Introduction
Osteosarcoma is the most common malignant bone tumor
in young adults and children (1).
Treatment of osteosarcoma is limited to chemotherapy followed by
surgery, as these types of tumor remain poor candidates for
radiotherapy due to their high resistance to irradiation (2). However, surgical removal of the tumor
together with the surrounding normal tissue may seriously impair
the affected site. Furthermore, it may be impossible to resect the
tumor with a wide and oncologically safe margin.
X-ray irradiation is a widely used treatment
modality that can control local malignant tumors without causing
severe damage to adjacent normal tissue. Irradiation-induced DNA
damage and double-strand breaks are lethal to cells when the damage
cannot be repaired (3). The outcome
of irradiation therapy is improved when a higher dose of
irradiation can be applied. However, as the dose increases, the
side effects and late complications resulting from exposure of the
surrounding normal tissue to irradiation increase to an
unacceptable level, limiting the usefulness of high doses of
irradiation. Therefore, it is important to find agents that
sensitize malignant tumor cells to RT, thereby minimizing radiation
toxicity to surrounding organs and allowing for lower effective
therapeutic doses.
Numerous mechanisms are involved in the development
of radio-resistance in tumor cells, and one of the possible
mechanisms is activation of the nuclear factor (NF)-κB signaling
pathway (4–6). NF-κB is a heterodimeric transcription
factor that is induced in response to a variety of stress stimuli,
such as exposure to ionizing radiation, and plays an important role
in the regulation of cell survival, apoptosis and the cell cycle
(4,5,7).
Inhibition of the NF-κB signaling pathway is considered to be a
potential therapeutic approach to enhance the effect of irradiation
therapy (8).
Previously, it was shown that NF-κB is
constitutively active in LM8 (9), a
highly metastatic subclone of the Dunn murine osteosarcoma cell
line (10), which may be
responsible for the intrinsic radio-resistance of LM8 cells.
Parthenolide is a sesquiterpene lactone that is
responsible for the activities of the plant feverfew. It is a
traditional folk remedy that has long been used for various
inflammatory conditions in Europe (11). Several studies have proposed that
the effect of parthenolide is due to the inhibition of NF-κB
activity. Parthenolide has been shown to inhibit growth or induce
apoptosis in a number of tumor cell lines (12–16).
In addition, parthenolide has been reported to show antitumor
activity through inhibition of NF-κB DNA binding and other
mechanisms (17–20) in various in vivo models.
This study aimed to investigate the
radio-sensitizing activity of parthenolide to Luc-LM8, a stable
transfectant reporter construct of the NF-κB transcriptional
activity into LM8, in vitro and in animal models by
subcutaneous (s.c.) inoculation of Luc-LM8 cells.
Materials and methods
Cell culture
The cloned murine osteosarcoma cell line LM8, which
shows a high metastatic incidence to the lung after s.c.
inoculation into mice, was cultured in DMEM containing 10% fetal
bovine serum and a 1% penicillin/streptomycin mixture in an air
incubator with 5% CO2 at 37°C. We established Luc-LM8, a
stable transfectant with pNF-κB-Luc (Stratagene, La Jolla, CA, USA)
5 times tandem repeats of the consensus sequence of NF-κB binding
site fixed with the luciferase gene into LM8, for evaluation of
NF-κB transcriptional activity by luciferase reporter assay in
vitro and in vivo. LM8 cells were transfected by
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) with
pNF-κB-Luc and pc-DNA3.1, and these clones were placed for 3 weeks
in culture medium containing 0.5 mg/ml G418 (Gibco-BRL,
Gaithersburg, MD, USA). G418-resistant clones were cultured in
medium with 10 ng/ml TNF-α (R&D, Minneapolis, MN, USA) for 3 h
and selected by quantifying luciferase activities using the
Single-Luciferase assay (Promega, Madison, WI, USA) to identify a
stable transfectant.
Animals
C3H male mice (age, 5 weeks) were purchased from
Japan Oriental Yeast Co., Ltd. (Tokyo, Japan) for in vivo
tumor growth assay. The mice were housed under specific
pathogen-free conditions with a 12-h light and dark cycle. The
housing care rules and experimental protocols were approved by the
Animal Care and Use Committee of Osaka University.
Tumorigenicity and metastatic
potential
Luc-LM8 was investigated to determine whether it
could form a tumor in vivo. Its metastatic potential to the
lung as compared to LM8 was also investigated. Luc-LM8 cells
(1×106) were suspended in 100 μl PBS and inoculated s.c.
into the right thigh of the mice. Mice were examined for s.c. tumor
formation twice a week and sacrificed at 4 weeks after cell
inoculation for histological examination of lung metastasis.
