Introduction
Gastric carcinoma is the fourth most prevalent
malignancy worldwide (1) and is one
of the most common causes of cancer-related mortality in China
(2). The only effective way of
improving the survival rate of patients suffering from gastric
carcinoma is an early diagnosis. More than half of patients
presenting with gastric carcinoma diagnosed from a biopsy were in
an advanced stage of cancer progression. Thus, a more critical
understanding of preneoplastic lesions in the gastric mucosa may
prove helpful in the detection of early-stage carcinoma in a
clinical setting. The development of gastric carcinoma is closely
associated with the presence of atrophic gastritis as well as
intestinal metaplasia (IM). Furthermore, it has been shown that the
presence of IM significantly increases the risk of gastric
carcinoma (3). However, not all
individuals with intestinal metaplasia progress to gastric cancer.
Therefore, the issue as to which IM patients should be clinically
followed has yet to be resolved (4).
Three types (I–III) of IM have been described
according to morphology and mucin histochemistry (5,6).
Findings of a number of studies revealed that type III was closely
involved in intestinal gastric carcinoma (7–10),
whereas investigations that have been conducted conclude that
sulphomucin-positive IM does not identify a high-risk group and is
of no value in the surveillance of gastric cancer (11). Additionally, Petersson et al
suggest that type III IM is a less sensitive indicator of future
development of gastric carcinoma than previously anticipated
(12).
As in our previous study, in this study IM was
divided into two subtypes according to morphology of the
metaplastic epithelium: simple intestinal metaplasia (SIM), in
which no or minimal polymorphism was found and atypical intestinal
metaplasia (AIM), in which metaplastic cells presented
significantly more polymorphisms or atypical changes (13). The predominant feature of AIM is the
appearance of immature goblet cells scattered in the epithelium of
IM already present in the gastric mucosa. These cells exhibit an
enlarged cell nucleus with several mucin vacuoles in the cytoplasm
and the nucleus loses its polarity. As with the immature goblet
cells, changes are also observed in some columnar cells. In
contrast, no or minimal nuclear pleomorphism and polarity change of
goblet and columnar cells in SIM has been observed. The key to
distinguishing the two groups of IM is the identification of the
immature goblet cells since there was little difficulty in
comparing the columnar cell atypical changes in the two groups. The
growth pattern in AIM was more focal and showed fewer Paneth cells
than those in SIM; partially serrated growth with predominant
nucleoli was observed in AIM.
Different subtypes of IM in relation to tumor
suppressor gene p53 (expressed in AIM) were investigated in
the present study and compared with the expression of its
downstream target gene murine double minute gene 2 (mdm2).
Under normal physiological conditions, the wild-type p53 protein
suppresses cell proliferation, stops cell division at the G1
checkpoint, and promotes the repair of damaged DNA. p53 protein
also initiates apoptosis in case of failed DNA repair. In the
absence of normally functioning wild-type p53 protein, cells are
sensitive to the S-phase entry with injured DNA and the genetic
changes, resulting in cell malignant change and tumor formation. As
a negative regulator of p53, mdm2 interacts with p53 protein to
inhibit the transcriptional activation of p53, leading to cell
proliferation in a tumor. The interaction between p53 and mdm2
plays a crucial role in cell cycle arrest, as well as apoptosis
following DNA damage (14,15). The wild-type p53 gene induces
the expression of mdm2 protein, which, in turn, strictly regulates
the p53 protein level. By depriving p53 of antineoplastic activity,
a high expression of mdm2 can obstruct the p53-mediated
transactivation (16). This
p53-mdm2 feedback loop is vital for cell-cycle regulation, and the
elimination of p53-dependent checkpoints is associated with
carcinogenesis (17,18).
SIM and AIM classification may better indicate the
essential nature of IM. SIM, due to its hyperplastic nature induced
in gastritis, was devoid of cellular pleomorphism as well as
molecular diversity. On the other hand, AIM possessed atypia
initiated by an oncogene during carcinogenesis. This classification
was performed to determine which type of IM is more relevant to
gastric carcinoma.
Materials and methods
Subjects
Informed consent was obtained from each patient and
the study was approved by the institutional ethics committee of the
Shandong University School of Medicine. We obtained 58 samples of
IM from endoscopic biopsy and 30 gastric carcinoma specimens by
surgical resection at Qilu Hospital of Shandong University. Of 88
patients studied, 59 were males and 29 females, with ages ranging
from 30 to 80 years of age (median 54.56). Additionally, in the 58
cases of IM, 38 patients were male and 20 female, with an age range
of 30–76 years (median 55.88), while in the 30 gastric carcinoma
cases, 21 patients were male and 9 female, with an age range of
30–80 years (median 52.00).
