Introduction
Serum often contains abnormally expressed proteins
during tumorigenesis (1). Serum
proteins may be abnormally increased or decreased during the
occurrence and development of nasopharyngeal carcinoma (NPC)
(2). However, there are currently
no simple or effective methods to collect and differentiate these
abnormally secreted marker proteins from the numerous serum
proteins. In this study, acetonitrile was used to remove the
majority of high-abundance proteins from serum samples obtained
from patients with NPC. Surface-enhanced laser
desorption/ionization time-of-flight mass spectrometry
(SELDI-TOF-MS) with a CM10 (weak cation exchange) ProteinChip was
then used to detect proteins, and the results were compared with
those for non-acetonitrile-treated NPC sera. These techniques were
utilized to better understand the protein profiles of
acetonitrile-treated and -non-acetonitrile-treated sera from NPC
patients and provide a new method for collecting and identifying
serum proteins showing abnormal upregulation or downregulation in a
specific disease setting.
Materials and methods
Serum samples
Serum samples for acetonitrile treatment were
obtained from patients with NPC who were initially diagnosed at the
Otolaryngological Department of our hospital between November 2007
and April 2008. The inclusion criteria were: patients with
pathologically confirmed poorly differentiated squamous cell
carcinoma naïve to prior treatment and with no other malignant
disease. A total of 58 patients with NPC (30 males and 28 females)
aged 44.7±7.9 years were enrolled in this study. Serum samples were
also obtained from healthy controls who received physical
examinations at the Physical Examination Center of our hospital in
April 2008. Inclusion criteria were: physically healthy individuals
without a history of NPC or any other malignant disease. A total of
58 healthy controls (30 males and 28 females) aged 44.0±10.5 years
were enrolled in the study.
For acetonitrile treatment, serum samples were
obtained from patients with NPC who were first diagnosed at our
hospital between July 2009 and November 2009. A total of 30
patients with NPC (22 males and 8 females) aged 44.3±6.8 years were
enrolled. Serum samples were also obtained from 30 healthy controls
(22 males and 8 females) aged 43.0±9.2 years who donated blood at
Nanning Central Blood Station in January 2010. Four subjects (2
males and 2 females) were selected from each group to construct
four subgroups (gender-matched patients and healthy controls per
subgroup) for ProteinChip screening. The serum samples were
voluntarily donated, and all subjects provided written informed
consent.
ProteinChip, mass spectrometer and
reagents
The CM10 ProteinChip from Bio-Rad (Foster City, CA,
USA) was used with a ProteinChip Biomarker System. Data were
analyzed using ProteinChip 3.2 and Biomarker Wizard software
(Ciphergen Biosystems, Freemont, CA, USA). The reagents included U9
buffer (9 mol/l urea and 2% CHAPS) supplemented with 0.1% DTT,
fresh saturated sinapinic acid (SPA) solution (50% acetonitrile +
0.5% TFA) and CM10 binding buffer (50 mM NaAC, pH 4.0).
Preparation of non-acetonitrile-treated
serum samples
Venous blood samples were collected and left to
stand for 30 min to 1 h. Samples were then centrifuged at 3000 rpm
at 4˚C for 10 min. Non-hemolytic serum was collected, divided into
aliquots, and stored at −80˚C. Before use, the serum was
centrifuged at 10,000 rpm at 4˚C for 2 min. Each chip spot required
10 μl of serum. The serum was diluted with 2 volumes of U9 buffer,
agitated in an ice bath for 30 min, and 360 μl of the relevant
binding buffer was added, diluted 40 times and mixed. Then, 100 μl
was retrieved for loading on pre-treated chips.
Preparation of acetonitrile-treated serum
samples
Serum samples were collected and stored as described
above. Then, 1.2 volumes of acetonitrile was added to 200 μl of
serum, vortexed, and left to stand at 4˚C for 10 min. Following
centrifugation at 12,000 rpm for 35 min, the supernatant was
retained and stored at −80˚C until use. To prepare each sample for
use, 40 μl of 0.9% normal saline was added and 10 μl of the protein
solution was collected and treated as described for the preparation
of non-acetonitrile-treated samples.