In vitro NF-κB transcriptional activity
assay
Luc-LM8 cells (1×105) were incubated in
6-well plates at various concentrations (0, 0.5, 1.0 and 2.0 μg/ml)
of parthenolide (Sigma-Aldrich, St. Louis, MO, USA) for 24 h, and
luciferase activities were quantified using the Single-Luciferase
assay system and a luminometer. Total protein per sample was
determined using the BioRad protein assay (BioRad Laboratories,
Hercules, CA, USA), and luciferase activity was expressed as
relative light units (RLU)/mg total protein.
Cell proliferation assay
Cell proliferation was evaluated using the WST-1
assay (Takara Bio, Otsu, Japan). Luc-LM8 cells (1×103,
96-well plates) were incubated in 100 μl culturing medium with
parthenolide (0 and 1.0 μg/ml) for 24 h, and then irradiated with
0, 2, 4 and 6 Gy, 180 kVp X-rays. At 72 h after irradiation,
parthenolide-containing medium was replaced with 110 μl of that
containing WST-1 solution (10 μl of WST-1 solution and 100 μl of
culture medium), and 3 h later the absorbance was determined at 450
nm with a reference wavelength of 620 nm using a
multi-spectrophotometer (Viento, Dainippon Sumitomo Pharma, Osaka,
Japan). Relative cell viability was represented as the ratio of the
absorbance of each experimental group vs. mean absorbance of the
control (no parthenolide and no irradiation treatment) group, which
was standardized as 100%.
Apoptosis detection assay
Cell apoptosis was measured using the TACS Annexin
V-FITC Apoptosis Detection kit (R&D). Luc-LM8 cells
(1×104, 12-well plate) were incubated with parthenolide
(0 and 1.0 μg/ml) for 24 h and irradiated with 0, 2 and 8 Gy, 180
kVp X-rays. At 48 h after irradiation, cells were collected and
centrifuged at 500 × g for 5 min at room temperature. Cells were
washed by resuspending in 1X phosphate-buffered saline and pelleted
by repeat centrifugation. Cells were then gently resuspended in 100
μl Annexin V incubation reagent and incubated in the dark for 15
min. Following incubation, 400 μl 1X binding buffer was added to
each sample and the degree of apoptosis was assessed by the
FACSCaliber® flow cytometer (Becton-Dickinson
Immunocytometry Systems, San Jose, CA, USA).
Tumor homogenate-based NF-κB
transcriptional activity assay
To investigate whether the NF-κB transcriptional
activity in Luc-LM8 cells was inhibited by parthenolide in
vivo, mice were inoculated s.c. with Luc-LM8 cells
(1×106) and divided into three groups (n=6 in each
group). The control group was injected intraperitoneally (i.p.)
with a vehicle every day starting from day 7 to 14. Parthenolide
was injected i.p. at a dosage of 1 and 2 mg/kg daily in the other
two groups from day 7 to 14. The mice were sacrificed on day 14,
and each primary tumor was collected and frozen in liquid nitrogen
for tissue homogenate-based luciferase assay (21) (Fig.
1A). To extract luciferase protein, the tumor was placed in 300
μl 1X RLB buffer (Promega, Southampton, UK) and homogenized using a
Fast-Prep homogenizer (Thermo Fisher Scientific, Waltham, MA, USA)
set to 60 m/sec for 30 sec followed by 15-min incubation at room
temperature. The supernatant was removed and transferred to a
QIAshredder column (Qiagen, Crawley, UK) and centrifuged (2 min at
16,000 × g). Luciferase activity was measured in the supernatant.
Luciferase activity was expressed as relative light units (RLU)/mg
total protein.
Tumor growth assay
Luc-LM8 cells (1×106) were inoculated
s.c. into the right thigh of 18 mice. To investigate whether
parthenolide enhances the radio-sensitivity of tumors, mice were
divided into four groups: Par (parthenolide alone), RT (irradiation
alone), Par+RT and the control (n=4–5 per group). The control group
was injected i.p. with a vehicle every day starting from day 7,
when tumor establishment was usually identified. Parthenolide was
injected i.p. at a dosage of 2 mg/kg daily from day 7 in the Par
and Par+RT groups. Irradiation with 4 Gy was administered to
primary tumors on day 14 in the RT and Par+RT groups. The mice were
sacrificed on day 28, and primary tumors were collected for tumor
size and histological evaluation by hematoxylin and eosin staining
(Fig. 1B). Tumor size was evaluated
by measuring the three dimensions of the excised tumor.
Statistical analysis
Data are presented as mean ± SD for in vitro
studies and the tumor growth model. Groups were compared by one-way
analysis of variance, and individual groups were compared using the
two-tailed Student’s t-test. All analyses used a P-value with a 95%
confidence interval.
Results
Establishment of the Luc-LM8 cell
line
We established 11 transfectants with pNF-κB-Luc into
LM8. Among them, we selected one cell line that was most similar to
the wild-type LM8 cell line in terms of local tumorigenicity and a
high metastatic potential to the lung after s.c. inoculation. We
named this cell line Luc-LM8.
