese Foundation for Cancer Research, Japan
between April, 2004 and March, 2009. Of these patients, CPT-11
alone was administered in 92 patients who were CDDP-refractory or
in whom adequate hydration proved difficult; a combination regimen
of CPT-11 plus CDDP was administered in the remaining 42 patients,
who were CDDP-naïve or sensitive. Patients were selected according
to the following criteria: i) histologically confirmed gastric
cancer with metastatic or recurrent disease; ii) an Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of 0 to
2; iii) failure of prior chemotherapy with a fluoropyrimidine-based
regimen, either in an adjuvant setting or for metastatic disease;
iv) no findings of peritoneal metastasis with radiologically
confirmed intestinal stenosis or massive ascites; v) age ≥18 years;
vi) adequate function of bone marrow, liver and kidney; vii) no
synchronous double cancer or other serious disease; and viii)
availability of informed consent prior to the administration of
treatment.
Treatment
In patients receiving CPT-11 alone, the regimen
consisted of infusional 150 mg/m2 CPT-11 on days 1 and
15 every 4 weeks (Arm I). In patients receiving CPT-11 plus CDDP,
the regimen consisted of infusional 70 mg/m2 CPT-11 on
days 1 and 15 and 80 mg/m2 CDDP by intravenous drip
infusion on day 1 with adequate hydration, to be repeated every 4
weeks (Arm IP). After 500 mg/m2 CDDP was administered,
CPT-11 alone was continued every 2 weeks. Patients were
pre-medicated intravenously with 5-HT3 blocker and
dexamethasone. Chemotherapy was administered until disease
progression, occurrence of intolerable toxicity, or withdrawal from
treatment at the patient’s request.
Response and toxicity evaluation
A CT scan was carried out every 2 cycles of therapy
to document the extent of disease and evaluate the response to
treatment. Objective responses in measurable lesions were evaluated
according to the guidelines of the Response Evaluation Criteria in
Solid Tumors Committee (RECIST 1.0). Symptomatic toxicities and
laboratory data were monitored every 2 weeks at the outpatient
department. Toxicities were evaluated according to the Common
Toxicity Criteria for Adverse Events, version 3.0 (CTCAE v.3.0).
Dose reduction and treatment delays were recommended according to
the extent of hematological and non-hematological toxicities.
Statistical analysis
Progression-free survival was calculated from the
first day of CPT-11 treatment until the time of the first
occurrence of progression, death from any cause, or the date of
last follow-up if none of the preceding events had occurred.
Overall survival was calculated from the first day of CPT-11
treatment to the date the patient succumbed to the disease or the
date of the last follow-up visit. Survival curves were obtained
using the Kaplan-Meier method. Univariate analysis of PFS and OS
was performed using the log-rank test. Correlations between
independent factors, treatment, and PFS and OS were determined by
multivariate analysis using the Cox proportional hazards regression
model in each arm. Statistical analyses were performed using SPSS
(SPSS Inc., IL, USA). P-values were two-sided, with P<0.05
indicating statistical significance.
Results
Patient characteristics
The patients were evaluable for survival parameters
and toxicity. Patient characteristics are shown in Table I. Median ages of patients were 61
years (range 19–82) in Arm I and 57.5 years (range 28–73) in Arm
IP, and the majority of the study population was male (72.4%). A
total of 133 patients (99.3%) had an ECOG PS of 0 or 1. Primary
lesions were present in 41 of 92 patients (44.6%) in Arm I and in 8
of 42 patients (19.0%) in Arm IP. The patients had received prior
chemotherapies with regimens containing S-1, S-1 plus CDDP,
capecitabine plus CDDP, or 5-FU plus methotrexate. Prior treatment
with S-1 was administered in an adjuvant setting in 10 of 92
(10.9%) patients in Arm I and in 10 of 42 (23.8%) patients in Arm
IP. Tumor response was evaluated in 109 (81.3%) patients with
measurable target lesions. Lymph nodes (42.5%), peritoneum (39.6%),
and liver (35.8%) were the most common metastatic sites.
