Introduction
Various treatment strategies for esophageal
carcinoma have been adopted in China and other countries.
Concurrent chemoradiotherapy (CRT) is considered to be one of the
standard regimens for esophageal carcinoma in the US (1,2) and
Japan (3), although it is generally
reported that the side effects of CRT are much more severe than
those of radiotherapy (RT) alone. This observation was confirmed in
the Radiation Therapy Oncology Group (RTOG) 85-01 trial (1). RT as a monotherapy is now widely
preferred in China (4–7). Late-course accelerated
hyperfractionation radiotherapy (LAFR) has achieved a 5-year
overall survival rate of 33% in Chinese patients with esophageal
carcinoma (4). In their study, Zhao
et al (7) found that LAFR
offers similar survival and local control rates compared to
standard chemotherapy plus RT, as in that delivered in the RTOG
85-01 (1,2) and 94-05 studies (8). Moreover, LAFR is more cost-effective
in China. If LAFR provides successful treatment results comparable
to those obtained with CRT and with fewer side effects, LAFR is
likely to become a more feasible treatment regime for patients with
esophageal carcinoma and one of the standard treatments used for
Chinese individuals. However, to the best of our knowledge, no
studies have focused on a direct comparison of the efficacy of LAFR
and CRT thus far. Before a multi-center phase III trial is
conducted to confirm the role of LAFR, this small-sample,
preliminary, prospective exploratory study was performed to compare
the efficacy of LAFR and CRT. The maximal tolerated dose (MTD) of
CRT for Chinese patients with esophageal carcinoma was used as the
CRT regimen (9).
Patients and materials
Eligibility
Patients (age range 18–70 years) for whom primary
esophageal squamous cell carcinoma was proven by histology
(T1-4N0-1M0) and staged by thoracoabdominal helical computed
tomography (CT) were eligible for this study. All 47 patients
provided written informed consent. Due to endoscopic ultrasound not
being available in our center when the study commenced, clinical
staging was evaluated based on CT scans, using the same standard as
described in our previously published article (9). The T stage was defined by the maximal
transverse diameter of the esophageal tumor: T1 ≤2 cm, T2 >2 cm
and ≤4 cm, and T3 >4 cm. Tumors indicating an invasion of any
adjacent structures were classified as T4. If the minimal
transverse diameter of lymph nodes in mediastinal and celiac were
>1 cm, the lymph nodes were classified as N1; otherwise, they
were classified as N0. Inclusion criteria involved patients not
receiving any prior RT or chemotherapy. Patients were required to
have a Karnofsky performance status of ≥60. The required laboratory
test results included a neutrophil count of ≥2.0×109/l,
a platelet count of ≥100×109/l, a hemoglobin count of
≥100 g/l, and serum creatinine, aspartate aminotransferase, alanine
aminotransferase and total serum bilirubin ≤ upper limits of
normal. The exclusion criteria included any of the following:
pregnancy, lactation, tracheoesophageal fistula, a history of other
malignancies, with the exception of carcinoma in situ of the
cervix, non-melanomatous skin cancer or cancer from which the
patient had not been disease-free for 5 years, a general medical
condition preventing combined modality therapy and a known
hypersensitivity to cisplatin (CDDP) or 5-fluorouracil (5-FU), as
well as use of any other concurrent antineoplastic therapy.
Pre-treatment evaluation
The pre-treatment evaluation included a medical
history, a complete physical examination, barium esophagography, a
chest and abdominal helical CT scan, upper gastroesophageal
endoscopy, electrocardiography, bronchoscopy, a bone marrow scan
(if clinically indicated), complete blood count and a biochemical
profile. The pre-treatment tests were performed during the 2 weeks
prior to treatment initiation. Patients received physical
examinations and blood counts were obtained once a week or more
often as required. A biochemical profile and electrocardiography
was performed prior to each chemotherapy cycle.
Recruitment and treatment plan
A total of 22 patients were prospectively recruited
and treated with LAFR. A further set of 25 patients, who were
treated with CRT during the same time period, were selected as the
control group. The treatment scheme is shown in Table I. In the LAFR group, patients
received RT as a monotherapy. In the CRT group, RT began on Day 1,
concurrently with the first cycle of chemotherapy.
 | Table ITreatment plan. |
Table I
Treatment plan.
| A, LAFR group |
|
| Week | 1 | 2 | 3 | 4 | 5 | | |
| RT | ||||| | ||||| | ||||| | || || || || || | || || || || || | | |
|
| RT regimen: 30 Gy in
weeks 1–3: 2 Gy/f, 1 f/d, 5 f/w; 30 Gy in weeks 4 and 5: 1.5 Gy/f,
2 f/d, 10 f/w; total dose: 60 Gy |
|
| B, CRT group |
|
| Week | 1 | 2 | 3 | 4 | 5 | 9 | 13 |
| RT | ||||| | ||||| | ||||| | ||||| | ||||| | | |
|
| RT regimen: Week
1–5: 2 Gy/f, 1 f/d, 5 f/w; total dose: 50 Gy. Chemotherapy: CDDP
(52.5 mg/m2) x1x4; 5-FU (700 mg/m2) x5x4.
Total dose: CDDP 210.0 mg/m2, 5-FU 14,000
mg/m2. |
|
| CDDP | ♦ | | | | ♦ | ♦ | ♦ |
| 5-FU | □ | | | | □ | □ | □ |
Radiotherapy
Multifield, external-beam megavoltage radiation was
delivered using 6-MeV linear accelerators. All fields were treated
each day. Treatment was administered with a combination of
anterior-posterior, oblique or lateral fields, in a manner that the
dose-to-target volume did not differ from the dose specified at the
isocenter by >10%. The administered dose was prescribed to the
isodose line covering the volume at risk. Port films were taken of
2 fields once weekly or more often if clinically indicated. The
prescription dose was calculated without tissue heterogeneity
correction. The bounds of the gross target volume (GTV) were
delineated by esophagography, CT scan and esophagoscopy. The upper
and lower bounds of clinical target volume (CTV) were defined as an
extension of 3 cm outside the upper and lower bounds of GTV,
respectively, while the lateral bounds of CTV were defined as an
extension of 1 cm outside the lateral bounds of GTV. The planning
target volume (PTV) was obtained by expanding CTV by 1 cm in all
directions.