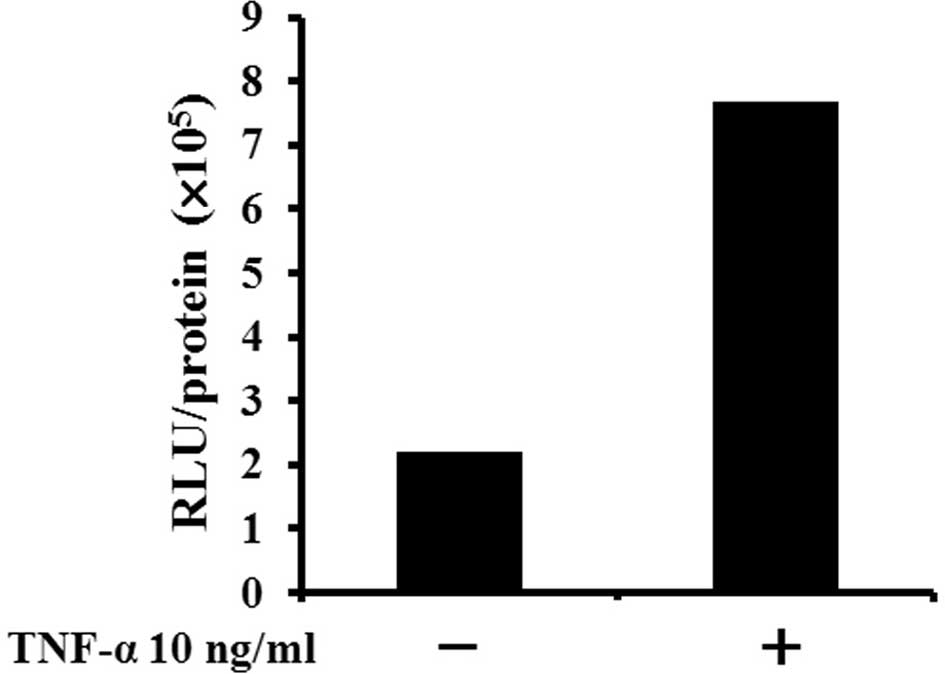
Relative luciferase activity corrected by protein
concentration (RLU/protein) was increased ~3-fold when Luc-LM8
cells were cultured with TNF-α for 3 h compared with cells without
TNF-α (Fig. 2A).
In the tumorigenicity and metastatic potential
assay, Luc-LM8 cells exhibited local tumor-forming ability and
spontaneous metastatic potential to the lung (Fig. 2B). The primary tumors at the right
thigh were identified by day 5 in all mice (n=24), and lung
metastases were found in lungs from all 6 histologically evaluated
mice. Results indicated that Luc-LM8, a clonal transfectant with
pNF-κB-Luc into LM8, maintained the original malignancy potential
of LM8.
To confirm that the transfected pNF-κB-Luc
functioned as a reporter construct of NF-κB transcriptional
activity and that NF-κB activity of Luc-LM8 was regulated by
parthenolide, Luc-LM8 cells were cultured in the presence of
various concentrations of parthenolide and subjected to luciferase
assay. RLU/protein exhibited a high expression when the Luc-LM8
cells were cultured without the NF-κB inhibitor. When treated with
parthenolide, the RLU/protein value of each sample decreased
inversely proportional to the dose of parthenolide (Fig. 2C). These results indicate that the
luciferase activity described the NF-κB transcriptional activity in
the Luc-LM8 cells and that NF-κB activity was inhibited by
parthenolide in a dose-dependent manner in vitro. Therefore,
all additional experiments were conducted using Luc-LM8 cells.
Parthenolide enhanced irradiation-induced
growth inhibition and apoptosis of Luc-LM8 cells in vitro
In the in vitro proliferation assay,
irradiation significantly inhibited the growth of Luc-LM8 cells.
Although parthenolide alone did not alter cell growth, we found
that parthenolide significantly enhanced the growth inhibitory
effect of RT at every dose tested (Fig.