Pre-treatment of the ProteinChip
The ProteinChip array cassette was placed in the
bioprocessor, and 200 μl of binding solution was added to each
well. The ProteinChip was incubated for 5 min at room temperature
and agitated vigorously. This was repeated once, and the
ProteinChip was kept moist until use.
Preparation of the ProteinChip
The ProteinChip was prepared in accordance with the
manufacturer's instructions. Following preparation of the serum
samples and pre-treatment of the ProteinChip, as described above,
100 μl of sample was added to each well. The ProteinChip was
agitated at 400–600 rpm at 4˚C for 2 h, washed with 200 μl of
deionized water, and then rapidly spun dry. Finally, 0.5 μl of SPA
was added to each well twice, and proteins were detected after the
ProteinChip had dried.
Protein detection
Proteins in the acetonitrile- and
non-acetonitrile-treated serum samples were detected using the
ProteinChip Biomarker System MS. Data were collected by averaging
the results of 130 laser shots with an intensity of 180, a detector
sensitivity of 8, data collection range of m/z 2,000–50,000, and an
optimization range of m/z 2,000–10,000. Data signals were collected
20 times per spot.
Normalization, collection and analysis of
protein profiles
Mass spectrograms from the same chip were combined
and normalized using ProteinChip Software version 3.2. The range of
