Introduction
Studies have indicated that proteases have
fundamental roles in pathological processes in cancer by
degradation of basal membranes and extracellular matrix (1). Matrix metalloproteinase (MMP)
expression has been linked to tumour aggressiveness and tumour
invasion in a wide range of cancers, such as colorectal cancer
(CRC) (2–4). Cathepsins, another class of proteases,
also play a role in invasion and metastasis of CRC (5). Serine protease inhibitors (serpins)
belong to a protein superfamily with inhibitory activity against
proteases. These serpins are involved in various physiological
processes and in the regulation of events associated with
inflammatory reactions and connective tissue turnover (6,7).
SerpinA3, also referred to as α-1-antichymotrypsin,
is a member of the serpin family (6) and is an acute phase protein which
increases in the blood during the inflammatory process (8,9). The
inhibitory effect of serpinA3 appears to be more specific against
neutrophil cathepsin G, mast cell chymases and pancreatic
chymotrypsin (8,9).
SerpinA3 is synthesised primarily by hepatocytes,
bronchial epithelial cells and monocytes but is also expressed in a
variety of organs such as kidney, brain and prostate (8,9).
Overexpression of proteinase inhibitors including serpinA3 was
found to result in poor prognosis and malignancy in gastric cancer
(10).
The serpinA3 gene is located on chromosome 14 in
humans, and a signal peptide polymorphism at codon 17 (A/T) in the
serpinA3 gene has been associated with susceptibility to
Alzheimer’s disease and may affect age-at-onset and disease
duration (11). A single nucleotide
polymorphism (SNP) (rs4934) is located in the signal peptide region
(at codon 15) in exon 2. It has been postulated that this genotype
affects the expression of serpinA3 protein (12–14).
Moreover, studies have proven that this genetic variant is a risk
factor for aneurysmal subarachnoid hemorrhage in a Polish (12), but not in a Chinese (13) or Japanese (14) population.
Data concerning the expression profile of serpinA3
in human CRC are limited. Therefore, the protein expression of
serpinA3 in CRC tissue and plasma from CRC patients and its
association with clinical parameters were determined. Screening for
the serpinA3 gene polymorphism (rs4934) was carried out to evaluate
a possible correlation with clinical outcome of CRC. Since
proteases can be associated with the tumour cells themselves
(1), an in vitro invasion
assay was used to investigate the effect of exogenous serpinA3 on
CRC cell (Caco2 and HT-29) invasiveness. Moreover, levels of
C-reactive protein (CRP) were investigated to assess the effect of
systemic inflammation (15) on the
level of serpinA3.
Materials and methods
Patients and controls
This study comprised blood samples from 311
consecutive CRC patients from southeastern Sweden. The samples were
collected from patients who underwent surgical resections for
primary colorectal adenocarcinomas at the Department of Surgery,
Ryhov County Hospital, Jönköping, Sweden. Clinicopathological
characteristics from the patients were obtained from surgical and
pathological records. The patient group comprised 166 males and 145
females with a mean age of 70 years (range 29–93). The tumours were
localised in the colon (n=162) and rectum (n=149) and were
classified according to Dukes’ classification system: stage A
(n=53), stage B (n=128), stage C (n=107) and stage D (n=23). Blood
donors (n=359), from Ryhov County Hospital, with no known CRC
history were from the same geographical region as the CRC patients
and were selected as controls. This control group consisted of 207
males and 152 females with a mean age of 67 years (range
50–80).
Blood samples were centrifuged to separate plasma
and blood cells and both were stored at −70°C.
Tissue samples and lysates
This study utilised tissue samples which were
available from 104 of the 311 CRC patients. The tumours and matched
normal tissue from 61 males and 43 females with a mean age of 68
years (range 29–83) were used. The tumours were classified
according to Dukes’ classification system: stage A (n=17), stage B
(n=45), stage C (n=33) and stage D (n=9). The tumours were
localised in the colon (n=61) and rectum (n=43). Tumour tissue and
adjacent normal mucosa (~5 cm from the tumour) were excised from
each patient and immediately frozen at −70°C until analysis.