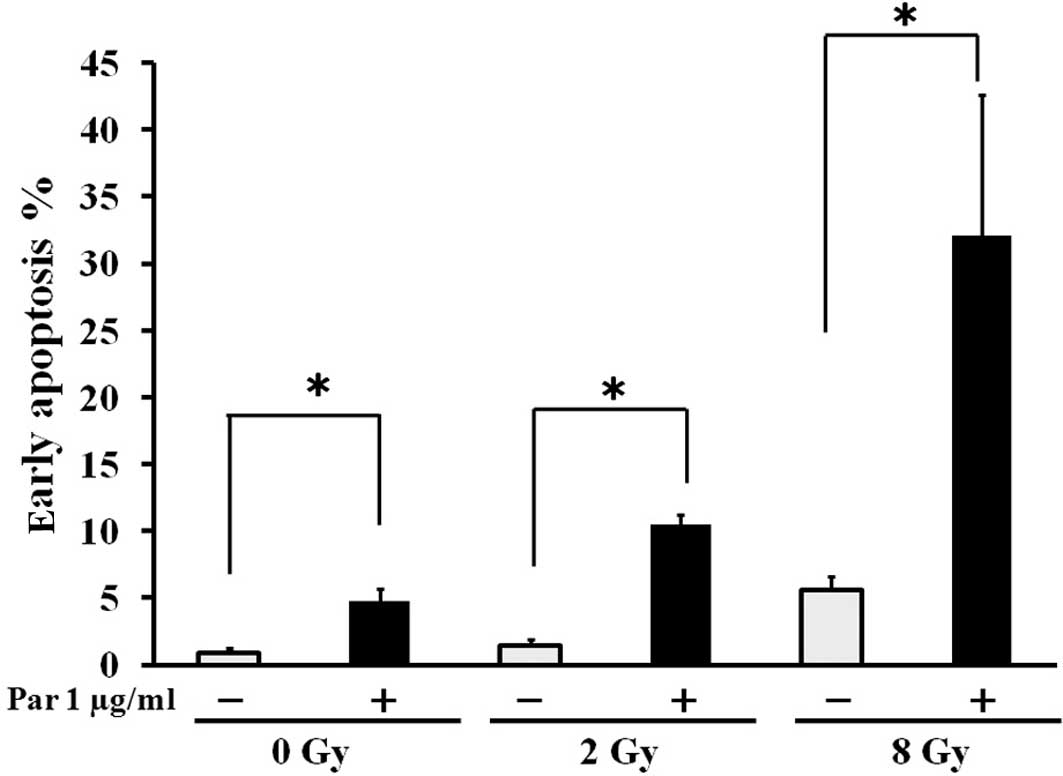
3). Furthermore, in the apoptosis detection assay, irradiation
markedly induced apoptosis of Luc-LM8 cells treated with
parthenolide in vitro in a synergistic manner (Fig. 4). These results suggest that
parthenolide sensitized Luc-LM8 cells to irradiation, most likely
through the inhibition of the NF-κB.
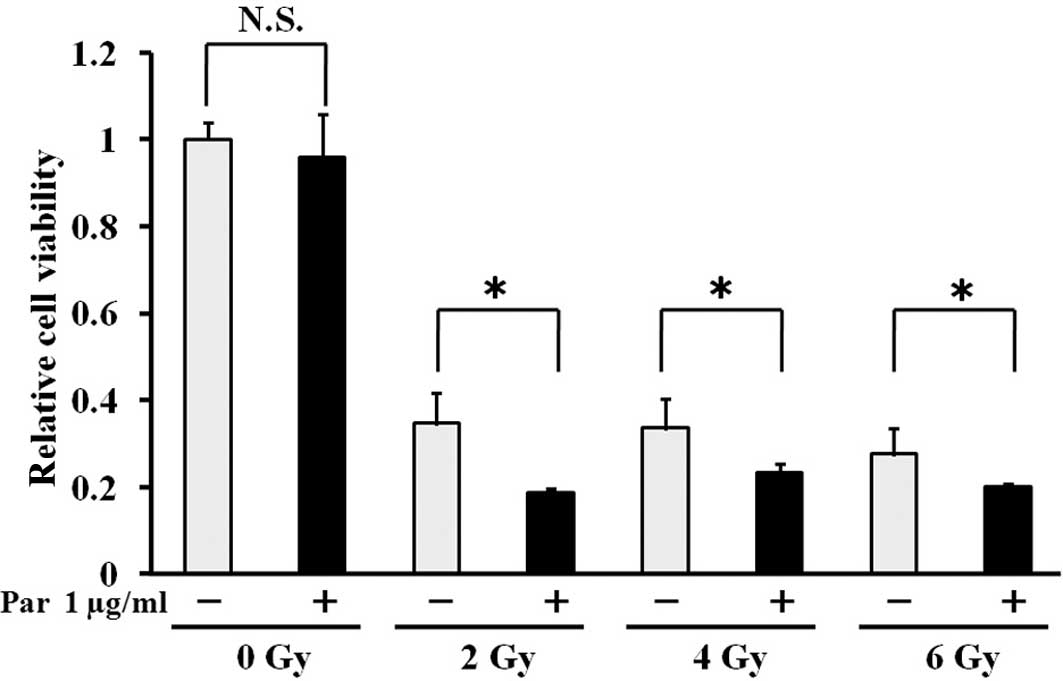 | Figure 3Parthenolide enhances
irradiation-induced growth inhibition of Luc-LM8 cells. Luc-LM8
cells were incubated with parthenolide (0 and 1.0 μg/ml) for 24 h,
and then irradiated with 0, 2, 4 and 6 Gy, 180 kVp X-rays. At 72 h
after irradiation, medium was replaced with that containing WST-1
reagent, and 3 h later, the absorbance was determined at 450 nm.
