Introduction
Non-Hodgkin’s lymphoma (NHL) is one of a large group
of cancers of the immune system. Incidence and mortality rates for
NHL are currently on the increase worldwide (1). The standard treatment for NHL is
chemotherapy. However, relapse eventually occurs and subsequent
failure of chemotherapy to control cancer has prompted the
development of alternative therapies (2). Mounting evidence shows that adoptive
immunotherapy with genetically modified T cells expressing chimeric
T-cell receptors targeting lymphoma-associated antigens is a
promising approach for treating this group of diseases. CD20 is
exclusively expressed in B-cell lineage and is minimally modulated
or shed from the cell surface on more than 95% of B-cell lymphomas
at high copy numbers. CD20 is also minimally internalized on
binding antibodies (Ab) (3). These
characteristics make CD20 an attractive target for immunotherapy.
Engineered T cells expressing a single chain anti-CD20 Ab fused to
the T-cell receptor complex CD3ζ chain display redirecting
MHC-unrestricted CD20-specific lymphoma cell cytolysis (4). The CD3ζ chain was previously shown to
be sufficient for mediating T-cell activation signals (5). CD3 and CD28 signals were found to be
fundamental for cellular proliferation and antigen-induced
interleukin-2 (IL-2) secretion of grafted T cells in an anti-CEA
scFv mediated T-cell adoptive immunotherapy study (6). The two signals can be delivered by one
recombinant receptor molecule (6,7).
Previously, we reported that engineered CD20-specific T cells,
particularly lysed CD20-positive target tumor cells, secreted IFN-γ
and IL-2 after binding to their target cells. However,
modifications of the cellular signaling pathways in target tumor
cells by engineered CD20-specific T cells have yet to be fully
elucidated.
In the present study, we observed that treatment of
NHL Raji cells with engineered CD20-specific T cells inhibited p38
MAPK and NF-κB activities and resulted in the inhibition of IL-10
secretion, phosphor (p)-STAT3 and Bcl-2 expression. In addition,
the activity of engineered T cells expressing anti-CD20scFvFc or
anti-CD20scFvFc/CD28/CD3ζ in vitro was investigated.
Materials and methods
Cell culture and plasmid DNA
The Burkitt lymphoma cell line, Raji, was cultured
in RPMI-1640 with 10% fetal bovine serum (FBS; Invitrogen,
Carlsbad, CA, USA) at 37°C in a humidified 5% CO2
incubator. The pLNCX vector containing anti-CD20scFvFc and
anti-CD20scFvFc/CD28/CD3ζ was constructed as previously described
(8).
Generation of genetically modified T
cells
Peripheral T-cell lymphomas (PTLs) were isolated
from normal donor blood as previously described (7,8).
Briefly, peripheral blood mononuclear cells from healthy donors
were isolated by Ficoll-Paque (GE Healthcare, Little Chalfont, UK)
density gradient centrifugation and cultured in RPMI-1640
containing 10% FBS, 1 μg/ml PHA-L (Roche, Basel, Switzerland), 30
ng/ml OKT3 (Wuhan Institute of Biological Products, Wuhan, China)
and 50 U/ml rhIL-2 (Sigma-Aldrich, St. Louis, MO, USA). After 10
days of sustainable culture, cells were analyzed by flow cytometry
using a Simultest Imk-Lymphocyte kit (BD, Franklin Lakes, NJ, USA).
Plasmid transfection of T cells occurred when >90% of the cells
were positive for CD3. PTLs (5×107) were mixed with 10
mg/ml salmon sperm DNA (Invitrogen) and 100 μg linearized plasmid
DNA. Cells were electroporated with Bio-Rad Gene Pulser Xcell at
300 V, 960 μA for 2 min. Approximately 48 h after electroporation,
cells were selected by 800 μg/ml G418 for 7 days and G418-resistant
PTLs were successfully transfected with recombinant gene for use in
this study.