Frozen tumour tissue and normal mucosa were thawed,
homogenised in ice cold lysis buffer containing PBS (9.1 mM dibasic
sodium phosphate, 1.7 mM monobasic sodium phosphate, 150 mM NaCl,
pH 7.4) and 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium
dodecyl sulphate (SDS), 100 μg/ml phenylmethylsulphonyl flouride
(PMSF), 2 μg/ml aprotinin, 1 mM sodium orthovanadate and 1 μg/ml
leupeptin. The lysate was placed on ice for 30 min and then
centrifuged at 13,000 × g for 10 min. Protein content of the
supernatant fluid was determined for each sample using the Bradford
protein assay (Bio-Rad Laboratories, UK).
Plasma samples
A total of 133 of CRC patients and 122 of the
controls were available for plasma collection. The CRC patient
group comprised 75 males and 58 females with a mean age of 68 years
(range 29–89). The patient tumours were classified according to
Dukes’ classification system: stage A (n=21), stage B (n=57), stage
C (n=45) and stage D (n=10). A total of 61 tumours were located in
the rectum and 72 in the colon. Controls consisted of plasma from
62 males and 60 females with a mean age of 63 years (range
56–68).
Assay for serpinA3 and C-reactive protein
concentrations
SerpinA3 was measured in tissue and plasma using a
commercially available enzyme-linked immunosorbent assay (ELISA)
kit (Immunology Consultants Laboratory, Inc., USA) and performed
according to the manufacturer’s instructions. The tissue levels of
serpinA3 from the tumour and paired normal tissue were expressed as
nanograms per milligram (ng/mg) of protein. CRP in plasma was
measured using an immunoturbidimetric method (ADVIA 1800 Chemistry
System, Siemens, USA). The plasma serpinA3 and CRP from CRC
patients and controls were expressed as micrograms per millilitre
(μg/ml).
Immunohistochemistry
Eleven tumour samples were obtained for
immunohistochemical staining. To study the cell type origin of
serpinA3 expression, staining was performed using a standard
protocol on 4-μm sections from formalin-fixed paraffin-embedded
tissue blocks. Antigen retrievel was performed by microwave
treatment in citrate buffer (pH 6.0). Endogenous peroxidase
activity was quenched by treatment with 3% hydrogen peroxide for 5
min. Sections were subsequently incubated with a primary goat
anti-human serpinA3 antibody (R&D Systems, Minneapolis, MN,
USA). After rinsing in Tris-buffered saline, sections were
incubated with secondary biotinylated horse anti-goat antibody
(Vector Laboratories, Burlingame, CA, USA). Avidin-biotin
peroxidase complexes (Dako Cytomation, Denmark) were added followed
by visualization with 3,3′-diaminobenzidine tetrahydrochloride
(Dako Cytomation). Sections were counterstained with Mayer’s
hematoxylin (Histolab Products, Sweden). As negative controls, the
primary antibodies were replaced by an isotype control IgG.
Staining procedures were performed according to the manufacturer’s
instructions.
DNA extraction and genotype
determination
DNA was isolated from blood from the patients and
blood donors using the QiaAmp DNA blood kit (Qiagen, CA, USA). DNA
samples were genotyped using the 5′-exonuclease allelic
discrimination assay (Applied Biosystems, CA, USA). The Taq Man SNP
genotyping assay was used for analysis of the serpinA3 genotype
rs4934 (Applied Biosystems). DNA (10 ng) was amplified in a total
volume of 12 μl containing Taq Man Universal PCR Master mix
(Applied Biosystems), included in the SNP genotyping assay.
Amplification was performed using an initial cycle at 50°C for 2
min followed by 1 cycle at 95°C for 10 min and finally 40 cycles at
95°C for 15 sec, then 60°C for 1 min. A post PCR endpoint reading
was performed on each plate using the 7500 Fast Real-Time PCR
system (Applied Biosystems). The manual calling option in the
allelic discrimination application ABI PRISM 7500 SDS software
version 1.3.1 was then used to assign genotypes.
Cell lines and invasion assay
Two established human colon cancer cell lines Caco-2
and HT-29 were purchased from American Type Culture Collection
(ATCC, Rockville, MD, USA). The cell lines were grown according to
the supplier’s instructions and the growth media were Eagle’s
minimum essential medium (Caco-2) and McCoy’s 5a (HT-29).