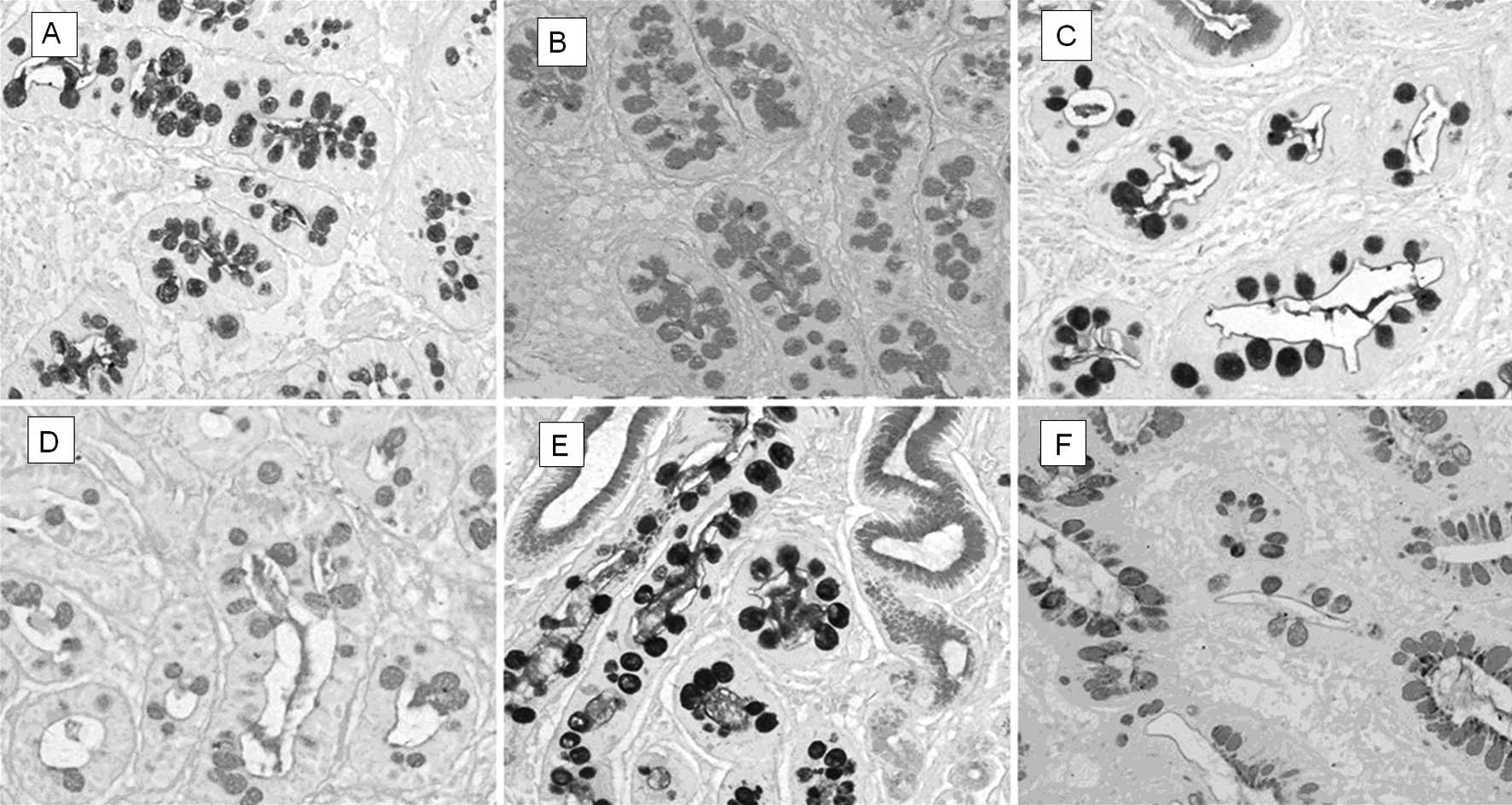
Mucin histochemical staining for IM subtypes was
performed. Serial sections were cut and stained with hematoxylin
and eosin. To determine mucin expression patterns, alcian blue pH
2.5/periodic acid-Schiff and high-iron-diamine (AB pH 2.5/PAS, AB
pH 2.5/HID) were performed to identify IM subtypes (19). A total of 58 cases of IM were
classified in accordance with the system used by Jass and Filipe
(6,20): type I (complete) IM goblet cells
secrete predominantly acid sialomucins; type II (incomplete) IM
goblet cells secrete predominantly sialomucins; columnar mucous
cells secrete non-sulfated mucins; type III (incomplete) IM
resembles type II except that columnar mucous cells predominantly
secrete sulfomucins (Fig. 1).