In the CRT group, RT was performed with conventional
fractionation on the first day of week 1. Patients were treated
with 5 daily fractions of 2.0 Gy per week over a 5-week period. The
total radiation dose was 50 Gy.
Details regarding the LAFR regimen were previously
published (10). In the LAFR group,
patients received conventional fractionation RT at 2 Gy per
fraction, to a dose of 30 Gy in 15 fractions over 3 weeks during
the first RT course, followed by accelerated fractionation RT,
twice daily, at 1.5 Gy per fraction, with a minimal interval of 6 h
between fractions and an overall treatment time of 5 weeks. The
total dose was 60 Gy.
Chemotherapy
Chemotherapy regimens, defined specifically for
Chinese individuals with esophageal carcinoma have been published
in detail (9). Chemotherapy was
commenced on Day 1 of RT using MTD: CDDP 52.5 mg/m2 on
Day 1 and 5-FU 700 mg/m2 on Days 1–5, repeated four
times every 28 days. The first and second cycles were concurrent
with CRT. CDDP was administered at an infusion rate of 1 mg/min on
Day 1, followed by a continuous daily intravenous infusion of 5-FU
(at least 8 h) from Day 1 to Day 5.
Evaluation of adverse events
Radiation-induced adverse events were graded
according to the RTOG criteria (11), which included acute reactions
occurring within the first 90 days of treatment or late reactions
occurring after 90 days of treatment. Chemotherapy-induced adverse
events were graded according to the National Cancer Institute
Common Toxicity Criteria (version 2.0) (12), with the exception of hand-foot
syndrome, which was graded using protocol-specific definitions.
Hand-foot syndrome grades were defined as (13): Grade 1: numbness,
dysesthesia/paresthesia, tingling, painless swelling or erythema.
Discomfort did not disrupt normal activities. Grade 2: painful
erythema, with swelling. Discomfort affected activities of daily
living. Grade 3: moist desquamation, ulceration, blistering and
severe pain. Severe discomfort made it impossible for the patient
to work or perform activities of daily living.
Dose attenuation
Dose modifications were based on the most serious
toxicities occurring on any day after treatment initiation.
The irradiation dose was not allowed to be modified.
However, RT was withheld for Grade 3 or higher toxicities until the
toxicities were no longer present. RT was continued, but
chemotherapy was withheld in cases where Grade 3 or higher
toxicities, unrelated to RT, occurred; for example, mucositis,
genitourinary toxicity and hand-foot syndrome. Chemotherapy was
resumed once these toxicities had been eradicated.
The CDDP dose was reduced by 50% if the serum
creatinine was between 1.6 and 2.0 mg/dl. Both CDDP and 5-FU were
stopped if the serum creatinine was >2 mg/dl, but RT was
continued. Chemotherapy was restarted once the toxicities had been
eradicated.
For hand-foot syndrome, only the 5-FU dose was
modified. For Grade 2 toxicity the 5-FU dose was reduced by 25% for
the following chemotherapy cycle, and for Grade 3 toxicity 5-FU was
stopped and the dose was reduced by 50% in the following
chemotherapy cycle.
In other cases, doses were also modified. If Grade
3–4 thrombocytopenia, Grade 3–4 anemia, Grade 4 neutropenia, or
Grade 3–4 non-hematologic toxicity occurred (with the exception of
Grade 3 nausea, vomiting and anorexia), both RT and CDDP with 5-FU
were withheld until the toxicities were no longer present. If this
did not occur within 2 weeks, the patient was withdrawn from the
study. The CDDP and 5-FU doses of the following chemotherapy cycle
were reduced by 25%. Prophylactic recombinant-human granulocyte
colony stimulating factor was used following the reduced
chemotherapy cycle. If Grade 3 neutropenia or Grade 2
thrombocytopenia alone occurred, chemotherapy was stopped and RT
was continued. The CDDP and 5-FU doses of the following
chemotherapy cycle were the same as those in the original regimen.
Prophylactic recombinant-human granulocyte colony stimulating
factor was used following that chemotherapy cycle.
Follow-up and therapeutic effects
evaluation
Following treatment, patients were followed up every
3 months for the first year, every 6 months for the second year and
annually thereafter. Each follow-up included history, physical
examination, complete blood count, blood biochemical examination,
chest X-ray or chest CT, esophageal barium radiography or
esophagoscopy. A biopsy of the primary tumor site was required if
locoregional recurrence was suspected following the X-ray or
CT.
The endpoints of this study were survival,
locoregional control and distant metastasis. Death from any cause
was calculated from the date of treatment until the patient
succumbed to the disease or the last follow-up evaluation. All
endpoints were observed from the first day of treatment until death
or the last follow-up time.
Statistical analysis
Statistical analyses were performed using the
SPSS13.0 software package. Constituent ratios were assessed using
the Chi-square test or the Fisher’s exact probability test. The
means between the two groups were compared using the t-test or
rank-sum test. The survival, local control and metastasis rates
were estimated using the Kaplan-Meier method. Statistical
significance was assessed using the log-rank test. P<0.05 was
considered to be statistically significant.
Results
Patient characteristics
Between July 2006 and June 2007, 22 sequential
untreated patients with pathologically confirmed esophageal
squamous cell carcinoma were treated with LAFR (LAFR group).