Histological types were intestinal in 47 patients (35.1%) and
diffuse in 87 patients (64.9%) according to the Lauren
classification. A total of 72 of 92 (78.3%) patients in Arm I, and
33 of 42 (78.6%) patients in Arm IP, received chemotherapy in a
third-line setting.
 | Table IPatient characteristics (n=134). |
Table I
Patient characteristics (n=134).
| Characteristics | Arm I, n
(%)
(n=92) | Arm IP, n
(%)
(n=42) |
|---|
| Gender |
| Male | 67 (72.8) | 30 (71.4) |
| Female | 25 (27.2) | 12 (28.6) |
| Median age,
(range) | 61 (19–82) | 57.5 (28–73) |
| ECOG PS |
| 0 | 73 (79.3) | 34 (81.0) |
| 1 | 18 (19.6) | 8 (19.0) |
| 2 | 1 (1.1) | 0 (0.0) |
| Previous gastrectomy
(+) | 51 (55.4) | 34 (81.0) |
| Histological
typea |
| Intestinal | 37 (40.2) | 10 (23.8) |
| Diffuse | 55 (59.8) | 32 (76.2) |
| Target lesions
(+) | 74 (80.4) | 35 (83.3) |
| Peritoneal metastasis
(+) | 36 (39.1) | 17 (40.5) |
| No. of involved
organs |
| 1 | 42 (45.7) | 19 (45.2) |
| ≥2 | 50 (54.3) | 23 (54.8) |
| Prior
chemotherapyb |
| S-1 | 21 (22.8) | 25 (59.5) |
| S-1 plus CDDP | 55 (59.8) | 4 (9.5) |
| CAP plus CDDP | 4 (4.3) | 0 (0) |
| 5-FU plus MTX | 2 (2.2) | 1 (2.4) |
| Others | 0 (0) | 2 (4.8) |
| Adjuvant S-1 | 10 (10.9) | 10 (23.8) |
| Third-line
chemotherapy (+) | 72 (78.3) | 33 (78.6) |
Response and survival
No complete response (CR) was observed in the 109
patients assessable for response; RR was 8.1% in Arm I and 20% in
Arm IP. Disease control (partial response, PR; and stable disease,
