Introduction
Topics such as the pathogenesis, clinical evaluation
and treatment of tumors of the gastroesophageal junction (GEJ) are
a controversial issue. This matter is likely due to the lower
incidence of these tumors as compared to that of cancers of the
esophagus or stomach, and to the fact that in clinical trials such
tumors have been never treated as a distinct disease entity, but
are generally grouped together with either esophageal or gastric
cancers. Therefore, whether cancers of the esophagus, GEJ and
stomach are a single disease, two diseases or more remains to be
clarified (1).
In western countries, there is a trend to an
increasing incidence of esophageal cancers as more of them are
distal and of gastric cancers as more of them are proximal
(2). In accordance with this
epidemiologic shift of the carcinomas of contiguous digestive
sections, the incidence of GEJ tumors was reported to have
increased by 5-fold over the last 20 years of the previous century
(3). A recent worldwide overview
documented a roughly homogeneous and steady incidence of cardia
cancers since 1980, despite the declining incidence of other
non-cardia carcinomas (4). However,
the identification of GEJ does not rely on universally accepted
criteria (5,6). Staging classification varies for
esophageal and gastric cancers and it is uncertain which staging is
more appropriate for GEJ cancers. Appropriate staging is crucial
since the clinical presentation of GEJ tumors is often locally
advanced, showing spreading potentiality to both mediastinal and
abdominal lymph nodes. Clinical investigations are currently
ongoing. This study offers a clinical and prognostic evaluation of
a series of GEJ carcinomas studied in a single institution during
the last 25 years compared to the carcinomas of the esophagus and
stomach diagnosed in the same period.
Patients and methods
Patients
Between January 1984 and December 2008, a
consecutive series of 613 patients with carcinoma of the upper
digestive tract were studied and treated in our Division. Of these,
64 patients presented with carcinoma of the esophagus, 58 of the
GEJ and 491 of the stomach. The large majority of these patients
underwent surgical resection with radical intent in the Surgical
Department of the Fondazione IRCCS Policlinico S. Matteo of Pavia,
Italy. In particular, 39/64 patients were operated with tumors of
the esophagus, 56/58 with GEJ tumors and 462/491 with gastric
tumors. The patients were followed up at the Department of Internal
Medicine of the same Hospital until 31 December 2009. The data
collected for each patient included: presenting signs and symptoms,
tumor location, analytical description of the surgical procedures,
radicality of the resection performed, macroscopic features of the
resected tumor, diameters of the tumoral mass, number of regional
metastatic lymph nodes, microscopic subtype of the tumor (presence
or absence of distinct squamoid differentiation), Lauren's
histological type (intestinal or diffuse), depth of penetration
into the bowel wall, cell differentiation (well, moderately or
poorly differentiated), grade of lymphatic invasion and main
laboratory data at presentation prior to surgery (blood cell count,
serum protein electrophoresis, liver and kidney function tests, and
tumoral markers).
Macroscopic evaluation and description of the entire
resected material and histological examination of the sampled
specimens were systematically performed centrally. Vascular and
lymphatic invasion was evaluated on paraffin sections stained with
H&E, while cases in which identification of endothelial
structures was uncertain underwent immunohistochemical studies for
CD34 and CD31 markers. The anti-CD31 antibody, which identifies the
antigen ER-MP12, identical to the vascular endothelial adhesion
molecule PECAM-1, and the anti-CD34 antibody, which stains normal
and endothelial cells, make the identification of vascular and
lymphatic vessels more straightforward. Systematic re-examination
of the specimens was carried out to verify the correct diagnostic
allocation into according to the categories of the WHO
classification (7).
For the purposes of this study, patients alive in
2009 who had not undergone a medical examination within the
preceding 6 months were recalled for a new clinical and
instrumental control. The vital status of those patients who did
not respond to this recall was ascertained by telephone
(information from relatives and/or from physician) or investigated
in the General Registry Offices of their last known municipality of
residence. The patients were staged according to the last tumor,
node and metastasis (TNM) classification (8), which stages tumors arising at the GEJ
using the same criteria as those applied to esophageal neoplasias.
These criteria include grading of cytologic differentiation, fix
sharp limits for the discrimination of tumor location, adopt
modified T, N and M categories and, use separate classifications
for squamous cell carcinoma and adenocarcinoma.