The cell invasion assay was carried out with 24-well
(lower chambers) and 12 transwell chambers (inserts) (BD BioCoat
Matrigel Invasion Chamber; BD Biosciences, Bedford, MA, USA)
following the manufacturer’s instructions. Briefly, for each type
of CRC cell line, a cell suspension (5×104 cells/ml) in
serum-free media in the absence or presence of full-length protein
serpinA3 (Immunology Consultants Laboratory) was used. The
concentrations of serpinA3 in the cell suspensions were 0, 10, 100
and 2000 ng/ml. The inserts were placed in the lower chambers
containing fetal bovine serum media serving as a chemoattractant.
Each insert contained an 8-micron pore size PET membrane with a
thin layer of Matrigel matrix serving as a basement membrane and an
invasion barrier. The cells that invaded the basement membrane
following incubation at 37°C in 5% CO2 for 22 and 44 h
were fixed, stained with hematoxylin and eosin and counted in 10
random microscopic (x200) fields. Experiments using Caco-2 were
performed twice and HT-29 four times in triplicates.
Statistical analysis
Differences in the frequencies of the serpinA3 gene
polymorphism between CRC patients and the control group and between
clinical data within the CRC subgroup were analyzed using the
Chi-square test. Hardy-Weinberg equilibrium was tested for the
genotypes. Differences in serpinA3 levels between the tumour and
paired normal tissues were examined using the Wilcoxon’s
signed-rank test. Differences in serpinA3 and CRP levels between
unpaired groups were tested using the Mann-Whitney U test, and
correlation coefficients were determined using the Spearman’s rank
correlation test. Statistical analyses were performed using SPSS
for Windows computer package, 2005 (Rel. 14.0, SPSS Inc., Chicago,
IL, USA). Results were considered significant at P<0.05.
Results
Protein levels of serpinA3 in colorectal
tissue
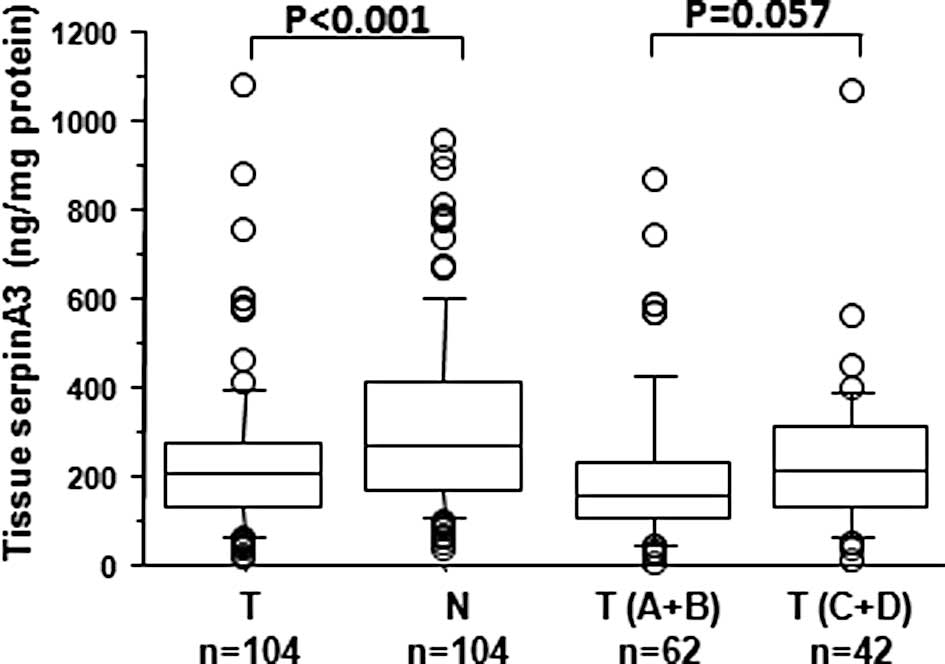
To investigate the potential role of serpinA3 as a
tumour marker the concentration of this protein in CRC and paired
normal tissue from 104 CRC patients was measured using ELISA. The
levels of serpinA3 in the tumour tissue (median 205 ng/mg; range
18–1082) showed a significantly lower (P<0.001) concentration in
comparison with the normal tissue (median 270 ng/mg; range 39–956)
(Fig. 1). Assessment of the
relative expression (tumour vs. normal tissue) showed that in 77%
of the cases the expression was down-regulated. Furthermore, the
protein levels of serpinA3 in the tumour and normal tissue were
found to be significantly positively correlated (r=0.50,
P<0.001) (data not shown).