SD) rate was 54.1% in Arm I and 54.3% in Arm IP. Median PFS was
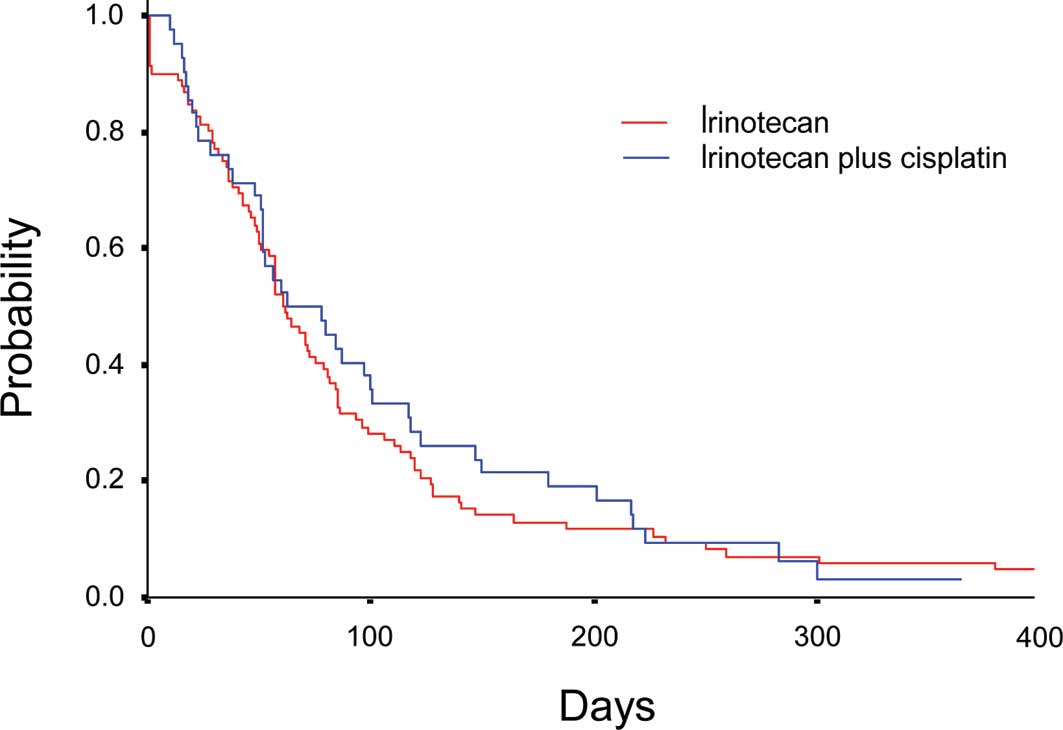
comparable between 2.6 months (95% CI, 2.2–3.0) in Arm I and 2.7
months (95% CI, 1.5–3.9) in Arm IP (P=0.73) (Fig. 1). Median OS was 9.8 (95% CI,
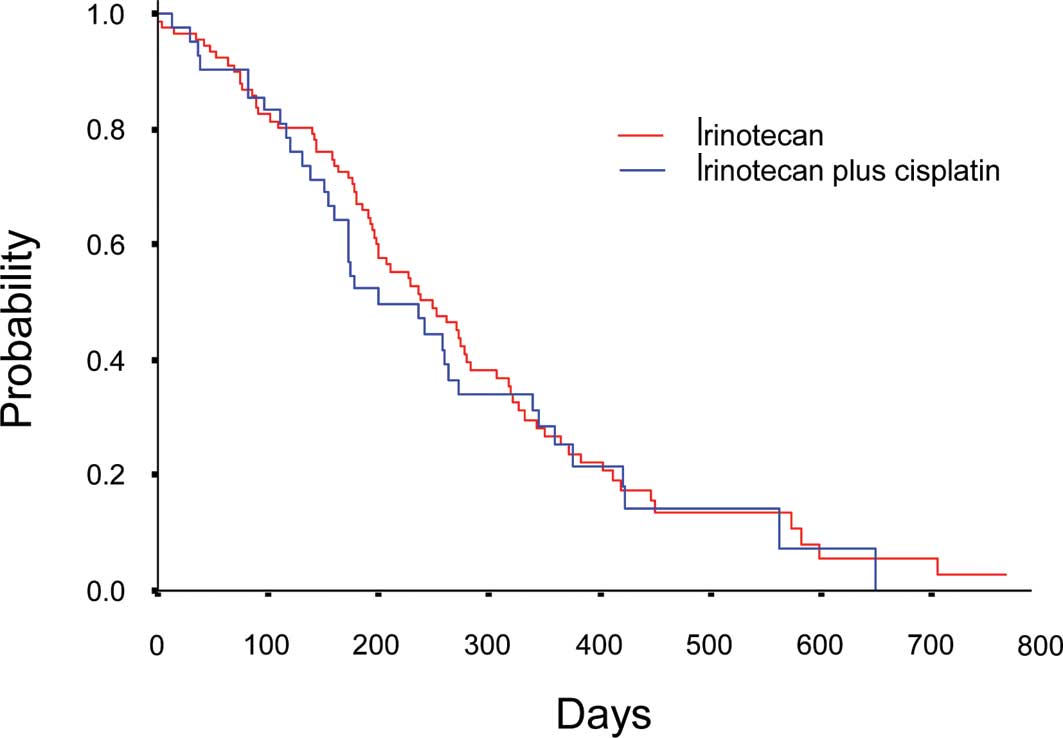
7.8–11.8) months in Arm I and 8 months (95% CI, 5.1–11.0) in Arm IP
(P=0.67) (Fig. 2). No significant
difference was observed between Arm I and Arm IP in either PFS or
OS.
Prognostic factor analysis
Multivariate analysis was performed to assess the
effects of age, adjuvant chemotherapy, degrees of response to
first- and second-line chemotherapy, histological type, prior
gastrectomy, measurable target lesions, peritoneal metastasis and
number of organs with metastasis at baseline. In Arm I, PFS was
significantly longer in patients aged <65 years (P=0.049,
HR=0.45) and with non-peritoneal metastasis (P=0.026, HR=0.46). In
Arm IP, PFS was significantly longer in patients who received
adjuvant chemotherapy (P=0.035, HR=0.27). The results showed that
OS in Arm I was significantly longer in patients in whom disease
control was obtained with second-line treatment (P<0.001,
HR=0.39). Moreover, histologically, intestinal type was an
independent prognostic factor for OS in Arm I (P=0.009, HR=0.45).
Disease control with second-line treatment (P<0.001, HR=0.13)
and one organ showing metastasis (P=0.012, HR=0.14) were
independently correlated with a longer OS in Arm IP.