Relative change in cell viability was represented as the ratio of
the absorbance of viable cells vs. the control (no parthenolide and
no irradiation treatment) group, which was standardized as 100%
(mean ± SD, n=3; *P<0.05). Irradiation significantly
inhibited the growth of Luc-LM8 cells. Although parthenolide (Par)
alone did not alter cell growth, parthenolide significantly
enhanced the growth inhibitory effect of irradiation at every dose
tested. |
Parthenolide suppressed NF-κB
transcriptional activity of the Luc-LM8 tumors and sensitized the
tumors to irradiation
To investigate whether our in vitro findings
of a radio-sensitization effect were also true for osteosarcoma
in vivo, we conducted animal experiments using a mouse model
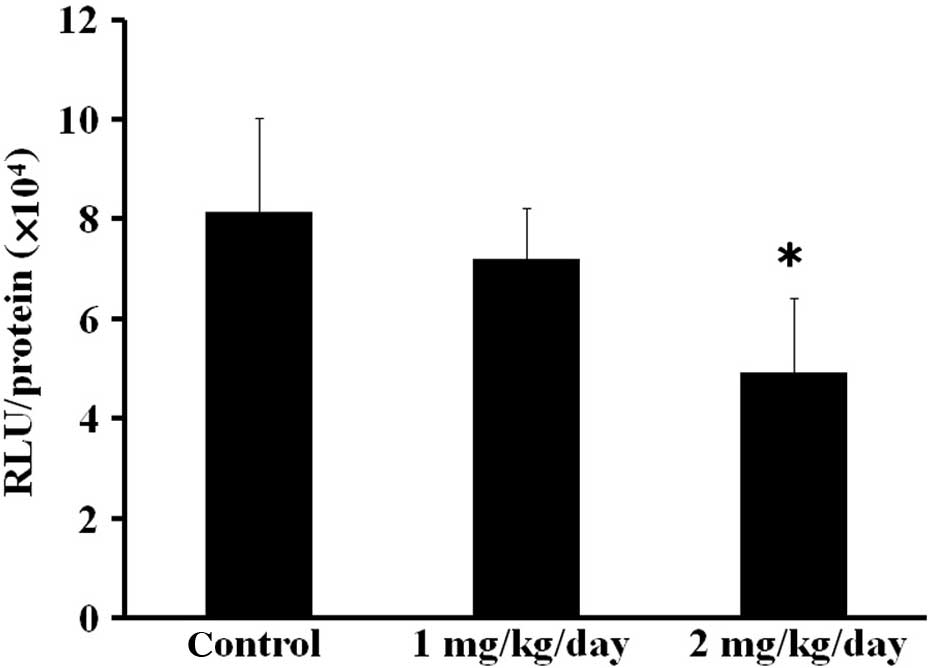
of s.c. tumor cell inoculation. In the tumor homogenate-based
luciferase assay, the NF-κB transcriptional activity in the primary
tumors was inhibited by parthenolide in a dose-dependent manner
(Fig. 5). The NF-κB activity of the
2 mg/kg/day parthenolide group was significantly suppressed
compared with the control group.
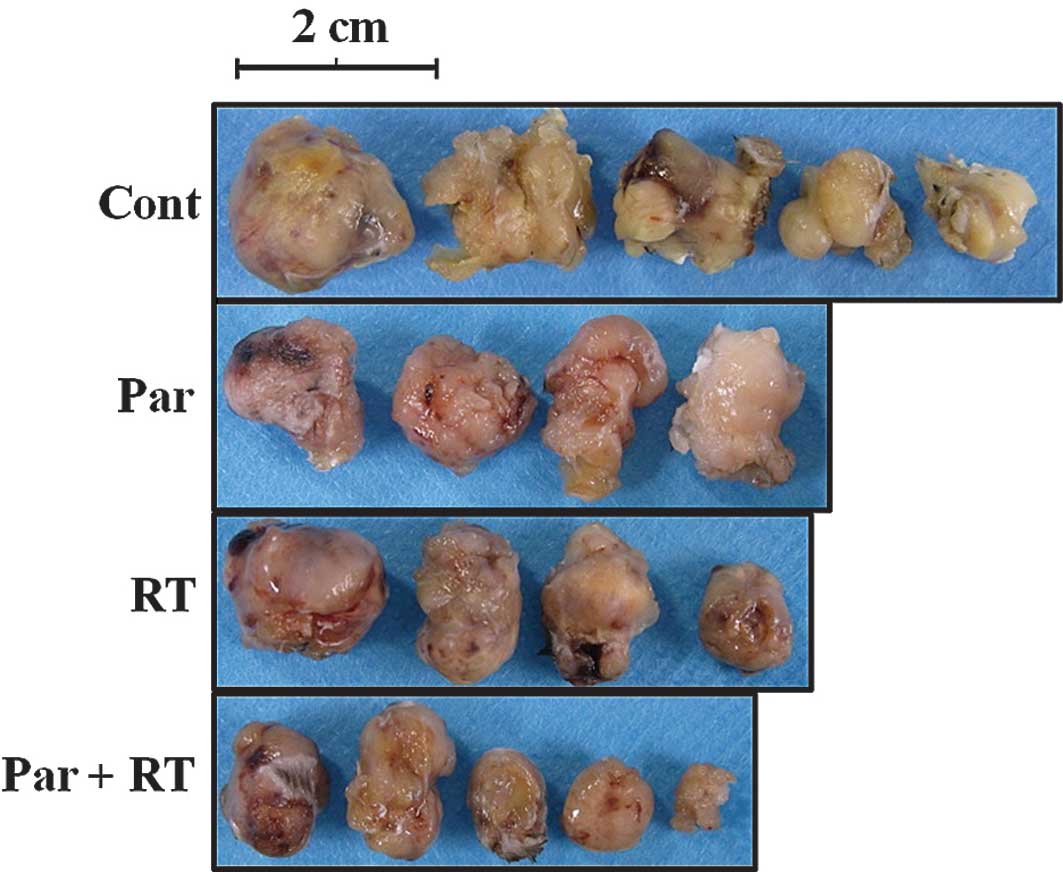
In the tumor growth assay, we found that tumor
growth was significantly suppressed in the Par+RT group compared to
all other groups. No other significant differences were observed
among the groups. These findings indicate the synergistic effect of
Par and RT on tumor growth inhibition (Fig. 6A and B). In addition, histological
analysis revealed the necrotic change in tumor tissue in all of the
experimental groups (Fig. 6C), and
the degenerative area was more extensive in the Par+RT group. These
findings suggest that parthenolide has a radio-sensitizing
potential on Luc-LM8 osteosarcoma in vivo.
Discussion
In the present study, we showed the
radio-sensitizing properties of parthenolide in vitro and
in vivo in Luc-LM8, a transfectant with pNF-κB-Luc into a
highly metastatic murine osteosarcoma cell line, LM8.
Radio-sensitization achieved by inhibition of NF-κB was previously
shown with similar effects in different types of cancer in
vitro and in vivo (4,22–25).
The majority of in vivo antitumor studies on the inhibition
of NF-κB activity used gene therapy, including the overexpression
of the IκB mutant that promotes the ubiquitine-proteasome
degradation of NF-κB. Eliseev et al (26) suggested that in the osteosarcoma
cell line, Saos2, inhibiting NF-κB activity by expressing the IκB
mutant induces radio-sensitization and intrinsic apoptosis after
ionizing radiation. Studies have shown the antitumor
radio-sensitizing activity of parthenolide in vitro.
Mondonca et al (27) found