IM subtyping according to morphology and
structural changes
A total of 58 IM cases were classified as AIM and
SIM according to the morphology with or without structural and
cellular changes of pleomorphism in the metaplastic epithelium.
Immunohistochemical staining
Immunohistochemical staining was performed using the
Envision method. Expression analyses of p53 and mdm2 proteins were
performed on formalin-fixed, paraffin-embedded specimens. p53
monoclonal antibody (ZM-0408) and mdm2 (ZM-0425) as well as PV-9000
2-step plus poly-HRP anti-mouse/rabbit IgG detection system were
purchased from ZSGB-Bio (Beijing, China).
Briefly, 4 μm sections were cut and dewaxed in
xylene and then rehydrated through graded alcohol. For the antigen
retrieval regimen, all slides were microwaved in 10 mmol/l sodium
citrate buffer (pH 6.0) at 10-min intervals for a total of 20 min.
The endogenous peroxidase activity was blocked by 10 min of
incubation with 3% hydrogen peroxidase (reagent A) at room
temperature. After washing in phosphate-buffered saline (PBS), the
sections were incubated with monoclonal mouse anti-human antibodies
p53 (ZM-0408) and mdm2 (ZM-0425) overnight at 4°C. The sections
were washed with PBS and incubated with polymerase auxiliary
(reagent B) for 20 min. After washing in PBS, the sections were
incubated with biotinylated HRP-conjugated secondary antibody
(reagent C) for 30 min at room temperature and 3,3′-diamino-
benzidine was visualized. Tissues were counterstained with
hematoxylin. A negative control used PBS instead of the primary
antibodies.
Immunohistochemical analysis
Positive nuclear staining of p53 and mdm2 proteins
appeared as brown granules. The percentage of positively stained
cells was estimated in an average of 100 cells counted in more than
5 high-magnification fields (high-power field, ×400). A positive
result was defined as ≥10% of the cells exhibiting positive
staining.
Statistical analysis
The significance of association was determined using
the analysis of variance (ANOVA) or χ2 test. P<0.05
was considered to be statistically significant.
Results
Subtype distribution of 58 IM
samples
Since IM was multifocally present, 19 (32.75%) cases
with foci of type I IM, 26 (44.82%) cases of type II IM, and 13