Adverse events
Adverse effects are shown in Table II. The most frequent grade 3/4
adverse hematological event was neutropenia, which was observed in
7 of 92 (7.6%) patients in Arm I and in 22 of 42 (52.4%) patients
in Arm IP (P<0.001). Leukopenia was observed in 4 of 92 (4.3%)
patients in Arm I and in 7 of 42 (16.7%) patients in Arm IP
(P<0.05). Septic shock and death within 30 days of the last
administration of CPT-11 were observed in one patient (1.1%)
receiving CPT-11 alone. The most common grade 3/4 adverse
non-hematological event was anorexia, which was observed in 5 of 92
(5.4%) patients in Arm I and in 7 of 42 (16.7%) patients in Arm IP
(P<0.05).
 | Table IIFrequency of grade 3/4 adverse
events. |
Table II
Frequency of grade 3/4 adverse
events.
| Adverse events
(AEs) | Arm I, n
(%)
(n=92) | Arm IP, n
(%)
(n=42) |
|---|
| Hematological
AEs |
| Leukopenia | 4 (4.3) | 7 (16.7) |
| Neutropenia | 7 (7.6) | 22 (52.4) |
| Anemia | 3 (3.3) | 2 (4.8) |
|
Thrombocytopenia | 1 (1.1) | 1 (2.4) |
| Febrile
neutropenia | 1 (1.1) | 3 (7.1) |
| Non-hematological
AEs |
| Anorexia | 5 (5.4) | 7 (16.7) |
| Nausea | 2 (2.2) | 3 (7.1) |
| Fatigue | 1 (1.1) | 2 (4.8) |
| Diarrhea | 3 (3.3) | 3 (7.1) |
| Hyperkalemia | 0 (0) | 1 (2.4) |
| Increased
transaminase | 0 (0) | 1 (2.4) |
| Treatment-related
deaths | 1 (1.1) | 0 (0) |
Discussion
Numerous studies have indicated a survival benefit
for treatment of advanced GC in a first-line setting. However,
although the SPIRITS trial demonstrated an OS of more than 1 year,
PFS with S-1 plus CDDP in a first-line setting was only 6 months,
and 74% of the patients received second-line chemotherapy (8). Moreover, in the JCOG9912 trial, OS and
PFS were 11.4 and 4.2 months, respectively, in the S-1 alone arm,
and 74% of the patients received second-line chemotherapy (16). On the other hand, in the Flags
global trial, OS and PFS were 8.6 and 4.8 months, respectively, in
the S-1 plus CDDP arm, and only 31% of the patients received
second-line chemotherapy (17).
This suggests that in certain cases, optimal second-line
chemotherapy contributes to the favorable OS observed with
first-line treatment. However, few well-designed, randomized trials
have been conducted for treatment in a second-line setting, and the
optimal regimen following failure of first-line chemotherapy
remains controversial. Recently, Thuss-Patience et al
reported that CPT-11 alone (250 mg/m2 every 3 weeks, to
be increased to 350 mg/m2, depending on toxicity) as
second-line treatment significantly improved OS compared to BSC
(HR=0.48; 95% CI, 0.25–0.92; P=0.023) in patients previously
treated with only one regimen (18). This suggests that CPT-11 is a key
novel agent in the treatment of advanced GC in a second-line
setting. In the current study, overall RR in Arm IP was 20%, as
compared with 8.1% in Arm I (P=0.65). The median PFS (Arm IP vs. I;
2.7 vs. 2.6 months) and median OS (Arm IP vs. I; 8 vs. 9.8 months)
did not differ between the two treatment groups. Although this was
a retrospective study, the results provide evidence that patients
in whom fluoropyrimidine- based first-line chemotherapy is
unsuccessful may derive a benefit from second-line treatment with
CPT-11.
Multivariate analysis revealed that in both arms,
patients with disease controlled by second-line CPT-11 treatment
had a significantly longer OS. The proportion of patients who
received third-line treatment in our study was 78.4%, and taxane
agents were administered in 94.3% of those patients. Third-line
chemotherapy including taxane agents may also have contributed to
the survival benefit. Patients with recurrent GC following adjuvant
S-1 monotherapy had a longer PFS in Arm IP. Nagashima et al
suggested that two or three favorable phenotypes, p53-negative,
bcl-2-negative and VEGF-positive, are favorable predictors of
therapeutic effects in patients treated with CPT-11 plus CDDP
(19). Expression of specific
chemosensitivity-related genes is currently being investigated in
patients enrolled in the JCOG9912 trial. It has been suggested that
certain CDDP-naïve populations with prior S-1 adjuvant treatment
may benefit from a CPT-11 plus CDDP regimen.