that parthenolide enhanced X-ray-induced cell killing in
radiation-resistant, NF-κB-activated CGL1 cells due to inhibition
of split-dose repair. Sun et al (28) showed that the radio-sensitization
effect of parthenolide in prostate cancer cells was mediated by
NF-κB inhibition and enhanced by the presence of PTEN. However, the
in vivo radio-sensitizing activity of parthenolide has yet
to be elucidated. In a previous study, we showed that parthenolide
effectively blocked the development of lung metastasis of LM8
(29). Parthenolide is currently
used commonly as a food supplement for the treatment of migraines
and reportedly was found to have no severe side effects when
compared with the placebo group. Therefore, it may be more
effective than gene therapy in vivo (30).
In our in vitro study, we investigated the
radio-sensitization effects of parthenolide on Luc-LM8 cells.
First, we showed that the NF-κB transcriptional activity in Luc-LM8
cells was inhibited by parthenolide in a dose-dependent manner.
Then, in the proliferation assay, parthenolide significantly
enhanced the growth inhibitory effect of radiation therapy at every
dose tested. These results indicate that parthenolide increases the
radio-sensitivity of Luc-LM8 cells. We hypothesized that this
radio-sensitization effect of parthenolide was due to an apoptotic
response exerted via inhibition of the NF-κB pathway. To test this
hypothesis, we assessed early apoptotic reactions in Luc-LM8 cells
treated with parthenolide and irradiation. In the apoptosis
detection assay, parthenolide induced apoptosis of Luc-LM8 cells,
and the cell apoptosis rate synergistically increased in a
dose-dependent manner with irradiation. Our findings suggest that
parthenolide induces an apoptotic response exerted via inhibition
of the NF-κB pathway and increases the radio-sensitivity of Luc-LM8
cells, and accordingly inhibits the proliferation of Luc-LM8
cells.
In the in vivo s.c. tumor model, the tissue
homogenate-based luciferase assay revealed that 7 days of
parthenolide injection reduced the NF-κB activity in Luc-LM8 tumor
tissue on the day of irradiation (day 14) in a dose-dependent
manner. Furthermore, our in vivo tumor growth study showed
that 14 days after irradiation, tumor growth was significantly
suppressed in the parthenolide-treated group compared with the
control group. Histologically, necrotic changes in tumor tissue
were found in all experimental groups and the area of necrosis was
more extensive in the irradiation with parthenolide group. Thus, it
appears that parthenolide has the potential to enhance the
necrotizing effect of irradiation on in vivo tumor
masses.