Following subdivision of the patients according to
localised (Dukes’ A+B) and disseminated (Dukes’ C+D) disease, the
tumour level of serpinA3 in Dukes’ C+D disease (median 227 ng/mg;
range 22–1082) was higher compared to the level in Dukes’ A+B
disease (median 170 ng/mg; range 18–881) (P=0.057) (Fig. 1). The tissue serpinA3 protein level
in localised disease was not significantly different (P=0.669)
compared with the level in disseminated disease (data not shown).
No association was found with other clinical characteristics such
as age, gender and tumour location (data not shown).
Plasma levels of serpinA3 and C-reactive
protein
In order to search for a tumour marker and the state
of inflammation, the plasma levels of serpinA3 and CRP were
measured using ELISA in 133 CRC patients and 122 controls. No
significant difference (P=0.129) was found in the levels of plasma
serpinA3 in the patients (median 148 μg/ml; range 50–690) in
comparison with the controls (median 160 μg/ml; range 100–345).
Plasma serpinA3 concentrations from CRC patients were not related
to age, gender, tumour location or Dukes’ stage. In agreement with
another study (15), a higher
concentration of plasma CRP (median 3 μg/ml; range 0–136) was found
in the CRC patients in comparison with the controls (median 1
μg/ml; range 0–27), with a statistical difference between these
groups (P<0.001). Moreover, plasma serpinA3 in the CRC patients
showed a positive correlation with CRP (r=0.54, P<0.001) and in
the controls (r=0.30, P<0.01) (data not shown).
Location of serpinA3 expression in
colorectal cancer tissue
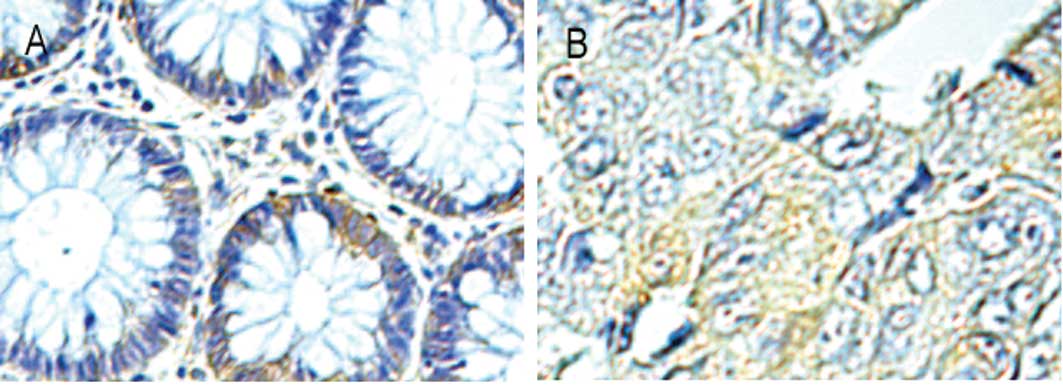
Immunohistochemistry was performed to confirm and
detect localisation of serpinA3 expression. Focal immunoreactivity
from absent to detectable was noted in epithelial cells of the
cancer and normal tissue (Fig. 2).
A heterogeneous extracellular distribution of serpinA3 was also
observed within bands of stroma and in some stromal cells with the
morphological characteristics of leukocytes. No staining was
observed with the isotypic IgG antibody which was used as a
negative control (data not shown).