Defining the optimal CPT-11 regimen from the results
of the present study is difficult. However, the selection of the
CDDP combination in a second-line setting appears to be unlikely,
as no benefit was noted in terms of PFS or OS in Arm IP as compared
to Arm I. Moreover, neutropenia, leukopenia and anorexia, the most
common grade 3/4 adverse events, occurred more frequently in Arm IP
than in Arm I.
The results suggest that, in chemotherapy for
advanced GC in a second-line setting, CPT-11 is a key novel agent
and that serial CPT-11 monotherapy is beneficial as compared to
CDDP combination therapy. Further prospective clinical trials may
be useful in developing individualized optimal treatments,
providing evidence concerning the efficacy of molecularly targeted
agents and the utility of biological markers for the treatment of
advanced GC in a second-line setting.
References
|
1
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995.PubMed/NCBI
|
|
3
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: a
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohtsu A, Shimada Y, Shirao K, et al:
Randomized phase III trial of fluorouracil alone versus
fluorouracil plus cisplatin versus uracil and tegafur plus
mitomycin in patients with unresectable, advanced gastric cancer:
The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol.
21:54–59. 2003. View Article : Google Scholar
|
|
5
|
Vanhoefer U, Rougier P, Wilke H, et al:
Final results of a randomized phase III trial of sequential
high-dose methotrexate, fluorouracil, and doxorubicin versus
etoposide, leucovorin, and fluorouracil versus infusional
fluorouracil and cisplatin in advanced gastric cancer: A trial of
the European Organization for Research and Treatment of Cancer
Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol.
18:2648–2657. 2000.
|
|
6
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and fluorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin
Oncol. 24:4991–4997. 2006.PubMed/NCBI
|
|
7
|
Cunningham D, Starling N, Rao S, et al:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rougier P, van Cutsem E, Bajetta E, et al:
Randomised trial of irinotecan versus fluorouracil by continuous
infusion after fluorouracil failure in patients with metastatic
colorectal cancer. Lancet. 352:1407–1412. 1998. View Article : Google Scholar
|
|
10
|
Cunningham D, Pyrhönen S, James RD, et al:
Randomised trial of irinotecan plus supportive care versus
supportive care alone after fluorouracil failure for patients with
metastatic colorectal cancer. Lancet. 352:1413–1418. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Futatsuki K, Wakui A, Nakao I, et al: Late
phase II study of irinotecan hydrochloride (CPT-11) in advanced
gastric cancer. CPT-11 Gastrointestinal Cancer Study Group. Gan To
Kagaku Ryoho. 21:1033–1038. 1994.PubMed/NCBI
|
|
12
|
Kanat O, Evrensel T, Manavoglu O, et al:
Single-agent irinotecan as second-line treatment for advanced
gastric cancer. Tumori. 89:405–407. 2003.PubMed/NCBI
|
|
13
|
Boku N, Ohtsu A, Shimada Y, et al: Phase
II study of a combination of irinotecan and cisplatin against
metastatic gastric cancer. J Clin Oncol. 17:319–323.
1999.PubMed/NCBI
|
|
14
|
Im CK, Rha SY, Jeung HC, et al: A phase II
study of a combined biweekly irinotecan and monthly cisplatin
treatment for metastatic or recurrent gastric cancer. Am J Clin
Oncol. 33:56–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ajani JA, Baker J, Pisters PW, et al:
Irinotecan/cisplatin in advanced, treated gastric or
gastroesophageal junction carcinoma. Oncology. 16:16–18.
2002.PubMed/NCBI
|
|
16
|
Boku N, Yamamoto S, Fukuda H, et al:
Fluorouracil versus combination of irinotecan plus cisplatin versus
S-1 in metastatic gastric cancer: a randomised phase 3 study.
Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|
|
18
|
Thuss-Patience PC, Kretzschmar A, Deist T,
et al: Irinotecan versus best supportive care (BSC) as second-line
therapy in gastric cancer. A randomized phase III study of the
Arbeitsgemeinschaft Internistische Onkologie (AIO). J Clin Oncol.
27(Suppl 15): 45402009.
|
|
19
|
Nagashima F, Boku N, Ohtsu A, et al:
Biological markers as a predictor for response and prognosis of
unresectable gastric cancer patients treated with irinotecan and
cisplatin. Jpn J Clin Oncol. 35:714–719. 2005. View Article : Google Scholar : PubMed/NCBI
|