(22.41%) cases of type III IM were identified. In 31 (53.44%) SIM
samples, there were 9 type I, 15 type II and 7 type III IM samples.
In 27 (46.55%) AIM samples, there were 10 type I, 11 type II and 6
type III IM samples (Table I).
 | Table IThe distribution of 58 IM cases with
different subtypes of intestinal metaplasia. |
Table I
The distribution of 58 IM cases with
different subtypes of intestinal metaplasia.
| Type I | Type II | Type III | Total (%) |
|---|
| SIM | 9 | 15 | 7 | 31 (53.45) |
| AIM | 10 | 11 | 6 | 27 (46.55) |
| Total (%) | 19 (32.76) | 26 (44.83) | 13 (22.41) | 58 (100.00) |
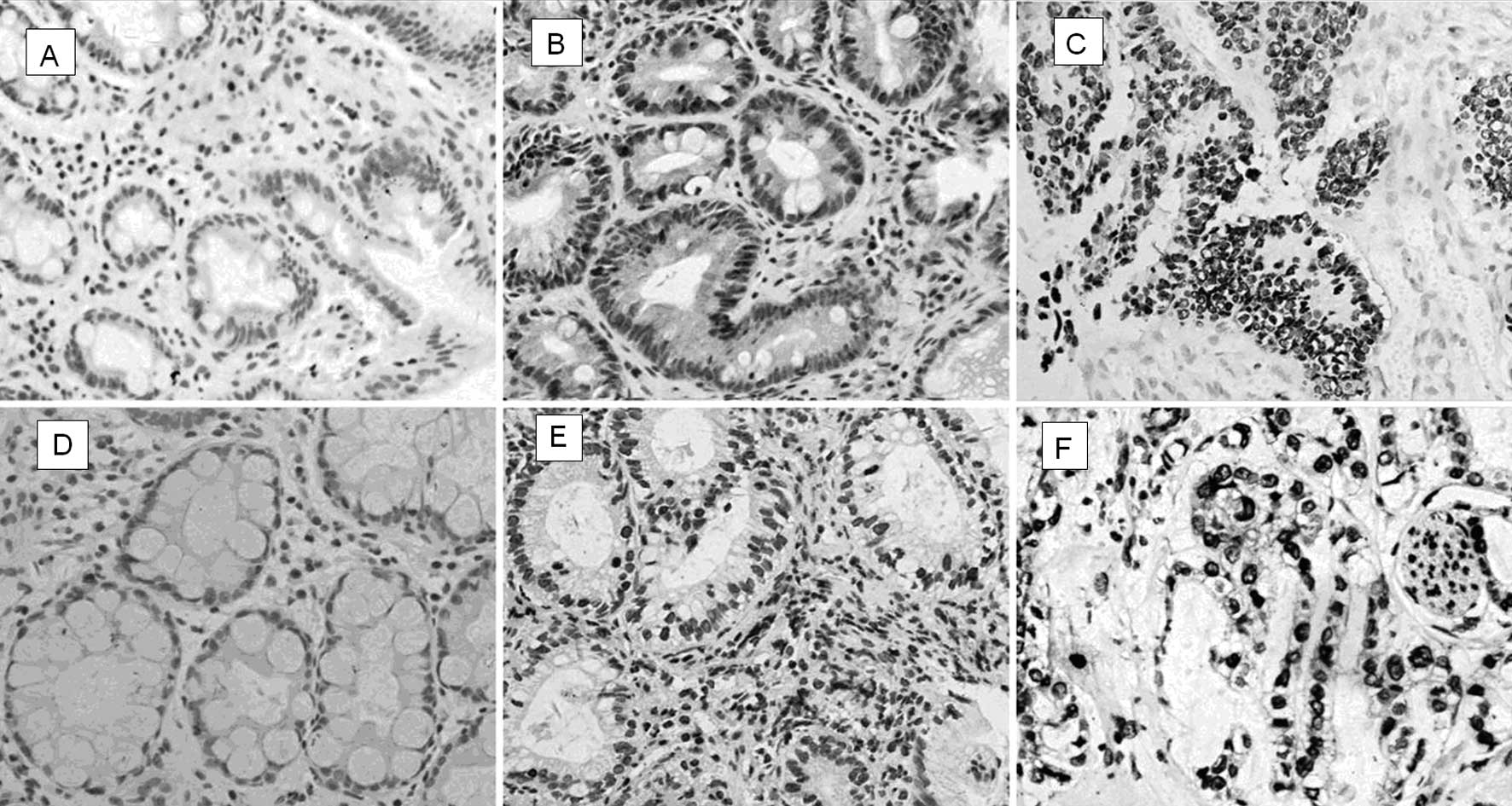
Expression of p53 protein
A positive expression of p53 was observed in 25.81%
(8/31) of SIM, 51.85% (14/27) of AIM and 56.67% (17/30) of gastric
carcinomas. Expression of p53 in gastric carcinomas and AIM was
higher than that in SIM (P<0.05) while no significant difference
was observed between AIM and gastric carcinomas (P>0.05).
p53 observed in the conventional IM was expressed in
26.32% (5/19) of type I, 42.31% (11/26) of type II and 46.15%
(6/13) of type III IM. A reduced expression of p53 was only found
in type I IM (P<0.05) while no difference was observed between
gastric carcinomas and types II or III IM (P>0.05) (Table II; Fig.
2).
 | Table IIExpression of p53 and mdm2 proteins in
different subtypes of IM and gastric carcinoma. |
Table II
Expression of p53 and mdm2 proteins in
different subtypes of IM and gastric carcinoma.
| Total (n) | p53 | mdm2 |
|---|
|
|
|---|
| Positive (%) | Negative | Positive (%) | Negative |
|---|
| SIM | 31 | 8a (25.80) | 23 | 6a (19.35) | 25 |
| AIM | 27 | 14 (51.85) | 13 | 14 (51.85) | 13 |
| Type I | 19 | 5b (26.31) | 14 | 7 (36.84) | 12 |
| Type II | 26 | 11 (42.30) | 15 | 10 (38.46) | 16 |
| Type III | 13 | 6 (46.15) | 7 | 3 (23.07) | 10 |
| Gastric
carcinoma | 30 | 17 (56.66) | 13 | 16 (53.33) | 14 |
Expression of mdm2 protein
Positive expression rates of mdm2 were observed in
SIM 19.35% (6/31), AIM 51.85%, (14/27) and gastric carcinomas
53.33% (16/30), respectively. The expression of mdm2 in gastric
carcinomas and AIM was significantly higher than that in SIM
(P<0.05), while no significant difference was found between AIM
and gastric carcinomas (P>0.05) (Table II, Fig.
2).