SerpinA3 genotype
To analyse the effect of the serpinA3 gene
polymorphism on colorectal carcinogenesis a Taq Man system was used
to ascertain the allelic and genotype frequencies in the CRC
patients and a control group. No significant difference in genotype
distribution was noted between the CRC patients and control
subjects or in allelic frequencies (Table I).
 | Table IGenotypic and allelic distribution
expressed as the percentage and frequency of the serpinA3 gene
polymorphism (rs4934) in CRC patients and controls. |
Table I
Genotypic and allelic distribution
expressed as the percentage and frequency of the serpinA3 gene
polymorphism (rs4934) in CRC patients and controls.
| Genotype G→A, %
(n)a | Allele, % (n)b |
|---|
|
|
|
|---|
| G/G | G/A | A/A | G | A |
|---|
| CRC | 25.7 (80) | 50.2 (156) | 24.1 (75) | 50.8 (316) | 49.2 (306) |
| Controls | 23.1 (83) | 52.7 (189) | 24.2 (87) | 49.4 (355) | 50.6 (363) |
Significant differences were not observed following
subdivision of the patients into groups according to rectal and
colonic cancer or localised (Dukes’ A+B) and disseminated (Dukes’
C+D) disease (Table II). When
assessing the levels of serpinA3 protein in the analysed tissue and
plasma samples or subdividing the patients into groups according to
clinicopathological characteristics, we were unable to identify any
significant differences in genotype or in allelic frequencies (data
not shown). Neither the patient nor the control group showed
significant deviation from the Hardy-Weinberg equilibrium.
 | Table IIGenotype and allelic frequency of the
serpinA3 gene polymorphism (rs4934) in regards to the
clinicopathological characteristics of the CRC patients. |
Table II
Genotype and allelic frequency of the
serpinA3 gene polymorphism (rs4934) in regards to the
clinicopathological characteristics of the CRC patients.
| Genotype | Allele |
|---|
|
|
|
|---|
| G/G | G/A | A/A | G | A |
|---|
| Tumour site |
| Rectum (n=149) | 38 | 75 | 36 | 151 | 147 |
| Colon (n=162) | 42 | 81 | 39 | 165 | 159 |
| Duke’s stage |
| A+B (n=181) | 46 | 90 | 45 | 182 | 180 |
| C+D (n=130) | 34 | 66 | 30 | 134 | 126 |
Cancer cell invasiveness
To evaluate the effect of serpinA3 on the
invasiveness of Caco-2 and HT-29 cells, serpinA3 was added to the
cell suspensions at various concentrations (0, 10, 100 and 2,000
ng/ml). Neither of the cell lines exposed to serpinA3 for 22 or 44
h exhibited a significant difference in invasiveness through the
Matrigel basement membrane compared with that of the unexposed
controls.
Discussion
It has been widely reported that proteinases that
are expressed in cancerous tissue promote invasiveness and tumour
progression (1–5). Recently, it has been reported that
cathepsin G activates pro-MMP-9 (16) and that negative MMP-9 expression is
associated with a longer survival time in CRC patients (17).
Observations suggest that serine protease inhibitors
exert inhibitory activity against proteases in the control of
events associated with inflammatory reactions and connective tissue
turnover (6,7). Recently it has been reported that
serpinA3 is aberrantly expressed in metastatic melanoma (18).
The expression profile of serpinA3 in human CRC has
rarely been studied. Immunohistochemistry was used to identify the
cellular source and localisation of serpinA3 protein in CRC
patients, and immunoreactivity was noted in the compartment of the
cancer and normal tissue and also in the stroma and stromal cells
such as leukocytes and fibroblasts. Moreover, CRC patients assessed
using ELISA were shown to have a significantly lower level of
serpinA3 protein in cancer tissues in comparison with paired normal
tissues. The protein level of serpinA3 was found to be
significantly positively correlated in the cancerous and normal
tissue. This indicates that there may be some basic individual
differences. To assess the results according to disease severity
the patients were divided according to localised (Dukes’ A+B) and
disseminated (Dukes’ C+D) disease. We found that serpinA3
expression was higher in disseminated disease.