Parthenolide has been reported to have
microtubule-interfering properties (31) that induce apoptotic cell death by
multiple pathways, including oxidative stress, endoplasmic
reticulum stress, intracellular thiol depletion, caspase
activation, and mitochondrial dysfunction (15,17,18),
inhibit 5-lipoxygenase and cyclooxygenase (32) and sensitize cancer cells to
chemotherapeutic drugs such as paclitaxel and docetaxel (33,34).
Despite widely documented anti-cancer activity and the absence of
major adverse effects, clinical development of parthenolide is
hampered by its poor water solubility, (35) thus limiting its potential as a
promising clinical agent. Previous studies investigated the in
vitro and in vivo activities of the water-soluble
parthenolide analogue dimethylaminoparthenolide (DMAPT) (20,35,36)
and reported that this analogue suppressed tumor growth by
targeting NF-κB and generating reactive oxygen (37–39).
The water-soluble parthenolide analogue may become a more readily
available radio-sensitizing agent, but further investigation is
needed to elucidate its efficacy and spectrum as a
radio-sensitizing agent.
In the present study, parthenolide suppressed
Luc-LM8 cell growth, induced apoptosis in vitro, and
inhibited tumor growth in vivo synergistically with
irradiation treatment, suggesting that parthenolide sensitizes
Luc-LM8 to irradiation. It is conceivable that the mechanism of
radio-sensitization may be the inhibition of NF-κB activity since
NF-κB has been shown to be associated with cancer resistance to RT.
Parthenolide is a potential candidate for use as a potent
radio-sensitizing drug for use in cancer RT.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research (no. 20591754) from the Ministry of Education,
Culture, Sports, Science and Technology, Japan grants. We also
would like to thank Dr Satoaki Nakamura for the invaluable advice
and Mrs. Mari Shinkawa for the excellent technical assistance in
the histological study.
References
|
1
|
Unni KK: Osteosarcoma of bone. J Orthop
Sci. 3:287–294. 1998. View Article : Google Scholar
|
|
2
|
Fuchs B and Pritchard DJ: Etiology of
osteosarcoma. Clin Orthop Relat Res. 40–52. 2002. View Article : Google Scholar
|
|
3
|
Bernier J, Hall EJ and Giaccia A:
Radiation oncology: a century of achievements. Nat Rev Cancer.
4:737–747. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung M and Dritschilo A: NF-kappaB
signaling pathway as a target for human tumor radiosensitization.
Semin Radiat Oncol. 11:346–351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Habraken Y and Piette J: NF-kappaB
activation by double-strand breaks. Biochem Pharmacol.
72:1132–1141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKenna WG and Muschel RJ: Targeting tumor
cells by enhancing radiation sensitivity. Genes Chromosomes Cancer.
38:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asai T, Tomita Y, Nakatsuka S, et al: VCP
(p97) regulates NFkappaB signaling pathway, which is important for
metastasis of osteosarcoma cell line. Jpn J Cancer Res. 93:296–304.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asai T, Ueda T, Itoh K, et al:
Establishment and characterization of a murine osteosarcoma cell
line (LM8) with high metastatic potential to the lung. Int J
Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knight DW: Feverfew: chemistry and
biological activity. Nat Prod Rep. 12:271–276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu CA, Wang MJ, Chi CW, Wu CW and Chen
JY: Rho/Rhotekin-mediated NF-kappaB activation confers resistance
to apoptosis. Oncogene. 23:8731–8742. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shanmugam R, Jayaprakasan V, Gokmen-Polar
Y, et al: Restoring chemotherapy and hormone therapy sensitivity by
parthenolide in a xenograft hormone refractory prostate cancer
model. Prostate. 66:1498–1511. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Won YK, Ong CN and Shen HM:
Anti-cancer potential of sesquiterpene lactones: bioactivity and
molecular mechanisms. Curr Med Chem Anticancer Agents. 5:239–249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koprowska K and Czyz M: Molecular
mechanisms of parthenolide’s action: old drug with a new face.
Postepy Hig Med Dosw (Online). 64:100–114. 2010.(In Polish).
|
|
16
|
Oka D, Nishimura K, Shiba M, et al:
Sesquiterpene lactone parthenolide suppresses tumor growth in a
xenograft model of renal cell carcinoma by inhibiting the
activation of NF-kappaB. Int J Cancer. 120:2576–2581. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen J, You KR, Lee SY, Song CH and Kim DG:
Oxidative stress-mediated apoptosis. The anticancer effect of the
sesquiterpene lactone parthenolide. J Biol Chem. 277:38954–38964.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Ong CN and Shen HM: Critical
roles of intracellular thiols and calcium in parthenolide-induced
apoptosis in human colorectal cancer cells. Cancer Lett.
208:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Liu L, Lee SO, Kim YT, You KR and
Kim DG: Susceptibility of cholangiocarcinoma cells to
parthenolide-induced apoptosis. Cancer Res. 65:6312–6320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guzman ML, Rossi RM, Karnischky L, et al:
The sesquiterpene lactone parthenolide induces apoptosis of human
acute myelogenous leukemia stem and progenitor cells. Blood.
105:4163–4169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Griesenbach U, Meng C, Farley R, et al:
In vivo imaging of gene transfer to the respiratory tract.
Biomaterials. 29:1533–1540. 2008. View Article : Google Scholar
|
|
22
|
Wang CY, Mayo MW and Baldwin AS Jr: TNF-
and cancer therapy-induced apoptosis: potentiation by inhibition of
NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mukogawa T, Koyama F, Tachibana M, et al:
Adenovirus-mediated gene transduction of truncated IkappaBalpha
enhances radiosensitivity in human colon cancer cells. Cancer Sci.
94:745–750. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magne N, Toillon RA, Bottero V, et al:
NF-kappaB modulation and ionizing radiation: mechanisms and future
directions for cancer treatment. Cancer Lett. 231:158–168. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mauro C, Zazzeroni F, Papa S, Bubici C and
Franzoso G: The NF-kappaB transcription factor pathway as a
therapeutic target in cancer: methods for detection of NF-kappaB
activity. Methods Mol Biol. 512:169–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eliseev RA, Zuscik MJ, Schwarz EM, O’Keefe
RJ, Drissi H and Rosier RN: Increased radiation-induced apoptosis
of Saos2 cells via inhibition of NF-kappaB: a role for c-Jun
N-terminal kinase. J Cell Biochem. 96:1262–1273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mendonca MS, Chin-Sinex H, Gomez-Millan J,
et al: Parthenolide sensitizes cells to X-ray-induced cell killing
through inhibition of NF-kappaB and split-dose repair. Radiat Res.
168:689–697. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, St Clair DK, Fang F, et al: The
radiosensitization effect of parthenolide in prostate cancer cells
is mediated by nuclear factor-kappaB inhibition and enhanced by the
presence of PTEN. Mol Cancer Ther. 6:2477–2486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kishida Y, Yoshikawa H and Myoui A:
Parthenolide, a natural inhibitor of nuclear factor-kappaB,
inhibits lung colonization of murine osteosarcoma cells. Clin
Cancer Res. 13:59–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murphy JJ, Heptinstall S and Mitchell JR:
Randomised double-blind placebo-controlled trial of feverfew in
migraine prevention. Lancet. 2:189–192. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miglietta A, Bozzo F, Gabriel L and Bocca
C: Microtubule-interfering activity of parthenolide. Chem Biol
Interact. 149:165–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sumner H, Salan U, Knight DW and Hoult JR:
Inhibition of 5-lipoxygenase and cyclooxygenase in leukocytes by
feverfew. Involvement of sesquiterpene lactones and other
components. Biochem Pharmacol. 43:2313–2320. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patel NM, Nozaki S, Shortle NH, et al:
Paclitaxel sensitivity of breast cancer cells with constitutively
active NF-kappaB is enhanced by IkappaBalpha super-repressor and
parthenolide. Oncogene. 19:4159–4169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sweeney CJ, Mehrotra S, Sadaria MR, et al:
The sesquiterpene lactone parthenolide in combination with
docetaxel reduces metastasis and improves survival in a xenograft
model of breast cancer. Mol Cancer Ther. 4:1004–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neelakantan S, Nasim S, Guzman ML, Jordan
CT and Crooks PA: Aminoparthenolides as novel anti-leukemic agents:
discovery of the NF-kappaB inhibitor, DMAPT (LC-1). Bioorg Med Chem
Lett. 19:4346–4349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guzman ML, Rossi RM, Neelakantan S, et al:
An orally bioavailable parthenolide analog selectively eradicates
acute myelogenous leukemia stem and progenitor cells. Blood.
110:4427–4435. 2007. View Article : Google Scholar
|
|
37
|
Shanmugam R, Kusumanchi P, Cheng L, et al:
A water-soluble parthenolide analogue suppresses in vivo
prostate cancer growth by targeting NF-kappaB and generating
reactive oxygen species. Prostate. 70:1074–1086. 2010.PubMed/NCBI
|
|
38
|
Shanmugam R, Kusumanchi P, Appaiah H, et
al: A water soluble parthenolide analog suppresses in vivo
tumor growth of two tobacco-associated cancers, lung and bladder
cancer, by targeting NF-kappaB and generating reactive oxygen
species. Int J Cancer. July 28–2010.(Epub ahead of print).
|
|
39
|
Yip-Schneider MT, Wu H, Ralstin M, et al:
Suppression of pancreatic tumor growth by combination chemotherapy
with sulindac and LC-1 is associated with cyclin D1 inhibition
in vivo. Mol Cancer Ther. 6:1736–1744. 2007. View Article : Google Scholar : PubMed/NCBI
|