The positive expression rates of mdm2 in types I, II
and III of IM were 36.84% (7/19), 38.46% (10/26) and 23.07% (3/13),
respectively. No statistical significance was found in the
expression of mdm2 among the gastric carcinomas and the three types
(I, II and III) of IM (P>0.05).
Discussion
Intestinal metaplasia (IM) occurs in benign lesions
as well as in carcinoma of the gastric mucosa, but only a small
proportion of cases are disposed towards or develop into carcinoma.
The relationship between IM and gastric carcinoma has yet to be
elucidated, as has the issue of which type of IM is closely related
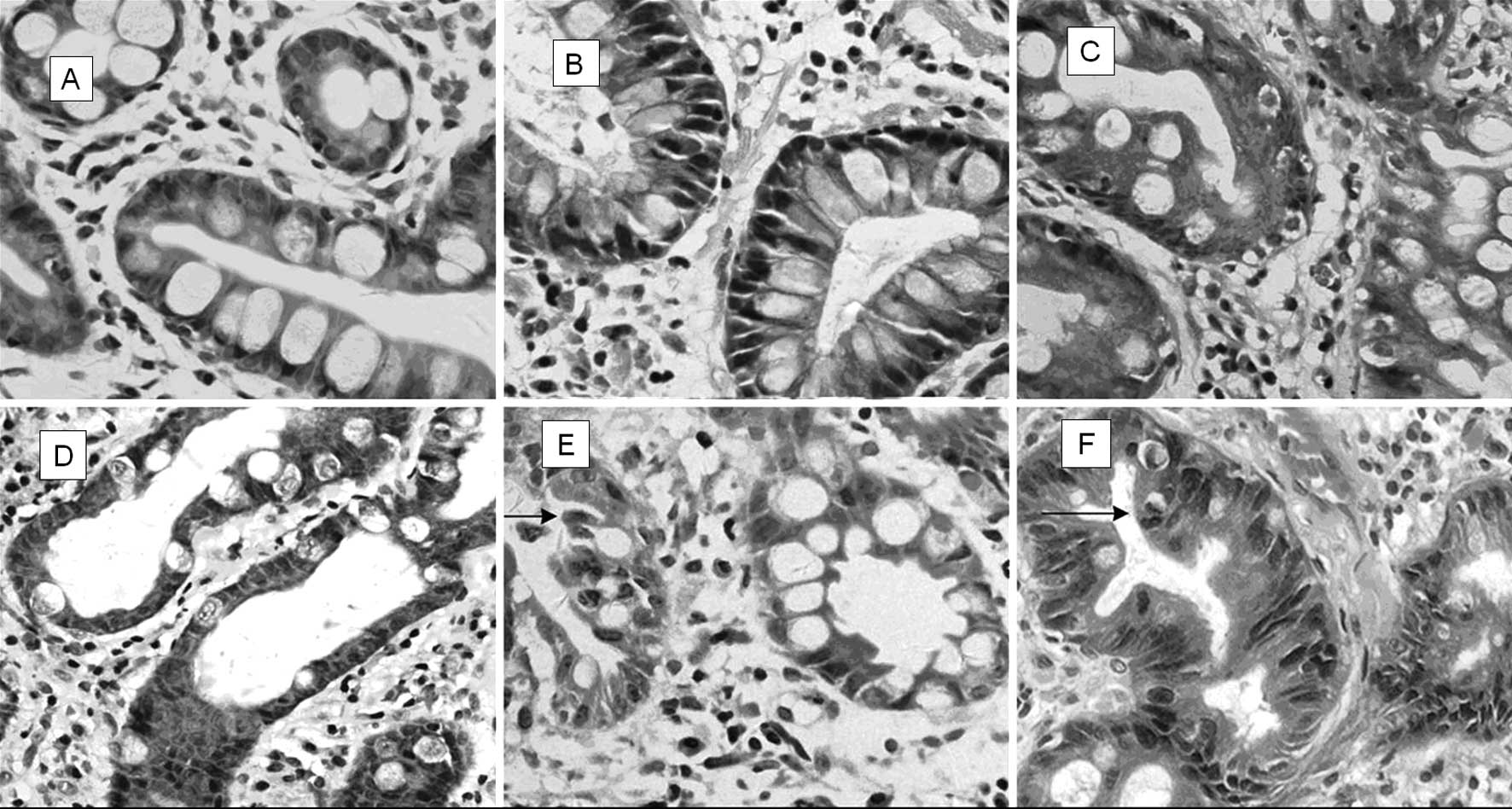
to gastric carcinoma. In this study, IM in the gastric mucosa was
classified into two groups: SIM (simple intestinal metaplasia, IM
with minimal or no pleomorphism), and AIM (atypical intestinal
metaplasia, IM with atypical changes) (13). Various differences between the two
groups were observed (Table III,
Fig. 3).