Based on the balance between its proteolytic and
inhibitory activity in tissue and our results that serpinA3
expression in cancer tissue is lower than that in paired normal
tissues it may be suggested that a high activity of proteinases is
linked to tumour growth and aggressiveness. Proteinases play a role
in cancer metastasis (1–5). Notably, the down-regulated serpinA3
expression in cancer tissue noted in the present study appears to
depend on regulatory factors secreted from tumour cells. However,
our results appear in part to be inconsistent due to the fact that
a higher serpinA3 concentration was associated with malignant
potency such as disseminated (Dukes’ C+D) disease. Further
investigation is required to explain this discrepancy. Notably,
serpinA3 uses conformational change, a change-based trapping
mechanism, to inhibit target enzymes (6). Moreover, serpinA3 is an inflammatory
protein and is regulated by cytokines among which the inflammatory
cytokine interleukin-6 (IL-6) is the major regulator (19). This cytokine is also elevated in CRC
(20). Considering the relationship
between cancer and inflammation, cytokines appear to contribute to
tumour growth, but also to effective antitumour immunity (21,22).
Studies related to structure-function relationships, the regulatory
pathway for serpinA3 expression in CRC and the method by which
serpinA3 affects invasion and metastasis in tumour cells are
warranted. However, in the present study, we found that cells
derived from CRC, such as Caco-2 and HT-29, did not reveal any
change in invasiveness following incubation with serpinA3 in
vitro. Thus, other mechanisms may be involved in
vivo.
Studies indicate that the tissue expression of
serpinA3 in gastric cancer (10)
and melanoma (18) is correlated
with a higher stage of disease and is inversely correlated with
overall survival. In a forthcoming study, it may be informative to
investigate the manner in which tissue levels of serpinA3 affect
the survival rate of CRC patients.
To investigate serpinA3 as a potential tumour marker
and the state of inflammation, the plasma levels of serpinA3 and
CRP were measured. No significant difference was noted in the
levels of plasma serpinA3 in patients in comparison to the controls
and no associations were found between these levels and a series of
clinical characteristics. The functional consequence of this
relationship may disqualify circulating serpinA3 as a tumour marker
with impact on CRC. In agreement with another study (15), a higher concentration of plasma CRP
was found in CRC patients compared to the controls. Moreover,
plasma serpinA3 and CRP of the CRC patients and controls were
significantly positively correlated. SerpinA3 concentrations in
plasma appear to be correlated with systemic inflammatory response
expressed by the inflammatory protein CRP, which appears not to be
specific to CRC patients. On the other hand, it has been questioned
whether circulating levels of CRP are a useful indicator of CRC
(23).
No studies have been carried out in CRC patients to
elucidate the consequences of the common SNP variant (rs4934) in
the serpinA3 signal sequence gene. We aimed to determine whether an
association exists between this gene variant and CRC. The genotype
distributions and allelic frequencies for the gene variant were not
significantly associated with CRC. Furthermore, we investigated
whether this SNP is a potential candidate affecting the expression
of serpinA3. However, we were unable to detect any association
between the genotype and plasma or tissue concentration of
serpinA3.
Overall, the present study aimed to assess the
significance of serpinA3 levels in CRC patients. SerpinA3 was found
to be down-regulated in cancerous tissue as compared to paired
normal tissue, while this suppression was lower at a severe disease
stage. Moreover, in vitro, exogenous serpinA3 had no impact
on the invasiveness of CRC cells.
Further studies are warranted to improve our
understanding of the role of serpinA3 in CRC. The data presented in
this study are prerequisite to a forthcoming study on progression
and the 5-year survival rate in CRC.
Acknowledgements
This study was supported by grants from Futurum The
Academy of Healthcare, County Council of Jönköping, Sweden, the
Foundation of Clinical Cancer Research, Jönköping, Sweden and the
University College of Health Sciences, Jönköping Sweden.
References
|
1
|
Lopez-Otin C and Matrisian LM: Emerging
roles of proteases in tumour suppression. Nat Rev Cancer.
7:800–808. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
3
|
Murray GI, Duncan ME, O’Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikkola J, Vihinen P, Vlaykova T,
Hahka-Kemppinen M, Kahari VM and Pyrhonen S: High expression levels
of collagenase-1 and stromelysin-1 correlate with shorter
disease-free survival in human metastatic melanoma. Int J Cancer.