The anatomical discrimination of Siewert (9), substantially accepted in the recent
version of the TNM classification, was used for the identification
of GEJ tumors. In particular, type I tumors arise in the distal
esophagus 1–5 cm from GEJ, type II is the true junctional tumors
arising within 1 cm proximal and 2 cm distal to GEJ, and type III
tumors are located from 2 to 5 cm distal to GEJ. Patient
characteristics are shown in Table
I.
 | Table IMain clinical characteristics of the
study population related to the tumor location (percentages in
brackets). |
Table I
Main clinical characteristics of the
study population related to the tumor location (percentages in
brackets).
| Esophagus | GEJ | Stomach |
|---|
| Total no. | 64 | 58 | 491 |
| M/F |
| No. | 53/11 | 37/21 | 284/207 |
| Ratio | 4.82 | 1.76 | 1.37 |
| Age (years) |
| Median | 64.3 | 65.3 | 64.1 |
| Range | 43–93 | 43–84 | 19–91 |
| Histology |
| Squamous or
adenosquamous | 54 (84) | 3 (5) | 1 (0.002) |
| Stage |
| 0 | 0 | 0 | 15 (3) |
| I A | 0 | 0 | 58 (12) |
| I B | 0 | 1 (2) | 57 (12) |
| II A | 5 (8) | 1 (2) | 61 (12) |
| II B | 2 (3) | 2 (3) | 53 (11) |
| III A | 3 (5) | 5 (9) | 40 (8) |
| III B | 15 (23) | 12 (21) | 52 (11) |
| III C | 10 (16) | 12 (21) | 36 (7) |
| IV | 29 (45) | 25 (43) | 119 (24) |
Supportive therapy alone, without any antitumoral
treatment, was administered to 10 patients with gastric tumors and
to 2 patients with GEJ tumors, due to wide disease dissemination
and/or poor performance status, or heavy comorbidity. Surgical
resection was performed in 61% of the esophageal, 97% of GEJ and in
94% of gastric tumors. Radical resection was performed in 80 of
esophageal, 70 of GEJ and 80% of gastric tumors, respectively.
Surgery was the only antitumoral treatment administered in 5 cases
of esophageal, in 8 of cardial and in 132 of gastric tumor.
Adjuvant chemotherapy was administered to 41
patients with esophageal, 48 with GEJ and 202 with gastric
tumor.
Radiotherapy was administered to 26 cases of
esophageal tumor (in 7 cases it was the only antitumoral treatment
adopted), while it was delivered in combination with chemotherapy
in 2 cases of tumor of the GEJ and in 16 of the stomach. Table II shows the treatments
administered, together with the chemotherapy regimens most
frequently utilized.
 | Table IITreatments adopted and chemotherapy
regimens delivered to patients according to the tumor site
(percentages in brackets). |
Table II
Treatments adopted and chemotherapy
regimens delivered to patients according to the tumor site
(percentages in brackets).
| Esophagus | GEJ | Stomach |
|---|
| Operated | 39 (61) | 56 (97) | 462 (94) |
| Radical
resection | 31 (80) | 40 (70) | 368 (80) |
| Radiotherapy | 26 (40) | 2 (4) | 16 (3) |
| Chemotherapy | 41 (64) | 48 (83) | 202 (41) |
| Type of
chemotherapy |
| CDDP, LF, FU | 29 (71) | - | - |
| CDDP, EPI, LF,
FU | 4 (10) | 28 (48) | 114 (56) |
| LF, FU | 5 (12) | 17 (30) | 74 (37) |
| CDDP, CPT-11 | 3 (7) | 3 (6) | 9 (4) |
| OXA, LF, FU | - | - | 5 (2) |
The median follow-up was 27 months on the whole
population (range 1–312), and 145 months for patients alive.