peak masses was analyzed between m/z 2,000 and 50,000. Peak
detection was performed using Biomarker Wizard software. The
settings for ‘auto-detect peaks to cluster’ were: the
signal-to-noise ratio was 5 and the minimum peak threshold was 15%
for the first pass. For cluster completion, the cluster mass window
was 0.3%, and the signal-to-noise ratio for the second pass was 2.
Finally, the protein profiles were obtained using Biomarker Wizard
software. The number of peaks in the spectra and the intensity of
each peak were recorded and analyzed in the acetonitrile- and
non-acetonitrile-treated serum samples.
Results
Small molecular proteins in serum were
enriched by acetonitrile precipitation
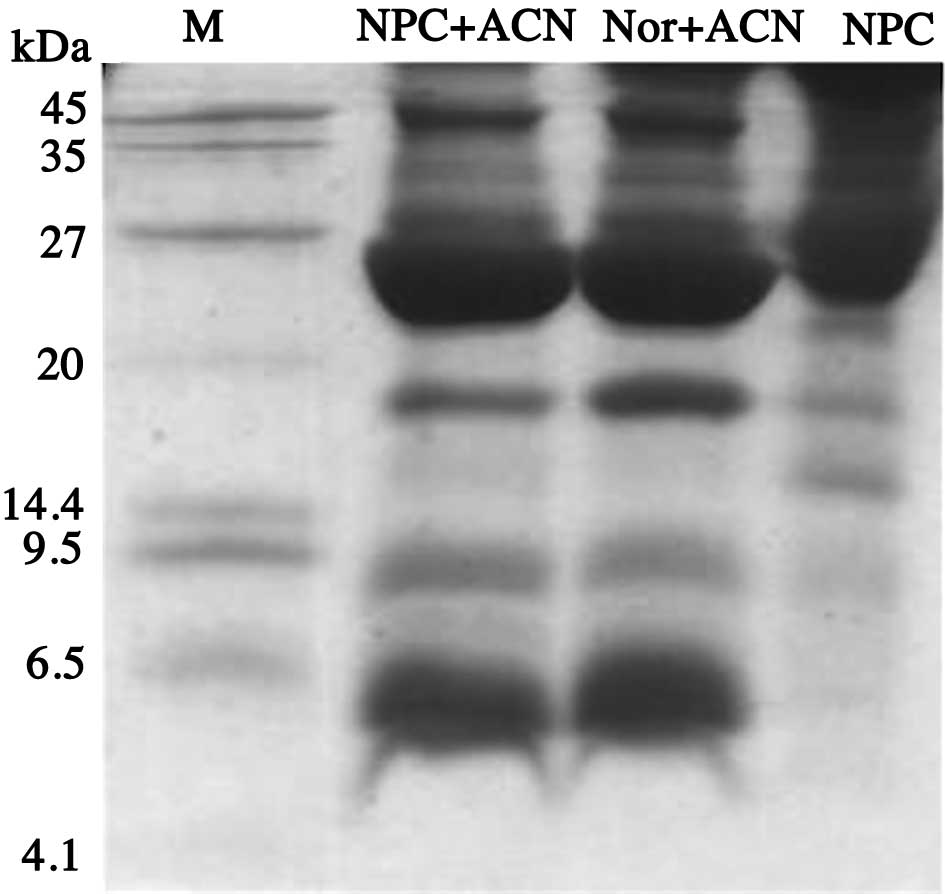
As shown in Fig. 1,
after precipitating 200 μl of serum with 1.2 volumes of
acetonitrile, Tricine-SDS-PAGE electrophoresis (3) indicated that proteins/polypeptides
<30 kDa were enriched.
Protein profiles of acetonitrile- and
non-acetonitrile-treated sera from patients with NPC and healthy
controls
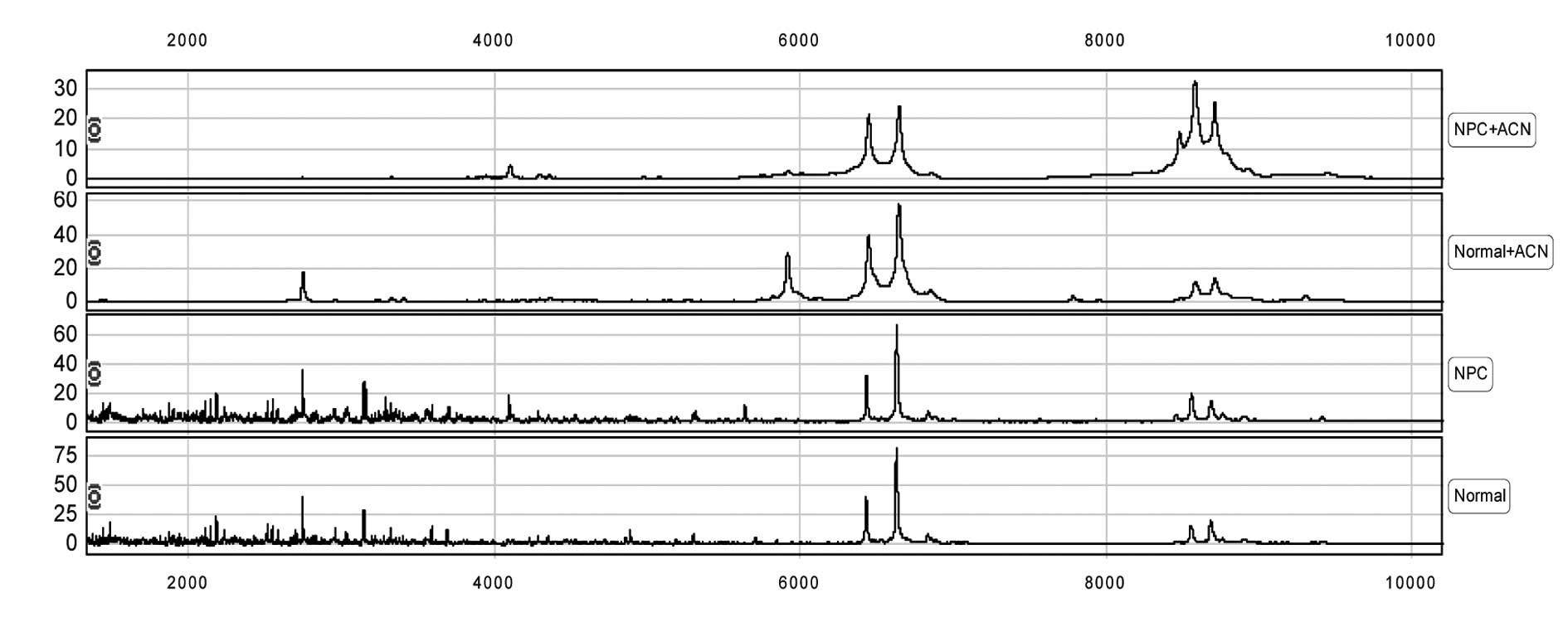
MS protein profiles were obtained for the
acetonitrile- and non-acetonitrile-treated sera from patients with
NPC or healthy controls using the CM10 ProteinChip (Fig. 2). Statistical analysis of the
protein profiles for the non-acetonitrile-treated NPC patient sera
(n=58) and normal healthy control sera (n=58) using the conditions