Cytotoxicity assays
The cytolytic activity of engineered CD20-specific T
cells was quantitated using a Cytotoxicity Detection kit (Roche,
Indianapolis, IN, USA) according to the manufacturer’s instructions
and by employing Raji cells as target cells. The target Raji cells
were co-cultured with engineered CD20-specific T cells (E/T ratio
was 10), and incubated at 37°C for 0, 4, 8, 12, 18, 24 or 48 h. The
cytotoxicity assay results were obtained from three independent
experiments performed in triplicate and the percentage of specific
cytotoxicity was calculated.
IL-10 secretion assay
Engineered CD20-specific T cells were co-cultured
with stimulator Raji cells in 24-well assay plates following
incubation. Culture supernatants were harvested and used to detect
the IL-10 level using Cytokine ELISA assays (R&D, Minneapolis,
MN, USA), according to the manufacturer’s instructions.
Western blotting
Raji cells were treated with engineered
CD20-specific T cells and CD3-negative Raji cells were sorted by
flow cytometry for the Western blot analysis. Western blots were
carried out as previously described (8,9). The
primary antibodies used were polyclonal antibodies against p38,
p-p38, STAT3, p-STAT3, Lyn, p-Lyn, GAPDH and BCL-2 (Cell Signal
Technology, MA, USA). Alternatively, whole cell lysates of
engineered CD20-specific T cells were probed with a mouse
anti-human CD3ζ mAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
(7).
Electrophoretic mobility shift assay for
NF-κB and SP-1
Nuclear extracts from Raji cells by NE-PER Nuclear
and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific Inc.,
Rockford, IL, USA) were used to detect Sp1 and NF-κB.
Electrophoretic mobility shift assay (EMSA) was performed using a
LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific
Inc., Rockford, IL, USA) according to the manufacturer’s
instructions. The following double-stranded oligonucleotides were
used as the probe, SP1: 5′-ATT CGA TCG GGG CGG GGC GAG-3′ and
3′-TAA GCT AGC CCC GCC CCG CTC-5′; NF-κB (2): 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and
3′-TCA ACT CCC CTG AAA GGG TCC G-5′.
Statistical analysis
The data of each series of experiments were