Statistical analysis
The time parameters taken into account were observed
survival and relative survival. The latter was calculated as the
ratio of the survival rate observed in the patients to the expected
survival rate drawn from the general reference population for
subjects similar to patients with respect to age, gender, calendar
year of initial observation and duration of observation (10). The age-, gender- and calendar
year-specific death rates available from the national Italian
mortality tables (ISTAT, Istituto Nazionale di Statistica) were
used to calculate the expected deaths and, thus, the expected
survival. Age changes according to individual birthdays in every
year of the follow-up were taken into account. Thus, a large
control group was ensured from the general population with
corresponding personal characteristics and with a well-defined
probability of succumbing to the disease. Consequently, the
relative survival, obtained by adjusting observed survival for
normal life expectancy, is considered to be a satisfactory estimate
of the possibility of surviving the effects of cancer. A detailed
example of the calculations required for each patient was provided
elsewhere, in a study of a population with colorectal cancer
(11). The observed deaths recorded
in the patient population at the end of the follow-up period and
the difference between the observed deaths and the cumulative
expected probability of death during the corresponding period
(i.e., excess mortality, which has to be taken into account for
relative survival) are the data used in both survival calculations
and multivariate analyses. The ratio of observed to expected deaths
was used to determine the standardized mortality ratio (SMR).
The Kaplan-Meier method (12) was used to evaluate survival, and
differences were analyzed by the log-rank test (13). The clinical and pathological
characteristics that showed statistically significant prognostic
values in univariate analyses were selected for multivariate
analyses. The latter were performed by multiple regressions applied
to a Cox proportional hazards model (14). A stepwise selection of factors was
applied to the multiple regressions.
Results