Concomitantly, 25 patients were treated with CRT (CRT group). The
47 patients, comprising 29 males and 18 females, were between the
ages of 40 and 70 years (median 64). Of the 47 patients, 19 had
stage II disease and 28 had stage III disease. As shown in Table II, although the percentage of stage
III patients in the LAFR group (63.6%) was slightly higher than
that in the CRT group (56%), the difference was not significant
(P=0.595). No significant statistical difference was noted in other
patient characteristics such as gender, age, location in the
esophagus, KPS score, weight loss and largest tumor diameter.
 | Table IIPatients characteristics. |
Table II
Patients characteristics.
|
Characteristics | LAFR group | CRT group | Statistical
value | P-value |
|---|
| Gender | | |
χ2=0.099 | 0.753 |
| Male | 14 | 17 | | |
| Female | 8 | 8 | | |
| Age (years) | | | t=2.425 | 0.635 |
| Range | 53–70 | 40–70 | | |
| Median | 66 | 64 | | |
| Location in the
esophagus | | |
χ2=0.619 | 0.445 |
| Upper | 9 | 6 | | |
| Median | 12 | 17 | | |
| Lower | 1 | 2 | | |
| Clinical stage | | |
χ2=0.283 | 0.595 |
| II | 8 | 11 | | |
| III | 14 | 14 | | |
| KPS score | | | t=1.340 | 0.187 |
| Range | 60–90 | 60–90 | | |
| Median | 80 | 80 | | |
| Weight loss | | |
χ2=0.091 | 0.763 |
| Yes | 12 | 10 | | |
| No | 10 | 15 | | |
| Largest tumor
diameter (cm) | 3.8±1.2 | 4.1±1.9 | t=0.457 | 0.639 |
All 47 patients were followed up until they
succumbed to the disease or until the time of the last follow-up
evaluation. Until August 31, 2008, no patients were lost to
follow-up. The median follow-up time for the patients was 17 months
(range 4–25).
Treatment compliance
In the LAFR group, one patient refused subsequent
treatment after receiving an irradiation dose of 57 Gy due to
personal reasons, while the remaining patients completed the
treatment as planned. In the CRT group, 5 patients failed to
complete all four cycles of chemotherapy (including one who
received only 32 Gy of irradiation). The planned treatment was
terminated for the following reasons: esophago-mediastinal fistula
in one patient who only completed one cycle of chemotherapy and
received only 32 Gy of irradiation; intolerable fatigue and
gastrointestinal adverse events in one patient who completed three
cycles of chemotherapy; disease progression in one patient who
completed one cycle of chemotherapy; and refusal of subsequent
treatment after symptom remission in two patients. Of note is that
although the patient developing esophago-mediastinal fistula
completed only one cycle of chemotherapy and received only 32 Gy of
irradiation, he was included in the evaluation of treatment
efficacy, since his symptom of swallowing difficulty was
significantly relieved. Subsequently, all 47 patients were
subjected to the evaluation of efficacy and toxicity.
Survival and causes of death
Survival rates
For all 47 eligible patients, the median survival
time from treatment initiation was 21 months, whereas the 1- and
2-year survival rates were 70.2 and 45%, respectively. The median
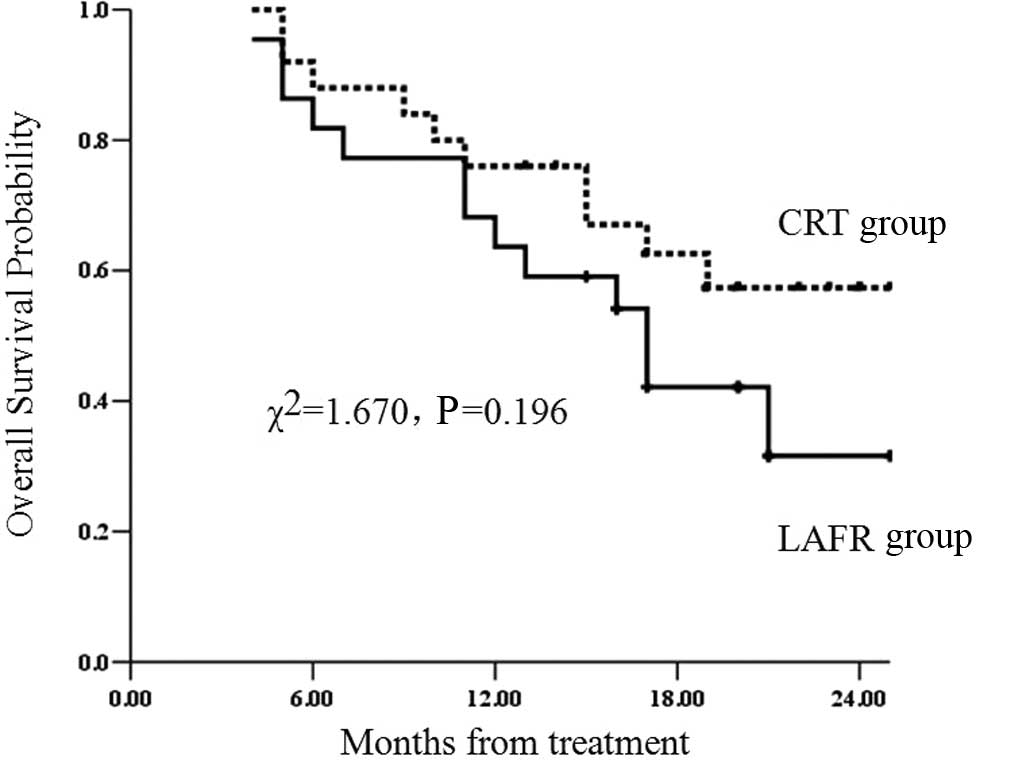
survival time in the LAFR group was 17 months, while that in the
CRT group was 21 months. The 1- and 2-year survival rates were 63.6
and 31.6%, respectively, in the LAFR group and 76 and 57.4%,
respectively, in the CRT group. The survival rate in the LAFR group
was lower than that in the CRT group, but the difference was not
significant (χ2=1.670; P=0.196) (Fig. 1).