expressed as the mean ± SD. Statistical differences between groups
were analyzed using ANOVA analysis. P<0.05 was considered to be
statistically significant.
Results
Cytotoxicity of engineered CD20-specific
T cells for targeting Raji cells
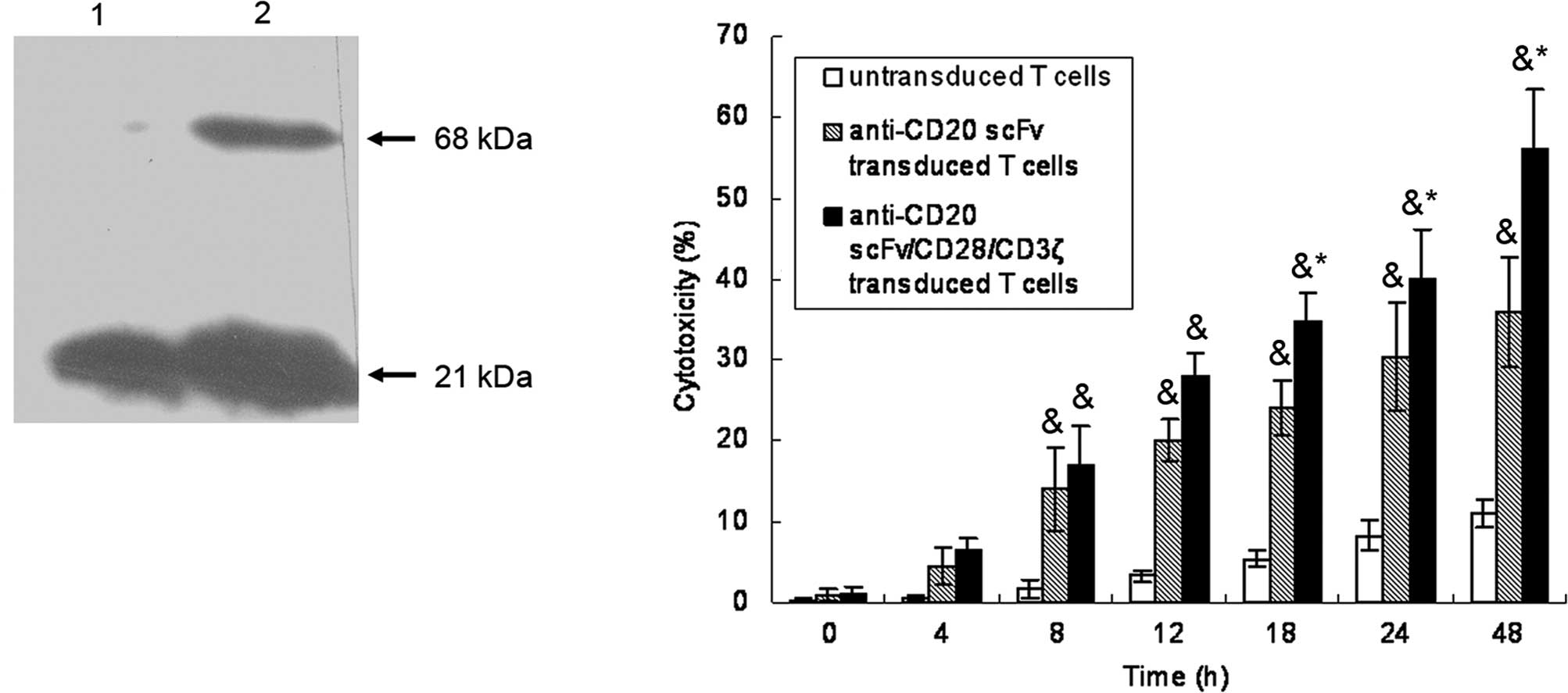
PTLs were transfected with vectors encoding
anti-CD20scFvFc or anti-CD20scFvFc/CD28/ζ. The successful
expression of the recombinant anti-CD20scFvFc/CD28/CD3ζ protein was
detected on anti-CD20scFvFc/CD28/CD3ζ-transfected PTLs by Western
blot analysis of CD3ζ expression (Fig.
1A). A 21-kDa band corresponding to wild-type CD3ζ was present
in the cell lysates of anti-CD20scFvFc or
anti-CD20scFvFc/CD28/CD3ζ-transfected PTLs. As shown in Fig. 1B,
anti-CD20scFvFc/CD28/CD3ζ-transfected PTLs lysed CD20-positive Raji
cells with higher efficacy than anti-CD20scFvFc-transfected PTLs,
whereas untransfected PTLs had little effect on targeted cells.
Treatment of Raji cells with engineered
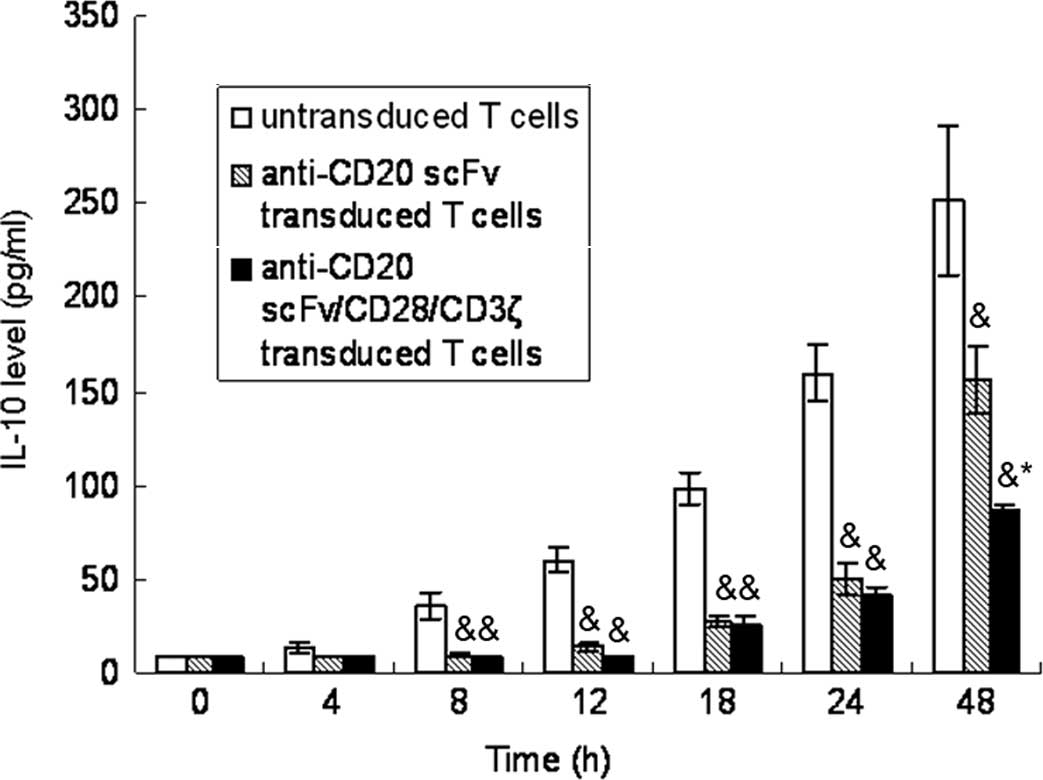
CD20-specific T cells inhibited IL-10 secretion
As shown in Fig. 2,