described in Materials and methods revealed a total of 99 protein
or polypeptide peaks in the non-acetonitrile-treated NPC sera, with
a detection rate of 18.1% for proteins with a molecular weight (MW)
of 6,000–10,000 kDa, 11.1% for proteins >10,000 kDa, and 5.1%
for proteins at a peak density of >5. In contrast, 80 protein or
polypeptide peaks were detected in the acetonitrile-treated NPC
sera, with a detection rate of 26.2% for proteins with a MW of
6,000–10,000 kDa, 37.5% for proteins with a MW of >10,000 kDa,
and 13.8% for proteins at a peak density of >5. Notably, a large
proportion of peaks in the non-acetonitrile-treated NPC sera were
<6,000 kDa, while the detection rate of peaks >6,000 kDa was
higher in the acetonitrile-treated NPC sera, accounting for over
half of the peaks (26.2+37.5%) (Table
I).
 | Table IDetection of proteins using a CM10
ProteinChip in acetonitrile- and non-acetonitrile-treated sera from
patients with NPC or healthy controls. |
Table I
Detection of proteins using a CM10
ProteinChip in acetonitrile- and non-acetonitrile-treated sera from
patients with NPC or healthy controls.
| MW range (kDa) | Peaks (n) | CM10 | Peaks (n) | CM10+ACN |
|---|
|
|
|---|
| NPC intensity | Normal intensity | NPC intensity | Normal intensity |
|---|
|
|
|
|
|---|
| <5 | 5–10 | >10 | <5 | 5–10 | >10 | <5 | 5–10 | >10 | <5 | 5–10 | >10 |
|---|