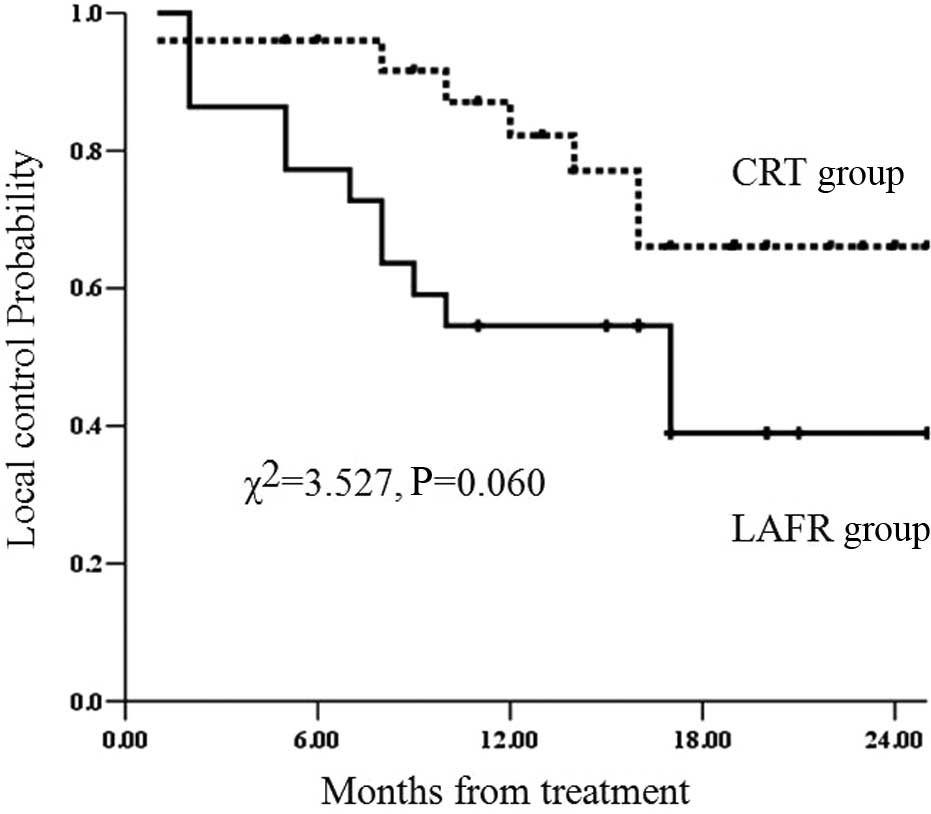
Local control rates
A total of 12 patients in the LAFR group and 7 in
the CRT group showed local control failure or recurrence. The
median local control in the LAFR group was 17 months, while the
corresponding result for the CRT group was not obtained. The 1- and
2-year local control rates were 54.5 and 39%, respectively, in the
LAFR group and 82.2 and 66.1%, respectively, in the CRT group. The
local control rate in the LAFR group was lower than that in the CRT
group, but the difference was not significant (χ2=3.527;
P=0.060) (Fig. 2).
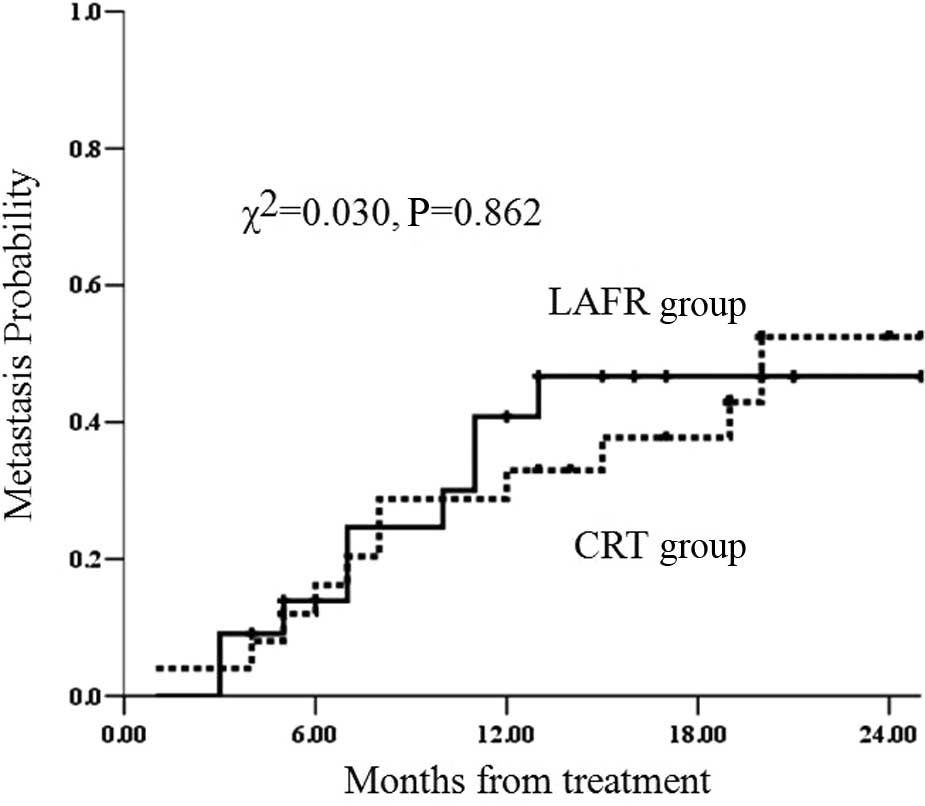
Metastasis rates
Nine patients in the LAFR group and 11 in the CRT
group showed distant metastasis. The median non-metastasis time in
the LAFR group was not obtained, while the value in the CRT group
was 20 months. The 1- and 2-year metastasis rates were 40.8 and
46.7%, respectively, in the LAFR group and 33 and 52.4%,
respectively, in the CRT group. The metastasis rates in the LAFR
and CRT groups were equal (χ2=0.030; P=0.862) (Fig. 3).
Causes of death
As of the last follow-up, 24 patients were alive and
23 had succumbed to the disease. A total of 13 patients in the LAFR
group had succumbed to the disease, including 6 (46.2%) who died of
local control failure, 2 (15.4%) of distant metastasis and 5
(38.5%) of a combination of the two causes. A total of 10 patients
in the CRT group had succumbed to the disease, including 5 (50%)
who died of distant metastasis, 2 (20%) of local control failure
and 3 (30%) of a combination of the two causes. Local control
failure was the major cause of death in the LAFR group, since it
contributed to 46.2% of deaths, while distant metastasis was the
major cause of death in the CRT group, contributing to 50% of
deaths.