IL-10 levels were up-regulated in a time-dependent manner in Raji
cells following co-cultivation with untransfected T cells, whereas
anti-CD20scFvFc or anti-CD20scFvFc/CD28/CD3ζ-transfected T cells
led to the inhibition of IL-10 secretary in Raji cells. The
treatment of Raji cells with anti-CD20scFvFc/CD28/CD3-transfected T
cells had a greater effect on the inhibition of IL-10 secretary
than the treatment of Raji cells with anti-CD20scFvFc-transfected T
cells.
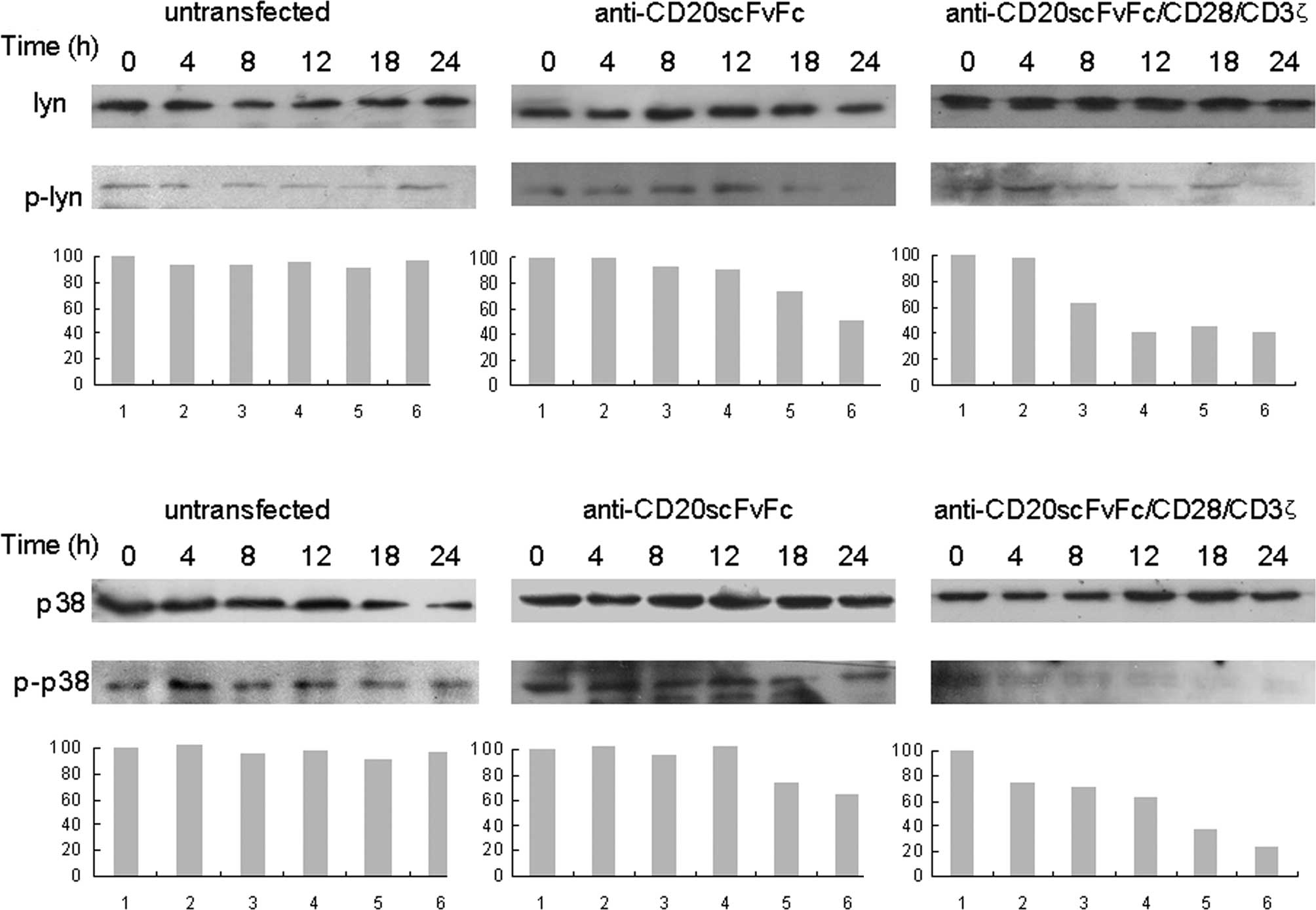
Treatment of Raji cells with engineered
CD20-specific T cells down-regulated p38 MAPK activity
Time kinetics analysis revealed that
anti-CD20scFvFc/CD28/CD3ζ-transfected T cells inhibited p-Lyn as
early as 8 h and was maximal at 24 h in Raji cells by Western
blotting (Fig. 3A).
Anti-CD20scFvFc-transfected T cells inhibited p-Lyn as early as 18
h and maximally at 24 h in Raji cells. Similarly, the treatment of
Raji cells with anti-CD20scFvFc/CD28/CD3ζ-transfected T cells
markedly inhibited p-p38 as early as 4 h, whereas the treatment of
Raji cells with anti-CD20scFvFc-transfected T cells slightly