| 2,000 | 23 | 22 | 0 | 1 | 21 | 1 | 1 | 4 | 3 | 1 | 0 | 3 | 0 | 1 |
| 3,000 | 19 | 19 | 0 | 0 | 19 | 0 | 0 | 9 | 9 | 0 | 0 | 9 | 0 | 0 |
| 4,000 | 15 | 14 | 0 | 1 | 13 | 2 | 0 | 9 | 8 | 0 | 1 | 9 | 0 | 0 |
| 5,000 | 13 | 12 | 1 | 0 | 11 | 2 | 0 | 7 | 6 | 0 | 1 | 6 | 0 | 1 |
| 6,000 | 5 | 3 | 1 | 1 | 3 | 1 | 1 | 6 | 4 | 0 | 2 | 4 | 0 | 2 |
| 7,000 | 3 | 3 | 0 | 0 | 3 | 0 | 0 | 4 | 4 | 0 | 0 | 3 | 1 | 0 |
| 8,000 | 6 | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 1 | 2 | 3 | 3 | 0 | 3 |
| 9,000 | 4 | 4 | 0 | 0 | 4 | 0 | 0 | 5 | 4 | 1 | 0 | 4 | 1 | 0 |
| 10,000 | 5 | 5 | 0 | 0 | 5 | 0 | 0 | 16 | 16 | 0 | 0 | 16 | 0 | 0 |
| 20,000 | 6 | 6 | 0 | 0 | 6 | 0 | 0 | 14 | 14 | 0 | 0 | 14 | 0 | 0 |
| Total | 99 | 94 | 2 | 3 | 91 | 6 | 2 | 80 | 69 | 4 | 7 | 71 | 2 | 7 |
| % of
6,000–10,000 | 18.1 | | | | | | | 26.3 | | | | | | |
| % of >10,000 | 11.1 | | | | | | | 37.5 | | | | | | |
| % of >5
intensity | 5.1 | | | | | | | 13.8 | | | | | | |
Effects of acetonitrile treatment on
serum protein profiles of patients with NPC and healthy
controls
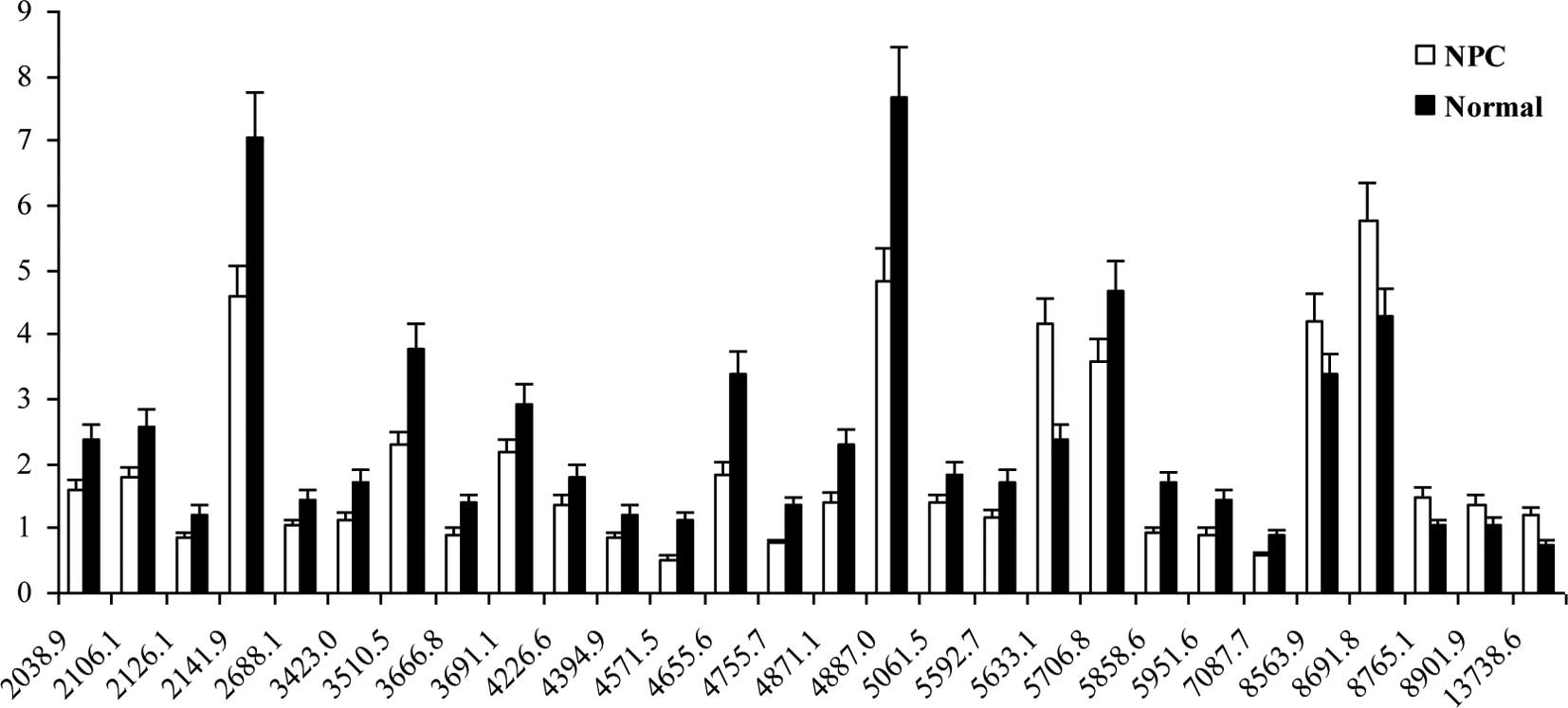
Statistical analysis of the protein profiles of the
non-acetonitrile-treated sera between NPC patients and healthy
controls showed that CM10 detected 28 differentially expressed
protein peaks at P<0.05 and mean > SD, of which 6 (5633.1,
8563.9, 8691.8, 8765.1, 8901.9 and 13738.6 kDa) were upregulated,
and 22 were downregulated (Fig. 3).