Survival rates of patients with
different clinical stages
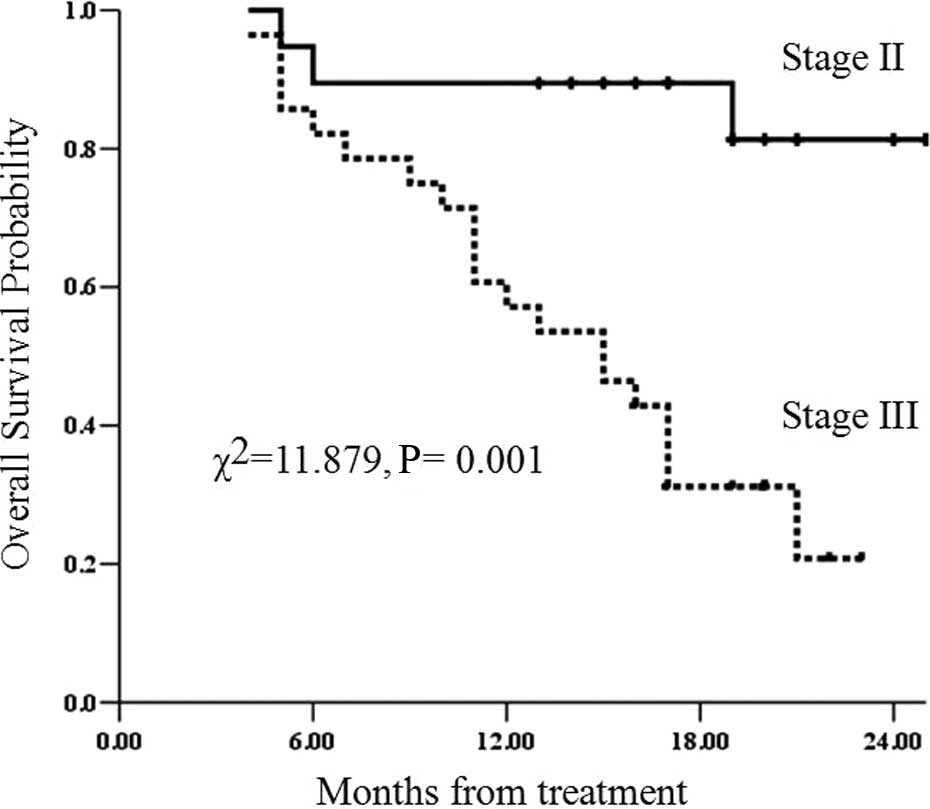
Of the whole group, the survival rate in patients
with stage II disease (CT-based staging) was significantly superior
to that of patients with stage III disease (χ2=11.879;
P=0.001) (Fig. 4).
Survival rates of patients with
clinical stage II
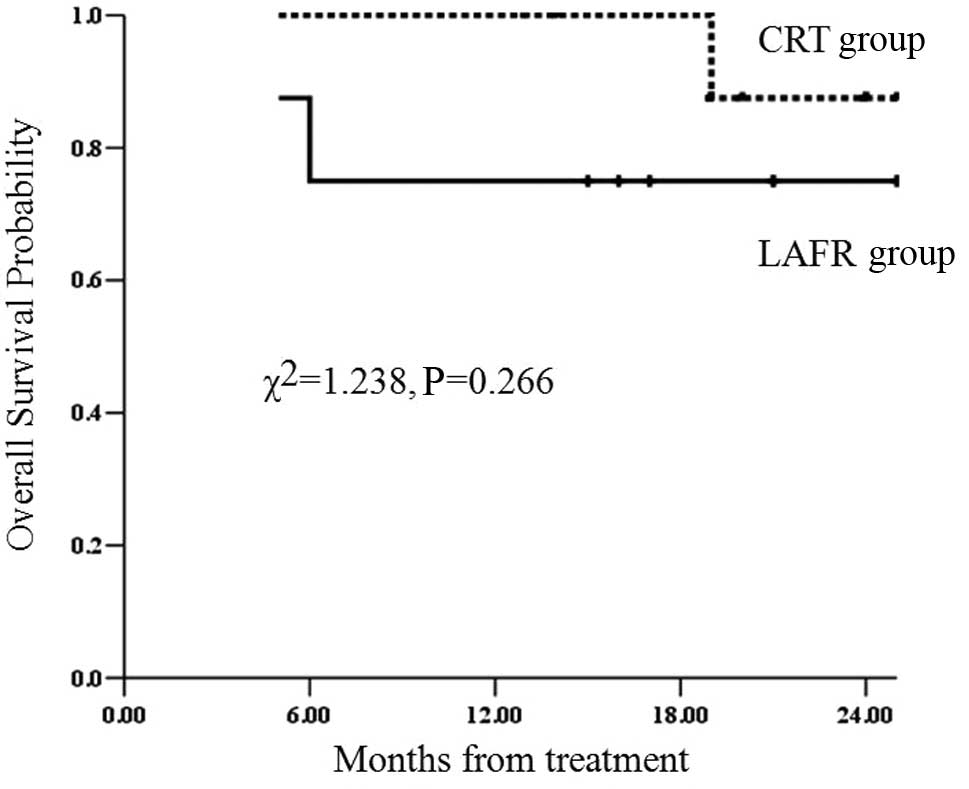
The 2-year survival rates of patients with clinical
stage II in the LAFR and CRT groups were 75 and 87.5%,
respectively. No significant difference was observed between the
two groups (χ2=1.238; P=0.266) (Fig. 5).