decreased p-p38 at 18 and 24 h (Fig.
3B). Treatment of Raji cells with modified or unmodified T
cells showed no effect on the expression of Lyn and p38.
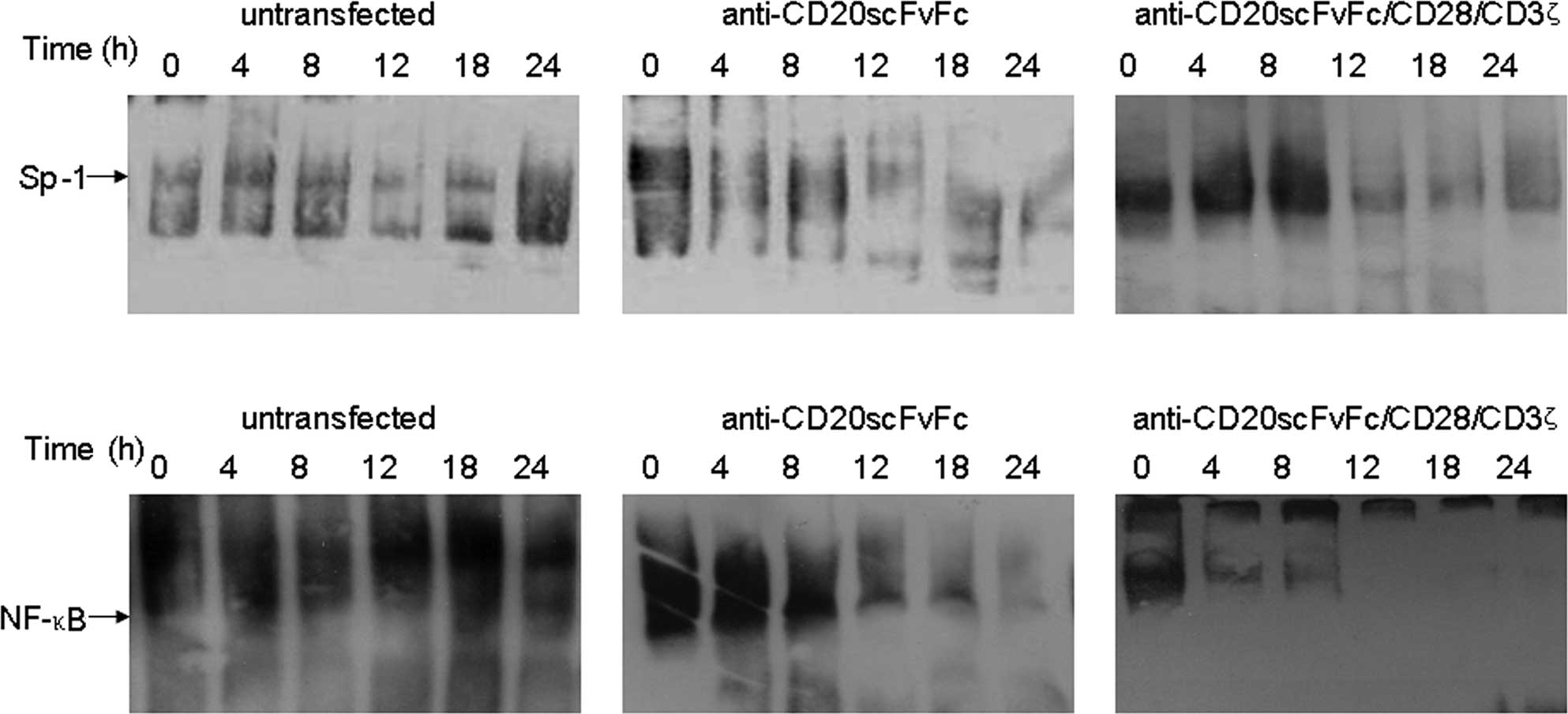
Treatment of Raji cells with engineered
CD20-specific T cells decreased Sp1 and NF-κB DNA-binding
activities
Gel shift assays revealed that the DNA-binding
efficiency of Sp1 was significantly reduced in Raji cells treated
with engineered CD20-specific T cells at 12 h (Fig. 4A). Additionally, the treatment of
Raji cells with engineered CD20-specific T cells inhibited NF-κB
DNA-binding activity. Anti-CD20scFvFc/CD28/CD3ζ-transfected T cells
inhibited Raji cell NF-κB DNA-binding activity as early as 4 h and
was maximal at 24 h (Fig. 4B). On
the other hand, anti-CD20scFvFc-transfected T cells inhibited Raji
cell NF-κB DNA-binding activity as early as 12 h. Treatment of Raji
cells with anti-CD20scFvFc/CD28/CD3ζ-transfected T cells showed a
higher inhibition of Sp1 and NF-κB DNA-binding activities than
treatment of Raji cells with anti-CD20scFvFc-transfected T
cells.