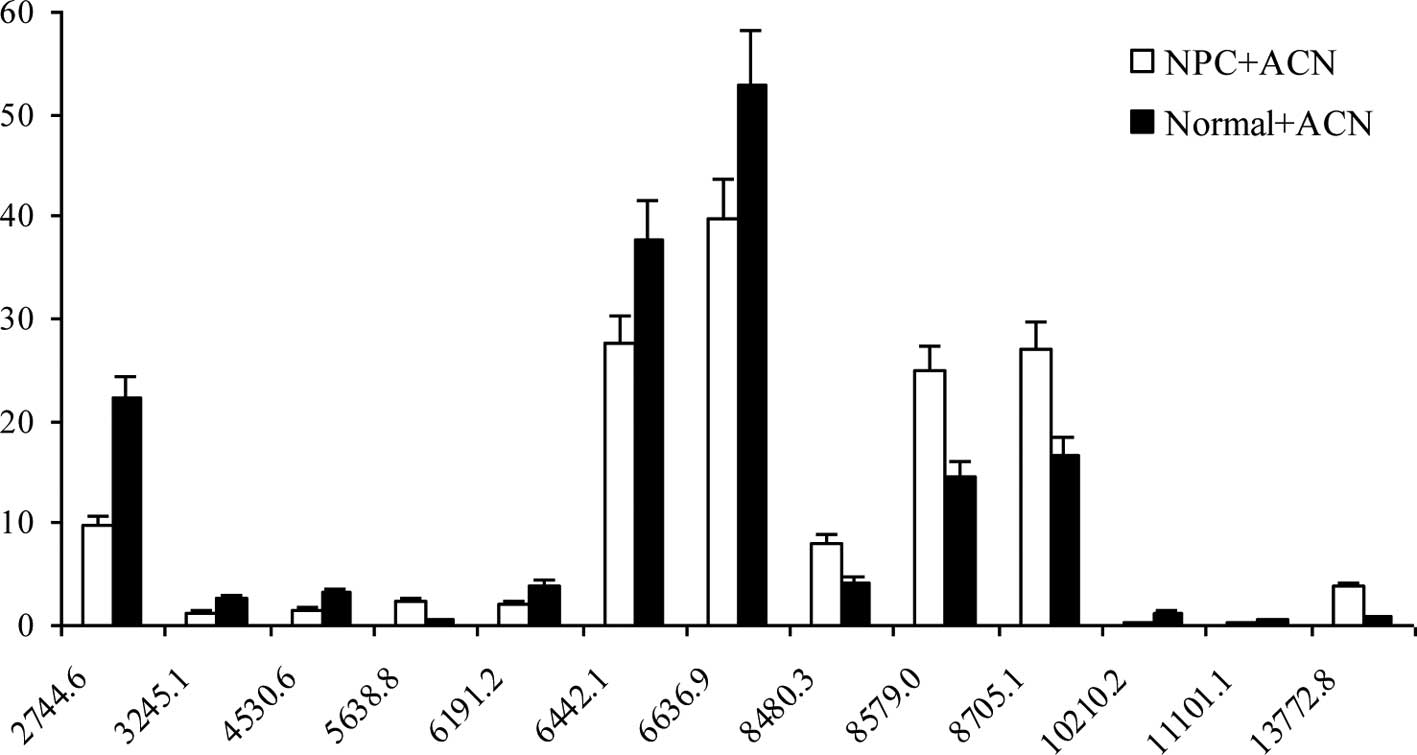
A comparison of the acetonitrile-treated sera between NPC patients
and healthy controls showed that CM10 detected 13 differentially
expressed protein peaks at P<0.05 and mean > SD, of which 5
(5638.8, 8480.3, 8579.0, 8705.1 and 13,772.8 kDa) were upregulated,
and 8 were downregulated (Fig.