Survival rates of patients with
clinical stage III
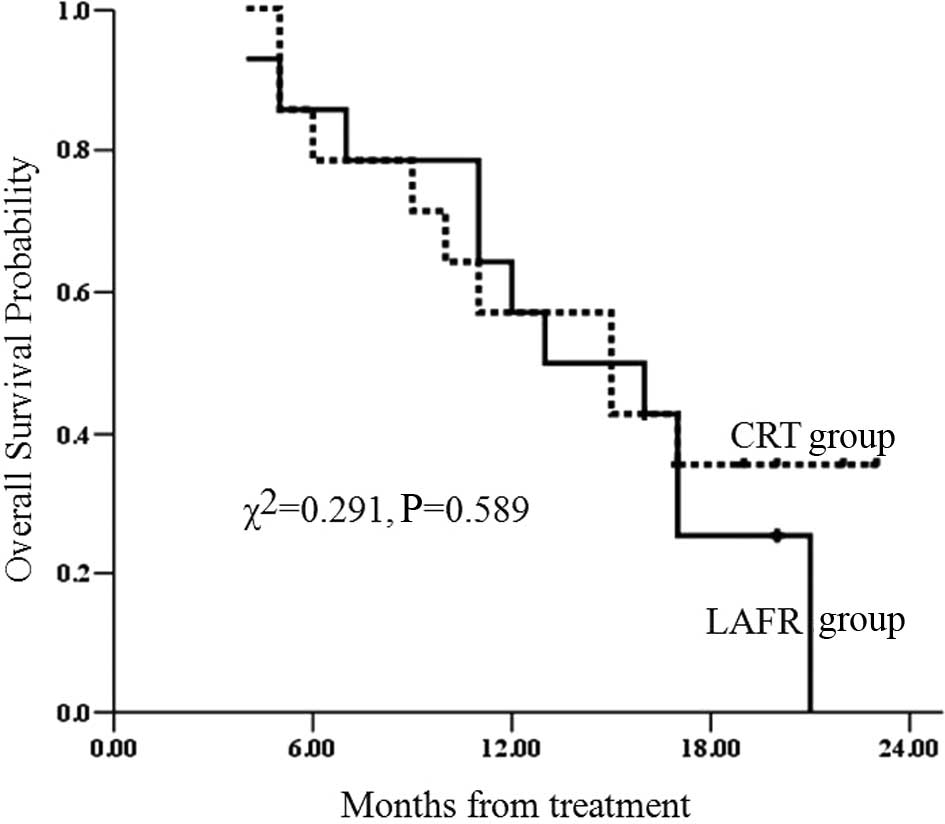
The 2-year survival rate of patients with clinical
stage III in the LAFR group was 0%. The 2-year overall survival
rate with clinical stage III in the CRT group has yet to be
determined; however, the 23-month survival rate was 35.7%. No
significant difference was observed between the two groups
(χ2=0.291; P=0.589) (Fig.
6).
Adverse events
The major acute adverse events observed in the two
groups were radiation-induced esophagitis and radiation-induced
pneumonia. No significant difference in the rates of these adverse
events was noted between the two groups. The rates of Grade III or
above radiation-induced esophagitis in the LAFR and CRT groups were
4.5 and 4%, respectively. Grade III or above radiation-induced
pneumonia was not observed. The rates of all hematological
toxicities, Grade III nausea and vomiting, and Grade III anorexia
were significantly lower in the LAFR group compared to the CRT
group. The results are shown in Table
III.
 | Table IIIAcute adverse events. |
Table III
Acute adverse events.
| Acute adverse
event | LAFR group (%) | CRT group (%) |
χ2-test | P-value |
|---|
| Radiation-induced
esophagitis | | | 1.177 | 0.734a |
| 0 | 6 (27.3) | 4 (16.0) | | |
| I–II | 15 (68.2) | 20 (80.0) | | |
| III–IV | 1 (4.5) | 1 (4.0) | | |
| Radiation-induced
pneumonia | | | 2.736 | 0.203a |
| 0 | 16 (72.7) | 13 (52.0) | | |
| I | 6 (27.3) | 10 (40.0) | | |
| II | 0 (0.0) | 2 (8.0) | | |
| Hematology | | | 16.780 | 0.000a |
| 0 | 13 (59.1) | 1 (4.0) | | |
| I–II | 9 (40.9) | 20 (80.0) | | |
| III–IV | 0 (0.0) | 2 (8.0) | | |
| Nausea and vomiting
III | 0 (0.0) | 7 (28.0) | 9.913 | 0.002 |
| Anorexia III | 0 (0.0) | 9 (36.0) | 13.236 | 0.002 |
| Sarcitis/myasthenia
III | 0 (0.0) | 1 (4.0) | | 1.000a |
Grade V late esophageal injury was observed in only
one patient in the CRT group, while no other Grade III or above
late adverse events were observed. No significant difference in
late injury in the esophagus and lungs was noted between the two
groups. Serious late adverse events, such as radiation-induced
myelitis and radiation-induced pericarditis, were not observed. The
results are shown in Table IV.
 | Table IVLate adverse events. |
Table IV
Late adverse events.
| Late adverse
event | LAFR group (%) | CRT group (%) |
χ2-test | P-value |
|---|
| Late esophageal
injury | | | 2.491 | 0.507a |
| 0 | 12 (54.5) | 11 (44.0) | | |
| I | 9 (40.9) | 9 (36.0) | | |
| II | 1 (4.5) | 4 (16.0) | | |
| V | 0 (0.0) | 1 (4.0) | | |
| Late lung
injury | | | 0.435 | 0.805 |
| 0 | 15 (68.2) | 15 (20.0) | | |
| I | 6 (27.3) | 8 (32.0) | | |
| II | 1 (4.5) | 2 (8.0) | | |
Discussion
LAFR for esophageal carcinoma has been widely
adopted in China. Early studies (4,5) of
LAFR from various individual medical centers have reported
achieving a 5-year survival rate of more than 30%, which is
significantly superior to conventional fraction RT. However, a
direct comparison cannot be made between these outcomes due to the
different recruitment criteria and study background used in the
studies.
Most early studies of LAFR have two obvious
shortcomings. First, there was a selection bias; for example, a
Karnofsky performance status of ≥80 (14), the ability to have a semi-liquid
diet (14), lesions of esophagus ≤8
cm (4,6) or esophageal lumen not completely
obstructed (4). Moreover, no
original data exist relating to disease stage in those studies. If
patients with a 5-year survival rate of 33% were recruited in the
early stage, a favorable prognosis may have been obtained. Those
cases therefore did not represent all esophageal carcinomas of
different stages. Secondly, data regarding the TNM stage were not
available when patients were randomized into treatment and control
groups, thus the process used for randomization did not guarantee
stage balance between the two groups. Since TNM stage is a crucial
factor related to prognosis (15),
randomization without the stage factor renders these outcomes
(4–6) discouraging. A previous LAFR study
using a large sample group of 201 cases showed that LAFR achieved
3- and 5-year overall survival rates of 34 and 26%, respectively,
while the 3-year overall survival rate in this study was only the
same as the previous 5-year overall survival rate of 33% reported
from the same cancer center (4).