The series of 613 patients with carcinoma of the
upper digestive tract included 64 carcinomas of the esophagus, 58
of the GEJ, and 491 of the stomach. The data shown in Table I suggest that the age at onset of
the three tumors is not different, while the gender ratio and
prevalence of carcinoma with squamoid differentiation decrease as
most distal is the location of the tumor. Notably, the anatomic and
prognostic classification at diagnosis showed a prevalence of
advanced stages (i.e., stages III and IV) among the esophageal
(89%) and GEJ tumors (94%), whereas advanced presentation showed
50% of the gastric tumors.
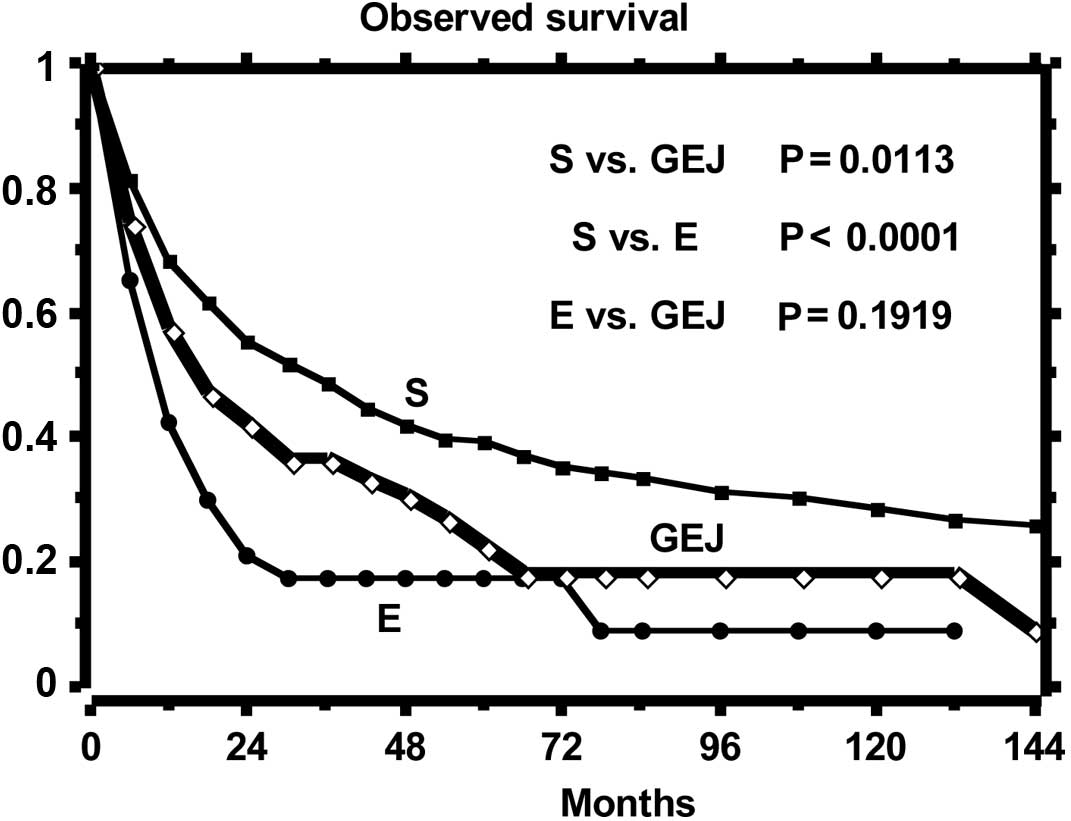
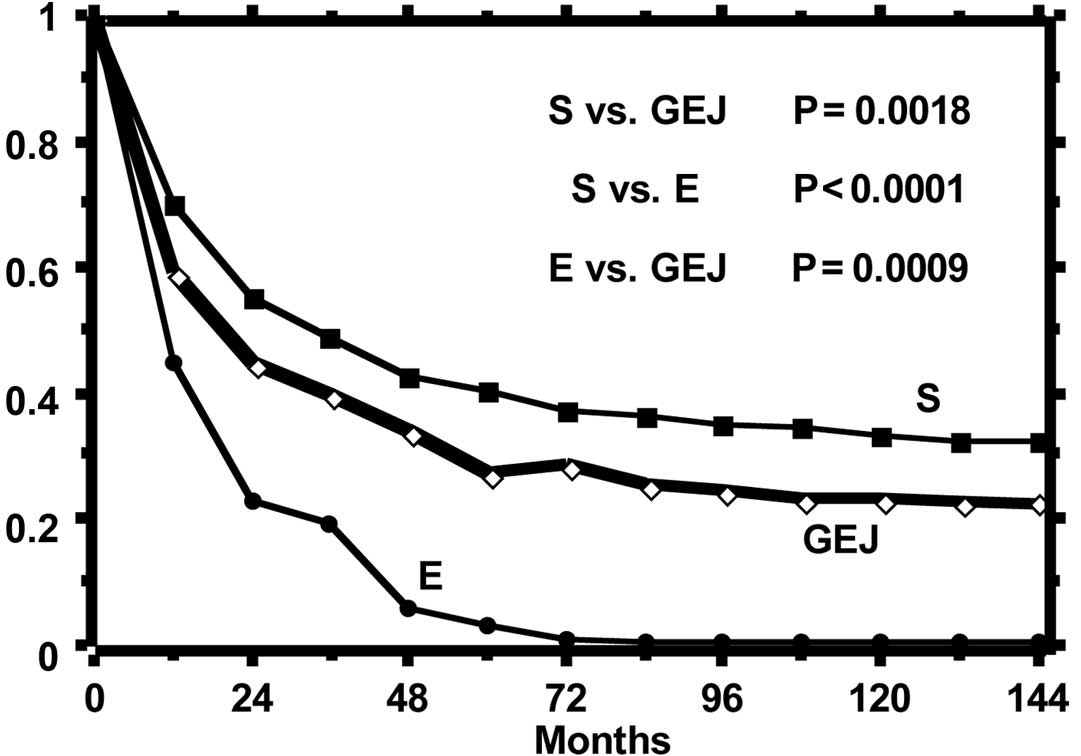
The curves of the observed and relative survival of
the three groups of patients are shown in Figs. 1 and 2, respectively. Observed survival shows a
statistically significant difference between gastric tumor and both
esophageal and GEJ tumors, while the relative survival curves show
a reduction of life expectancy statistically different among all
groups. The highest excess mortality is noted in the esophageal
tumor, while that of GEJ tumor is intermediate as compared to that
of the esophagus and stomach. The SMR, distinct per site of tumor
origin in the digestive tract, is reported in Table III, which shows that the mortality
of gastric tumor is approximately 5-fold that of the normal
reference population, that of the tumor of the GEJ is approximately
3-fold that of the stomach and, in turn, that of the esophageal
tumor is twice that of the GEJ tumor. Minor differences in the SMR
are observed within the three anatomical levels of origin of the
esophageal tumors and among the three Siewert classes of the GEJ
tumors.
 | Table IIIStandardized mortality ratio (SMR) of
the tumors of the esophagus, GEJ and stomach with further
distinction related to the three levels of origin for the
esophageal tumors and to the three Siewert's classes of the GEJ
tumors. |
Table III
Standardized mortality ratio (SMR) of
the tumors of the esophagus, GEJ and stomach with further
distinction related to the three levels of origin for the
esophageal tumors and to the three Siewert's classes of the GEJ
tumors.