4).
The MWs of the upregulated protein peaks (5633.1,
8563.9, 8691.8 and 13738.6 kDa) found in the protein profiles of
the non-acetonitrile-treated NPC sera were similar to those of the
acetonitrile-treated NPC sera (5638.8, 8579.0, 8705.1 and 13,772.8
kDa). Specifically, the patterns for 2 protein peaks (8563.9 and
8691.8 kDa) in the non-acetonitrile-treated NPC sera were the same
as those in the acetonitrile-treated NPC sera (8579.0 and 8705.1
kDa), although the peak intensity was 5–7 times higher in the
latter (Figs. 2–4). Since the equipment and methodology
utilized were the same in all of the tests, the four upregulated
proteins obtained from the acetonitrile-and
non-acetonitrile-treated NPC sera can be regarded as the same
proteins/polypeptide.
Discussion
Proteins perform a wide range of physiological
functions, and the existence of life is dependent on protein
activity. Pathophysiological changes and drug effects are closely
related to changes in the components or quantities of proteins.
Human serum contains many low-abundance proteins secreted by and
shed from tissue cells. These proteins may be important proteins
for signal conduction and regulation. Furthermore, necrosis,
apoptosis, hemolysis and tumorigenesis may cause intracellular
proteins to be released into the blood (1,4). Serum
proteomics research focusing on low-abundance proteins in NPC may
aid in both the identification of protein markers for NPC and a
better understand of its pathogenesis, and thus reveal new
techniques for the diagnosis of NPC. Furthermore, such proteins may
offer potential targets for treatment or serve as markers that
monitor disease course and treatment effectiveness.
Each milliliter of serum contains 60–80 mg of
protein, mainly albumin (50–70%), immunoglobulins (IgG, IgA, IgD,
IgM, and IgE; IgG accounts for 10–25% of the total protein
content), transferrin, haptoglobin, and lipoprotein. The majority
of the remaining proteins are of low abundance (5). When using SELDI-TOF-MS to analyze
serum proteins, high-abundance albumin and IgG can interfere with
the detection of low-abundance proteins, thereby affecting the
resulting profiles (6).
Acetonitrile can decrease the specific inductivity
of water, and biomacromolecules with hydrophilic surfaces may be
dehydrated causing them to aggregate and ultimately precipitate.