Two other studies (16,17) have reported long-term survival
outcomes from a well-known cancer center in China. The 5-year
overall survival rate was only approximately 20%. None of the
abovementioned survival outcomes are comparable to early results
(4,5), which have shown 5-year overall
survival rates of more than 30%. These survival rate results are
inconsistent. A significant reason for this inconsistency may be
the different stages of disease in the patients at the point when
they were recruited for the different studies.
Zhao et al (7) reported that LAFR provides a 5-year
overall survival rate of 26% and concluded that the LAFR regimen
offers a similar survival rate to standard chemoradiotherapy
(1,2). In this study, the esophageal
carcinomas were staged by CT and ultrasound, and more than 60% of
patients who began the study with stage I-IIA of the disease had
favorable survival outcomes. The survival rates of stage III
patients were not available in this study. Since most of the
patients with esophageal carcinoma had advanced disease at the time
of presentation (15,18), the results of this study do not
guarantee that LAFR provides a favorable outcome in the treatment
of esophageal carcinoma of all stages.
No results from multi-center randomized controlled
trials have confirmed the survival outcomes of LAFR thus far. The
role of LAFR in the treatment of esophageal carcinoma remains to be
evaluated. A multi-center prospective randomized controlled phase
III trial should therefore be conducted to compare the efficacy of
LAFR and CRT. Prior to this phase III trial, however, we conducted
this preliminary small-sample study to test the efficacy and side
effects of LAFR. Since no standard CRT schemas are currently
available in China, a phase I trial was performed to obtain the MTD
of CRT for Chinese patients with esophageal carcinoma (9). The MTD was used as the CRT regimen,
making it feasible to carry out this study.
Results of the present study showed that the overall
survival and local control rates of LAFR were lower than those of
CRT, but the difference was not significant. Due to the small
sample size and the relatively short follow-up period, the effects
of LAFR on survival remain to be elucidated. However, LAFR achieved
substantial efficacy in this study, considering that more than 60%
of patients were classified as having stage III disease. The 1- and
2-year overall survival rates in the LAFR group were 63.6 and
31.6%, respectively, while the 1- and 2-year local control rates
were 54.5 and 39%, respectively.
The survival rates of LAFR patients in our study was
not comparable to that reported previously (7). However, only 36.4% of patients were
classified as having stage II disease in our LAFR group, and this
proportion was much lower than that of the previous study, in which
more than 60% of patients had stage I–IIA of the disease. However,
the subgroup analyses showed that patients with stage II in the
LAFR group achieved favorable survival rates. The 2-year overall
survival rate in the LAFR group was 75%, similar to the 87.5% in
the CRT group (χ2=1.238; P=0.266). Therefore, LAFR was
found to be as effective as in a previous study for treating
early-stage esophageal carcinoma (7). The overall survival rate of stage II
patients in this study was also higher than that of stage III
patients (P=0.001). These results indicate that esophageal
carcinoma can be staged relatively accurately based on CT without
ultrasound. Moreover, stage II patients achieved similar survival
rates to those in a previous LAFR trial that recruited only T2N0M0
patients and for which the 2-year overall survival rate was 66.8%
(19). Therefore, LAFR results from
our study are consistent with those from previous studies (7–19), at
least with regards to early-stage esophageal carcinoma.
The incidence of hematological toxicities, severe
nausea and vomiting, and severe anorexia in the LAFR group was
found to be significantly lower than that in the CRT group,
indicating that LAFR is associated with fewer side effects in
patients, thus making it more clinically feasible. Since fewer side
effects were observed, patients in the LAFR group required less
supportive treatment. In addition, LAFR is more cost-effective than
CRT in China, which is of great significance to Chinese patients
presenting with esophageal carcinoma since the majority of patients
are from poverty-stricken regions.
In our study, the outcome of clinical stage III
patients was discouraging. The 2-year overall survival rate in the
LAFR group was 0%. On the other hand, although the 2-year overall
survival rate in the CRT group has yet to be determined, the
23-month survival rate was 35.7%. Consequently, it is imperative to
improve the outcome of clinical stage III patients. In patients
treated with LAFR, locoregional failure is one of the main patterns
of failure in esophageal carcinoma treatment, based on our study
and those of other authors (4,7).
Intensity-modulated radiotherapy (IMRT) technology improves the
irradiation dose for tumors while sparing normal tissues (20). Wang et al (21) have reported that the results of an
IMRT study for esophageal carcinoma and the preliminary survival
outcomes were encouraging. If IMRT technology is used in the LAFR
regimen, higher doses may be delivered to the tumors and better
local control may be obtained. More and more molecular-targeted
therapies are used for esophageal carcinoma due to the
over-expression of epidermal growth factor receptors associated
with this disease (22,23). In the future, LAFR with IMRT
technology combined with cetuximab (C225), gefitinib and erlotinib
is a potential treatment modality for patients with esophageal
carcinoma. However, in the present study, the major cause of death
in the CRT group was distant metastasis, indicating that for CRT
more attention should be focused on developing new chemotherapeutic
drugs, such as paclitaxel (24,25)
and irinotecan (26), to reduce
metastasis.
In our small-sample exploratory study, the overall
survival and local control rates of LAFR patients were lower than
those of CRT patients, but the difference was not significant. LAFR
achieved a positive outcome for clinical stage II patients, similar
to those for CRT. LAFR is also more cost-effective than CRT.
Considering that more than 60% of patients were classified as
having stage III disease, LAFR achieved substantial efficacy with
fewer side effects when compared to CRT. Based on this study, the
effectiveness of LAFR in treating esophageal cancer is currently
being evaluated in a prospective phase III trial.