| Esophagus | GEJ | Stomach |
|---|
|
|
|
|
|---|
| Upper | Middle | Lower | Siewert's class | |
|---|
| 1/3 | 1/3 | 1/3 | I | II | III | |
|---|
| No. | 17.0 | 27.0 | 20.0 | 10.0 | 36.0 | 12.0 | 491 |
| SMR | 42.5 | 39.8 | 37.4 | 18.1 | 12.6 | 17.1 | 5.3 |
| No. | | 64.0 | | | 58.0 | | 491 |
| SMR | | 39.2 | | | 14.9 | | 5.3 |
A multivariate analysis of the relative survival was
performed based on the clinical covariates of age, gender, stage
(the five categories of the seventh AJCC classification), histology
(presence vs. absence of squamoid differentiation), radicality of
the resection (yes vs. no), chemotherapy (yes vs. no) and site of
tumor origin (esophagus vs. GEJ vs. stomach). The final results of
the stepwise selection of the best covariates are reported in
Table IV. Tumor location,
histology and administration of chemotherapy do not significantly
affect the reduction of life expectancy, whereas stage, radical
resection, age and gender play a role in the reduction of life
expectancy. Stage and gender exhibit a substantially unbalanced
distribution among the three types of tumors, whereas age at
diagnosis, which is relatively similar in the three tumors, has a
limited but inversely proportional impact on relative survival, as
noted by the absolutely low value and the negative sign of its
coefficient.
 | Table IVMultivariate analysis according to the
proportional hazard model applied to the relative survival of the
613 patients studied. |
Table IV
Multivariate analysis according to the
proportional hazard model applied to the relative survival of the
613 patients studied.
| Coefficient | Standard error | Chi-square | P-value |
|---|
| Stage | 0.713 | 0.085 | 70.444 | <0.0001 |
| Radicality | 0.985 | 0.165 | 35.556 | <0.0001 |
| Age | −0.027 | 0.006 | 22.375 | <0.0001 |
| Gender | 0.409 | 0.139 | 8.711 | 0.0032 |
Discussion
In view of the number of patients analyzed and the
retrospective character of the study, firm conclusions cannot be
drawn. However, the long follow-up period of time, the absence of
selection biases in the series studied (including cases with any
age at diagnosis, as well as the inoperable cases), and the
analysis of relative survival for a more accurate prognostic
evaluation suggest certain clinical observations. In particular,
the choice of analyzing relative survival as the best estimate of
specific survival and expression of the excess mortality due to the
disease, is particularly suitable for patients that have i) a wide
age range at diagnosis, including elderly subjects, and ii) a
relatively long survival, and for these reasons are exposed to a
number of factors, such as co-morbidities, accidents or
complications, which compete with the tumor to reduce the life
expectancy.
Since the relative survival is inferred from the
expected survival from nationwide population life tables,
stratified by age, gender and calendar time, a direct consequence
of its consideration is the strong reduction of the prognostic
importance of age, up to the inversion of its correlation with
survival because of the correct weighing of the mortality due to
co-morbidity at various ages. In this series, the age distribution
at diagnosis did not present differences in the three cancer
groups, but the excess mortality showed a weak but inverse
correlation with age (the higher the age, the lower the excess
mortality due to the tumor).
The main conclusion from this study regarding the
excess mortality is that tumor location, histologic type and
administration of chemotherapy lose any prognostic weight when
analyzed with stage, radical resection, gender and age. As regards
the histology, it should be noted that the well-known different
pathogenesis of squamous cell carcinomas and adenocarcinomas is not
under investigation. However, we show that the two carcinomas share
a common prognosis that is secondary to other clinical factors.
Moreover, the prognosis of the GEJ tumors, intermediate as compared
to that of the esophageal and gastric ones, appears to be due not
to distinct clinical features associated with the specific anatomic
site, but with a combination of clinical factors that are common to
the tumors of the contiguous regions.
Gender is a parameter showing a different
distribution at presentation among the three tumors and may be
involved in the prognostic grading generally observed in the moving
from esophagus to stomach. However, the most significant factors
are stage and radical resection. The distribution of these factors
along the whole patient series elucidates the variation of life
expectancy in a more favorable manner than tumor location, its
histologic discrimination and the administration of
chemotherapy.