 | Table IIIThe differences in morphology between
SIM and AIM. |
Table III
The differences in morphology between
SIM and AIM.
| SIM | AIM |
|---|
| Tissue
organization | Glands arranged
neatly with uniform goblet cells | Glands arranged
irregularly, enlarged round nuclei with polarity changes |
| Goblet cells | Minimal changes
resemblance to normal intestine | Immature
differentiation, polarity changes and rare mitosis |
| Column cells | Minimal changes
resemblance to normal intestine | Enlarged nuclei
with foamy cytoplasm. |
| Gastric pit | The mucosa is
flat | Ruffled mucosa with
deeper pit, appeared to serrated change |
There are three substantial advantages to this novel
classification of IM into AIM and SIM as compared to conventional
classification. Firstly, the novel classification is easy and
practical for the pathologist to diagnose under microscope without
supplementary staining. Secondly, the cellular morphologic variants
of IM usually coordinate with molecular changes as well as
progression, since classification of AIM as such clarifies its
precancerous nature. Thirdly, the classification is not only a
diagnostic method, but also a theory that may promote the
understanding of IM along with clinically pragmatic activities in
the follow-up of IM patients such as ‘optical biopsy’ of gastric
mucosa, for which IM was diagnosed under confocal endoscopy without
histopathological biopsy (21,22).
As for the conventional classification of IM, our data show that
both AIM and SIM distributed in all three types, whereas intrinsic
precancerous information was obscured by the conventional
classification of IM based on mucous chemical staining.
p53, a tumor suppressor gene, plays an
important role in the regulation of gene expression in cell cycle
progression and apoptosis in response to DNA damage (23). Mutation in the p53 gene is
one of the most prevalent genetic abnormalities in human cancer
(24), including gastric carcinoma.
A number of studies suggest that gastric precancerous lesions
exhibit p53 abnormalities, particularly in the early stages
of intestinal metaplasia (25–27).
In this study, the expression of p53 was significantly higher in
gastric carcinoma than that in type I, but not in types II or III
IM. The positive expression rates of p53 in SIM, AIM and gastric
carcinomas were 25.81, 51.85 and 56.67%, respectively, and the
expression of p53 in gastric carcinomas was significantly higher in
AIM as compared to SIM. Results of various studies also showed that
the frequency of p53 mutations is consistent at around 40% in early
and advanced differentiated gastric carcinomas as well as advanced
undifferentiated carcinomas (28,29),
whereas it is low in early undifferentiated carcinomas (30,31).
The finding that p53 mutations were detected in IM and
gastric cancer (32) suggests that
IM is a precursor of differentiated carcinomas. Therefore, our data
confirm the hypothesis that p53 gene mutation is an early
event in the development of gastric carcinoma.
mdm2, a newly discovered oncogene, is located
at chromosome 12q13–14; the gene’s organization on the human
chromosome was evaluated in 1992 (33). Due to its association with the tumor
suppressor p53 gene and its implication in human cancer, the
biochemical activities of mdm2 in cancer cells have been
investigated thoroughly. A high expression of the mdm2 protein has
been found in 36% of all types of sarcomas and 10% of
well-differentiated gliomas, as well as esophageal cancer,
neuroblastoma, and anaplastic astrocytoma (34), suggesting that mdm2 expression is
one of the common causes of oncogenesis (35). In our study, the expression of mdm2
in gastric carcinomas was not significantly higher than that in
types I, II and III of IM, while it was significantly higher in AIM
as compared to SIM (the positive expression rates of mdm2 in SIM,
AIM and gastric carcinomas were 19.35, 51.85 and 53.33%,
respectively). Findings of previous studies have suggested that the
expression of mdm2 increases in gastric carcinoma (36,37).
Nakajima et al (3) reported
that expression of the mdm2 protein increased not only in gastric
cancer, but also in intestinal metaplasia as compared to chronic
gastritis. A distinct mdm2 expression was observed in our study,
indicating the precancerous nature of AIM more accurately than
mucous classification. Further investigation into the level of p53
and mdm2 expression through RT-PCR is required. However, the main
objective of this study was to observe p53 and mdm2 expression
through routine immunohistochemical studies as these methods are
readily available in the clinical environment.