Accordingly, acetonitrile treatment can precipitate and remove many
high-abundance proteins from serum and eliminate the
intra-molecular interactions between proteins. Acetonitrile can
also degenerate and disassociate low-abundance
proteins/polypeptides that initially bind to high-abundance
proteins such as albumin. As a result, these low-abundance proteins
or polypeptides are not removed when eliminating the high-abundance
macromolecules (7). In this study,
acetonitrile was used to treat serum samples obtained from NPC
patients to remove the high-abundance macromolecular proteins, and
the protein profiles were then compared with
non-acetonitrile-treated NPC sera using SELDI-TOF-MS technology
with a CM10 ProteinChip. Under standard conditions, 99 protein or
polypeptide peaks were detected in the non-acetonitrile-treated
sera from the NPC patients, with a detection rate of 18.1% for
proteins with a MW of 6,000–10,000 kDa, 11.1% for proteins with a
MW of >10,000 kDa, and 5.1% for proteins at a peak density of
>5. In contrast, 80 peaks were detected in the
acetonitrile-treated sera from the NPC patients, with a detection
rate of 26.2% for proteins with a MW of 6,000–10,000 kDa, 37.5% for
proteins with a MW of >10,000 kDa, and 13.8% for proteins at a
peak density of >5. Compared with non-acetonitrile-treated sera
from healthy subjects, 28 differentially expressed protein peaks
were detected in the non-acetonitrile-treated sera from NPC
patients (P<0.05; mean > SD), of which 6 peaks were
upregulated and 22 were downregulated. In comparison, for
acetonitrile-treated sera, 13 peaks were differentially expressed
in patients with NPC versus the healthy controls (P<0.05; mean
> SD), of which 5 peaks were upregulated and 8 were
downregulated in NPC patients. Four differentially expressed
proteins were upregulated in the acetonitrile- and
non-acetonitrile-treated NPC sera compared with the healthy
controls, although the latter had lower peak values, suggesting
these proteins are promising markers for NPC. However, the
downregulated proteins differed between the acetonitrile- and
non-acetonitrile-treated NPC sera profiles.
Using SELDI-TOF-MS in conjunction with a CM10
ProteinChip, striking differences were noted in the protein
profiles of the acetonitrile- and non-acetonitrile-treated NPC
sera. Of note is that a large proportion of the proteins from the
non-acetonitrile-treated NPC sera were <6,000 kDa, while the
detection rate of protein peaks >6,000 kDa was higher in the
acetonitrile-treated NPC sera, accounting for over half of all
protein peaks detected (26.2+37.5%). Four upregulated proteins were
found in both the acetonitrile- and non-acetonitrile- treated sera
from the NPC patients; these 4 proteins may be valuable markers for
NPC. Few upregulated proteins were lost, and the peak value
densities increased by 5–7-fold following acetonitrile treatment.
Therefore, acetonitrile is effective in eradicating the majority of
the high-abundance macromolecular proteins from serum samples of
patients with NPC. Acetonitrile treatment can be applied in serum
proteomics research and may facilitate the detection and
identification of significant differentially expressed
proteins.
Acknowledgements
This study was supported by the Local High Disease
Control and Prevention Research Laboratory Foundation of Guangxi,
China (no. 0630006-5E7Z; no. 0842009-Z14) and The Natural Science
Foundation of Guangxi, China (no. 06390-18).
References
|
1
|
Adkins JN, Varnum SM, Auberry KJ, et al:
Toward a human blood serum proteome: analysis by multidimensional
separation coupled with mass spectrometry. Mol Cell Proteomics.
1:947–955. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang YJ, Xuan C, Zhan BB, et al:
SELDI-TOF MS profiling of serum for detection of nasopharyngeal
carcinoma. J Exp Clin Cancer Res. 28:852009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schägger H: Tricine-SDS-PAGE. Nat Protoc.
1:16–22. 2006.
|
|
4
|
Ma PC, Blaszkowsky L, Bharti A, et al:
Circulating tumor cells and serum tumor biomarkers in small cell
lung cancer. Anticancer Res. 23:49–62. 2003.PubMed/NCBI
|
|
5
|
Steel LF, Trotter MG, Nakajima PB, et al:
Efficient and specific removal of albumin from human serum samples.
Mol Cell Proteomics. 2:267–270. 2003.
|
|
6
|
Li J, Zhang Z, Rosenzweig J, et al:
Proteomics and bioinformatics approaches for identification of
serum biomarkers to detect breast cancer. Clin Chem. 48:1296–1304.
2002.PubMed/NCBI
|
|
7
|
Chertov O, Biragyn A, Kwak LW, et al:
Organic solvent extraction of proteins and peptides from serum as
an effective sample preparation for detection and identification of
biomarkers by mass spectrometry. Proteomics. 4:1195–1203. 2004.
View Article : Google Scholar : PubMed/NCBI
|