This may mean that prognostic differences among
tumors of the upper digestive tract are more related to the
possible reasons for an advanced stage presentation and consequent
difficulties in surgical management, such as conditions for delayed
diagnosis, different opportunity of anatomic spreading and
different possibility of radical resection. This does not
necessarily mean we are dealing with the same type of tumor arising
from esophagus to stomach, since some distinctive characteristics
related to distribution of histological type, gender ratio and
pathogenetic mechanisms are clear. Our data suggest that these
differences are less important than stage and radical resection
when correlated with prognosis.
In this regard, the evaluation of the SMR appears to
indicate that the subdivision of the GEJ tumor into the three
classes identified by Siewert is less justified, since it was not
regarded as significant that a cardiac tumor develop within 5 cm
upward or downward of the GEJ. The significant factor is the origin
from GEJ, a site which shares the mentioned probabilities of
delayed diagnosis and advanced stage at presentation, of wide
pre-clinical spread and consequently more complex surgical needs.
This concept substantially agrees with the conclusions of Jin et
al (15) and of Maeda et
al (16), but further
confirmation is required.
Acknowledgements
This study was supported in part by grants from the
Fondazione IRCCS Policlinico S. Matteo, Pavia, and the
‘Ferrata-Storti Foundation’, Pavia. We are indebted to Dr Rachel
Stenner for the revision of the English of this paper.
References
|
1
|
Rusch VW: Are cancers of the esophagus,
gastroesophageal junction, and cardia one disease, two or several?
Sem Oncol. 31:444–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Apisarnthanarax S and Tepper J: Crossroads
in the combined-modality management of gastroesophageal junction
carcinomas. Gastrointest Cancer Res. 2:235–243. 2008.PubMed/NCBI
|
|
3
|
Devesa SS, Blot WJ and Fraumeni J Jr:
Changing patterns in the incidence of esophageal and gastric
carcinoma in the United States. Cancer. 83:2049–2053. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bertuccio P, Chatneoud L, Levi F, et al:
Recent patterns in gastric cancer: a global overview. Int J Cancer.
125:666–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato T, Kato Y, Matsuura M and Gagner M:
Significance of palisading longitudinal esophagus vessels:
identification of the true esophagogastric junction has
histopathological and oncological considerations. Dig Dis Sci.
55:3095–3101. 2010. View Article : Google Scholar
|
|
6
|
Takubo K, Vieth M, Aida J, Sawabe M,
Kumagai Y, Hoshihara Y and Arai T: Differences in the definition
used for esophageal and gastric disease in different countries.
Digestion. 80:248–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamilton SR and Aaltonen LA: Pathology and
Genetics. Tumors of the Digestive System. World Health Organisation
Classification of Tumours Lyon: IARC press; pp. 1–314. 2000
|
|
8
|
AJCC (American Joint Committee on Cancer).
Cancer Staging Manual. 7th edition. Edge SB, Byrd DR, Compton CC,
et al: Springer; New York: pp. 103–110. pp. 117–121. 2009
|
|
9
|
Siewert JR and Stein HJ: Classification of
adenocarcinoma of the esophageal junction. Br J Surg. 85:1457–1459.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armitage P and Berry G: Statistical
Methods in Medical Research. 2nd Edition. Blackwell Scientific
Publications; Oxford: pp. 403–405. 1987
|
|
11
|
Gobbi PG, Valentino F, Berardi E, et al:
New insights into the role of age and carcinoembryonic antigen in
the prognosis of colorectal cancer. Br J Cancer. 98:328–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaplan EL and Meier P: Non parametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
13
|
Peto R, Pike MC, Armitage P, et al: Design
and analysis of randomized clinical trials requiring prolonged
observation of each patient. II. Analysis and examples. Br J
Cancer. 35:1–39. 1977. View Article : Google Scholar
|
|
14
|
Cox DR: Regression models and life tables.
J R Stat Soc. 34:187–220. 1972.
|
|
15
|
Jin L, Yoshida M, Kilagawa Y, et al:
Subclassification of superficial cardia cancer in relation to the
endoscopic esophagogastric junction. J Gastroenterol Hepatol.
(Suppl 2): 273–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda H, Okabayashi T, Nishimori I, et al:
Clinicopathologic features of adenocarcinoma at the gastric cardia:
is it different from distal cancer of the stomach? J Am Coll Surg.
208:308–310. 2008.PubMed/NCBI
|