Since the expression of p53 and mdm2 proteins was
found to be significantly higher in AIM and gastric carcinomas as
compared to SIM, our findings revealed that AIM had more atypia at
the molecular level than that of SIM, together with more
morphological disturbances. Additionally, compared to SIM, AIM has
greater potential to progress to a severe precancerous lesion or
gastric cancer. Findings of the present study therefore suggest
that SIM is a response to stimuli such as inflammation, whereas AIM
may undergo malignant transformation and be considered a
preneoplastic lesion (13). This is
an effective criterion by which to diagnose early gastric
carcinomas in the clinical follow-up of AIM patients. However, the
exact pathogenesis or pathways of AIM in the multi-step
carcinogenesis of the gastric mucosa has yet to be elucidated.
Effective analysis of diagnostic samples is required to draw a
definitive conclusion. Therefore, further studies with a larger
patient population and a thorough retrospective follow-up using a
rigorous research design are required.
Acknowledgements
This study was funded by a program from Shandong
Province Science and Technology Committee Dept. of Health
(2006TK47).
References
|
1
|
Burkitt MD, Varro A and Pritchard DM:
Importance of gastrin in the pathogenesis and treatment of gastric
tumors. World J Gastroenterol. 15:1–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL
and Zhang X: Detection of micrometastasis of gastric carcinoma in
peripheral blood circulation. World J Gastroenterol. 10:804–808.
2004.PubMed/NCBI
|
|
3
|
Nakajima N, Ito Y, Yokoyama K, Uno A,
Kinukawa N, Nemoto N and Moriyama M: The expression of murine
double minute 2 (MDM2) on helicobacter pylori-infected intestinal
metaplasia and gastric cancer. J Clin Biochem Nutr. 44:196–202.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung WK and Sung JJ: Review article:
intestinal metaplasia and gastric carcinogenesis. Aliment Pharmacol
Ther. 16:1209–1216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jass JR: Role of intestinal metaplasia in
the histogenesis of gastric carcinoma. J Clin Pathol. 33:801–881.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jass JR and Filipe MI: A variant
intestinal metaplasia associated with gastric carcinoma: a
histochemical study. Histopathology. 3:191–199. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dixon MF: Campylobacter and chronic
gastritis. Campylobacter and Gastroduodenal Disease. Rathbone BJ
and Heatley RV: Blackwell Scientific Publications; Oxford: pp.
1061–1116. 1989
|
|
8
|
Sipponen P and Hyvainen H: Role of
helicobacter pylori in the pathogenesis of gastric peptic
ulcer and gastric cancer. Scand J Gastroenterol Suppl. 196:3–6.
1993.
|
|
9
|
Dixon MF: Pathophysiology of
helicobacter pylori infection. Scand J Gastroenterol Suppl.
201:7–10. 1994.
|
|
10
|
Rothery GA and Day DW: Intestinal
metaplasia in endoscopic biopsy specimens of gastric mucosa. J Clin
Pathol. 38:613–621. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ectors N and Dixon MF: The prognostic
value of sulphomucin positive intestinal metaplasia in the
development of gastric cancer. Histopathology. 10:1271–1277. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petersson F, Borch K and Franzén LE:
Prevalence of subtypes of intestinal metaplasia in the general
population and in patients with autoimmune chronic atrophic
gastritis. Scand J Gastroenterol. 37:262–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng Y, Wang L, Zhang JP, Yang JY, Zhao
ZM and Zhang XY: Expression of p53, c-erbB-2 and Ki67 in intestinal
metaplasia and gastric carcinoma. World J Gastroenterol.
16:339–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barak Y, Juven T, Haffner R and Oren M:
mdm2 expression is induced by wild type p53 activity. EMBO J.
12:461–468. 1993.PubMed/NCBI
|
|
15
|
Momand J and Zambetti GP: Mdm-2: ‘big
brother’ of p53. J Cell Biochem. 64:343–352. 1997.
|
|
16
|
Lev Bar-Or R, Maya R, Segel LA, Alon U,
Levine AJ and Oren M: Generation of oscillations by the p53-Mdm2
feedback loop: a theoretical and experimental study. Proc Natl Acad
Sci USA. 97:11250–11255. 2000.PubMed/NCBI
|
|
17
|
Leach FS, Tokino T, Meltzer P, Burrell M,
Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW and Vogelstein
B: p53 mutation and MDM2 amplification in human soft tissue
sarcomas. Cancer Res. 53:2231–2234. 1993.PubMed/NCBI
|
|
18
|
Leite KR, Franco MF, Srougi M, Nesrallah
LJ, Nesrallah A, Bevilacqua RG, Darini E, Carvalho CM, Meirelles
MI, Santana I and Camara-Lopes LH: Abnormal expression of MDM2 in
prostate carcinoma. Mod Pathol. 14:428–436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reis CA, David L, Nielsen PA, Clausen H,
Mirgorodskaya K, Roepstorff P and Sobrinho-Simões M:
Immunohistochemical study of MUC5AC expression in human gastric
carcinomas using a novel monoclonal antibody. Int J Cancer.