97:432–438. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuester D, Lippert H, Roessner A and
Krueger S: The cathepsin family and their role in colorectal
cancer. Pathol Res Pract. 204:491–500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Law RHP, Zhang Q, McGowan S, Buckle AM,
Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI
and Whisstock JC: An overview of the serpin superfamily. Genome
Biol. 7:2162006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gettins PG: Keeping the serpin machine
running smoothly. Genome Res. 10:1833–1835. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalsheker NA: Alpha-1-antichymotrypsin.
Int J Biochem Cell Biol. 28:961–964. 1996. View Article : Google Scholar
|
|
9
|
Janciauskiene S: Conformational properties
of serine proteinase inhibitors (serpins) confer multiple
pathophysiological roles. Biochim Biophys Acta. 1535:221–235. 2001.
View Article : Google Scholar
|
|
10
|
Allgayer H, Babic R, Grutzner KU, Beyer
BCM, Tarabichi A, Schildberg FW and Heiss MM: Tumor-associated
proteases and inhibitors in gastric cancer: analysis of prognostic
impact and individual risk protease patterns. Clin Exp Metastasis.
16:62–73. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamboh ML, Minster RL, Kenney M, Ozturk A,
Desai PP, Kammerer CM and DeKosky ST: Alpha-1-antichymotrypsin (ACT
or SERPINA3) polymorphism may affect age-at-onset and disease
duration of Alzheimer’s disease. Neurobiol Aging. 27:1435–1439.
2006.
|
|
12
|
Slowik A, Borratynska A, Turaj W, Pera JP,
Dziedzic T, Figlewicz DA, Betlej M, Krzyszkowski T, Czepko R and
Szczudlik A: Alpha-1-antichymotrypsin gene (SERPINA3) A/T
polymorphism as a risk factor for aneurysmal subarachnoid
hemorrhage. Stroke. 36:737–740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Zhu Y, Ge M, Pang Q and Yu Y:
Polymorphism rs4934 of SERPINA3 and sporadic intracranial aneurysms
in the Chinese population. Cerebrovasc Dis. 29:68–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krischek B, Akagawa H, Tajima A, Narita A,
Kasuya H, Hori T and Inoue I: The alanine/threonine polymorphism of
the alpha-1-antichymotrypsin (SERPINA3) gene and ruptured
intracranial aneurysms in the Japanese population. Cerebrovasc Dis.
23:46–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erlinger TP, Platz EA, Rifai N and
Helzlsouer KJ: C-reactive protein and the risk of incident
colorectal cancer. JAMA. 291:585–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson TJ, Nannuru KC and Singh RK:
Cathepsin G-mediated activation of pro-matrix metalloproteinase 9
at the tumor-bone interface promotes transforming growth factor-β
signaling and bone destruction. Mol Cancer Res. 7:1224–1233.
2009.PubMed/NCBI
|
|
17
|
Bendardaf R, Buhmeida A, Hilska M, Laato
M, Syrjänen S, Syrjänen K, Collan Y and Pyrhönen S: MMP-9
(gelatinase B) expression is associated with disease-free survival
and disease-specific survival in colorectal cancer patients. Cancer
Invest. 28:38–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Jiang H, Dai D, Su M, Martinka M,
Brasher P, Zhang Y, McLean D, Zhang J, Ip W, Li G, Zhang X and Zhou
Y: Alpha 1 antichymotrypsin is aberrantly expressed during melanoma
progression and predicts poor survival for patients with metastatic
melanoma. Pigment Cell Melanoma Res. 23:575–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castell JV, Gomez-Lechon MJ, David M,
Andus T, Geiger T, Trullenque R, Fabra R and Heinrich PC:
Interleukin-6 is the major regulator of acute phase protein
synthesis in adult human hepatocytes. FEBS Lett. 242:237–239. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung YC and Chang YF: Serum interleukin-6
levels reflect the disease status of colorectal cancer. J Surg
Oncol. 83:222–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balkwill F: Chemokine biology in cancer.
Semin Immunol. 15:49–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trichopoulos D, Psaltopoulou T, Orfanos P,
Trichopoulou A and Boffetta P: Plasma C-reactive protein and risk
of cancer: a prospective study from Greece. Cancer Epidemiol
Biomarkers Prev. 15:381–384. 2006. View Article : Google Scholar : PubMed/NCBI
|