References
|
1
|
Herskovic A, Martz K, al-Sarraf M,
Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L
and Emami B: Combined chemotherapy and radiotherapy compared with
radiotherapy alone in patients with cancer of the esophagus. N Engl
J Med. 326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer. Long-term
follow-up of a prospective randomized trial (RTOG 85-01). JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishida K, Ando N, Yamamoto S, Ide H and
Shinoda M: Phase II study of cisplatin and 5-fluorouracil with
concurrent radiotherapy in advanced squamous cell carcinoma of the
esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical
Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 34:615–619.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi XH, Yao W and Liu T: Late course
accelerated fractionation in radiotherapy of esophageal carcinoma.
Radiother Oncol. 51:21–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han C, Yang XR, Gao XS, Zhou DA and Wan J:
Late course accelerated hyperfractionated radiotherapy for
esophageal carcinoma. Chin J Radiat Oncol. 8:1921999.
|
|
6
|
Sheng XF, Mei BD, Chai MZ, Shen XM, Gu HG
and Song MZ: Late course accelerated hyperfractionation
radiotherapy (LCAHR) for middle esophageal carcinoma: clinical
phase III Trial. Chin J Radiat Oncol. 7:86–89. 1998.
|
|
7
|
Zhao KL, Shi XH, Jiang GL and Wang Y:
Late-course accelerated hyperfractionation radiotherapy for
localized esophageal carcinoma. Int J Radiat Oncol Biol Phys.
60:123–129. 2004. View Article : Google Scholar
|
|
8
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Q, Gao XS, Qiao XY, Zhou ZG, Zhang P,
Chen K, Zhao YN and Asaumi J: Phase I trial of escalating-dose
cisplatin with 5-fluorouracil and concurrent radiotherapy in
Chinese patients with esophageal cancer. Acta Med Okayama.
62:37–44. 2008.PubMed/NCBI
|
|
10
|
Gao XS, Qiao XY, Yang XR, Asaumi J, Zhou
ZG, Wang YD, Zhou DA, Wan J, Kuroda M, Kishi K, Kawasaki S and
Hiraki Y: Late course accelerated hyperfractionation radiotherapy
concomitant with cisplatin in patients with esophageal carcinoma.
Oncol Rep. 9:767–772. 2002.
|
|
11
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
Shipley W and Curran W: Common toxicity criteria: version 2.0. an
improved reference for grading the acute effects of cancer
treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys.
47:13–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blum JL, Jones SE, Buzdar AU, LoRusso PM,
Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS and Griffin T:
Multicenter phase II study of capecitabine in paclitaxel-refractory
metastatic breast cancer. J Clin Oncol. 17:485–493. 1999.PubMed/NCBI
|
|
14
|
Shi XH, Wu GD and Liu XW: Comparison of
treatment results between conventional fractionation and
late-course accelerated fractionation for esophageal carcinoma.
Chin J Radiat Oncol. 3:150–153. 1994.
|
|
15
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao XY, Zhou ZG, Gao XS, Ma GX and Wang
J: Comparison between conventional fractionation, late-course
accelerated hyperfractionation and external beam combined with
intracavitary radiation in the treatment of locally moderate and
advanced esophageal carcinoma. Chin J Radiat Oncol. 13:261–264.
2004.
|
|
17
|
Qiao XY, Gao XS, Zhou ZG, Wang X, Zhang J,
Zhang P and Yang XR: Retrospective analysis on results of
late-course accelerated hyperfractionation radiotherapy and
conventional fractionation radiotherapy plus brachytherapy for
esophageal carcinoma. Chin J Radiat Oncol. 15:182–184. 2006.
|
|
18
|
Ajani JA: Contributions of chemotherapy in
the treatment of carcinoma of the esophagus: results and
commentary. Semin Oncol. 21:474–482. 1994.PubMed/NCBI
|
|
19
|
Zhao KL, Wang Y and Shi XH: Late course
accelerated hyper-fractionated radiotherapy of upper and middle
thoracic esophageal T2N0M0 carcinoma. Chin J Radiat Oncol.
24:80–82. 2002.PubMed/NCBI
|
|
20
|
Nutting CM, Bedford JL, Cosgrove VP, Tait
DM, Dearnaley DP and Webb S: A comparison of conformal and
intensity-modulated techniques for oesophageal radiotherapy.
Radiother Oncol. 61:157–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Han C, Li XN, Gao C, Jia JH, Cai
BN, Zhang X and Xiao AQ: Short-term efficacy of intensity-modulated
radiotherapy on esophageal carcinoma. Chin J Cancer. 28:1138–1142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valverde CM, Macarulla T, Casado E, Ramos
FJ, Martinelli E and Tabernero J: Novel targets in gastric and
esophageal cancer. Crit Rev Oncol Hematol. 59:128–138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taira N, Doihara H, Oota T, Hara F, Shien
T, Takahashi H, Yoshitomi S, Ishibe Y and Shimizu N: Gefitinib, an
epidermal growth factor receptor blockade agent, shows additional
or synergistic effects on the radiosensitivity of esophageal cancer
cells in vitro. Acta Med Okayama. 60:25–34. 2006.
|
|
24
|
Safran H, Gaissert H, Akerman P, Hesketh
PJ, Chen MH, Moore T, Koness J, Graziano S and Wanebo HJ:
Paclitaxel, cisplatin, and concurrent radiation for esophageal
cancer. Cancer Invest. 19:1–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brenner B, Ilson DH, Minsky BD, Bains MS,
Tong W, Gonen M and Kelsen DP: Phase I trial of combined-modality
therapy for localized esophageal cancer: escalating doses of
continuous- infusion paclitaxel with cisplatin and concurrent
radiation therapy. J Clin Oncol. 22:45–52. 2004. View Article : Google Scholar
|
|
26
|
Ilson DH, Bains M, Kelsen DP, O’Reilly E,
Karpeh M, Coit D, Rusch V, Gonen M, Wilson K and Minsky BD: Phase I
trial of escalating-dose irinotecan given weekly with cisplatin and
concurrent radiotherapy in locally advanced esophageal cancer. J
Clin Oncol. 21:2926–2932. 2003. View Article : Google Scholar : PubMed/NCBI
|