74:112–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jass JR and Filipe MI: The mucin profile
of normal gastric epithelium, intestinal metaplasia and gastric
carcinoma. Histochem J. 13:831–939. 1981.
|
|
21
|
Hwang JH: To perform a biopsy or not to
perform a biopsy? Does confocal endomicroscopy provide the answer
for surveillance in Barrett’s esophagus? Gastrointest Endosc.
70:655–657. 2009.
|
|
22
|
Liu H, Li YQ, Yu T, Zhao YA, Zhang JP,
Zhang JN, Guo YT, Xie XJ, Zhang TG and Desmond PV: Confocal
endomicroscopy for in vivo detection of microvascular architecture
in normal and malignant lesions of upper gastrointestinal tract. J
Gastroenterol Hepatol. 23:56–61. 2008.PubMed/NCBI
|
|
23
|
Vogelstein B and Kinzler KW: p53 function
and dysfunction. Cell. 70:523–526. 1992. View Article : Google Scholar
|
|
24
|
Harris AL: Mutant p53 - the commonest
genetic abnormality in human cancer? J Pathol. 162:5–6. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Correa P and Shiao YH: Phenotypic and
genotypic events in gastric carcinogenesis. Cancer Res.
54:1941–1943. 1994.PubMed/NCBI
|
|
26
|
Tahara E, Kuniyasu H, Yasui W and Yokozaki
H: Gene alterations in intestinal metaplasia and gastric cancer.
Eur J Gastroenterol Hepatol. 6:97–101. 1994.
|
|
27
|
Shiao YH, Ruqqe M, Correa P, Lehmann HP
and Scheer WD: p53 alteration in gastric precancerous lesions. Am J
Pathol. 144:511–517. 1994.PubMed/NCBI
|
|
28
|
Uchino S, Noguchi M, Ochiai A, Saito T,
Kobayashi M and Hirohashi S: p53 mutation in gastric cancer: a
genetic model for carcinogenesis is common to gastric and
colorectal cancer. Int J Cancer. 54:759–764. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maesawa C, Tamura G, Suzuki Y, Ogasawara
S, Sakata K, Kashiwaba M and Satodate R: The sequential
accumulation of genetic alterations characteristic of the
colorectal adenoma-carcinoma sequence does not occur between
gastric adenoma and adenocarcinoma. J Pathol. 176:249–258. 1995.
View Article : Google Scholar
|
|
30
|
Tamura G, Sato K, Akiyama S, Tsuchiya T,
Endoh Y, Usuba O, Kimura W, Nishizuka S and Motoyama T: Molecular
characterization of undifferentiated-type gastric carcinoma. Lab
Invest. 81:593–598. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ranzani GN, Luinetti O, Padovan LS,
Calistri D, Renault B, Burrel M, Amadori D, Fiocca R and Solcia E:
p53 gene mutations and protein nuclear accumulation are early
events in intestinal type gastric cancer but late events in diffuse
type. Cancer Epidemiol Biomarkers Prev. 4:223–231. 1995.PubMed/NCBI
|
|
32
|
Ochiai A, Yamauchi Y and Hirohashi S: p53
mutations in the non-neoplastic mucosa of the human stomach showing
intestinal metaplasia. Int J Cancer. 69:28–33. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliner JD, Kinzler KW, Meltzer PS, George
DL and Vogelstein B: Amplification of a gene encoding a
p53-associated protein in human sarcomas. Nature. 358:80–83. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bueso-Ramos CE, Manshouri T, Haidar MA,
Huh YO, Keating MJ and Albitar M: Multiple patterns of MDM-2
deregulation in human leukemias: implications in leukemogenesis and
prognosis. Leuk Lymphoma. 17:13–18. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iwakuma T and Lozano G: MDM2, an
introduction. Mol Cancer Res. 1:993–1000. 2003.
|
|
36
|
Günther T, Schneider-Stock R, Häckel C,
Kasper HU, Pross M, Hackelsberger A, Lippert H and Roessner A: Mdm2
gene amplification in gastric cancer correlation with expression of
Mdm2 protein and p53 alterations. Mod Pathol. 13:621–626.
2000.PubMed/NCBI
|
|
37
|
Tanière P, Martel-Planche G, Maurici D,
Lombard-Bohas C, Scoazec JY, Montesano R, Berger F and Hainaut P:
Molecular and clinical differences between adenocarcinomas of the
esophagus and of the gastric cardia. Am J Pathol. 158:33–40.
2001.PubMed/NCBI
|