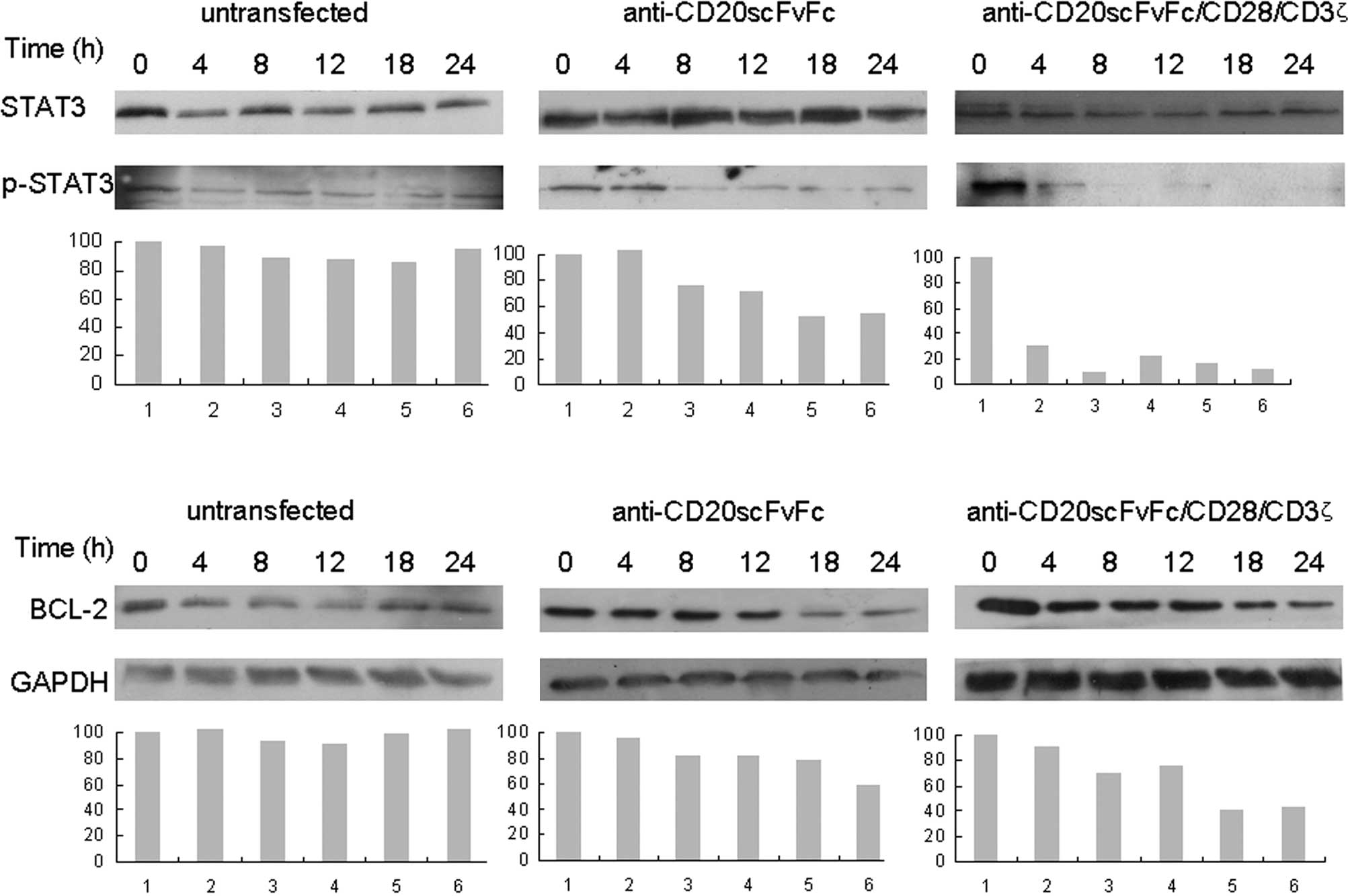
Treatment of Raji cells with engineered
CD20-specific T cells inhibited phosphor-STAT3 and Bcl-2
expression
Fig. 5 shows that
the treatment of Raji cells with anti-CD20scFvFc- or
anti-CD20scFvFc/CD28/CD3ζ-transfected T cells inhibited p-STAT3 as
early as 8 and 4 h, respectively, and was maximal at 24 h (Fig. 5A). We observed that treatment of
Raji cells with engineered CD20-specific T cells significantly
inhibited Bcl-2 expression (Fig.
5B). Additionally, treatment of Raji cells with
anti-CD20scFvFc/CD28/CD3ζ-transfected T cells demonstrated a higher
inhibition of p-STAT3 and Bcl-2 expression than treatment of Raji
cells with anti-CD20scFvFc-transfected T cells.
Discussion
Adoptive T-cell immunotherapy of cancer is based on
grafting cytotoxic T cells with chimeric antigen receptors
constituting a tumor-specific single chain antibody (scFv) and a
cellular activation intracellular signaling domain (10). Genetic modification of T cells with
integral membrane scFv chimeric signaling receptors reacted with
tumor-associated antigens in a non-MHC-restricted manner, thereby
bypassing the MHC/peptide complex loss, which is a significant
escape mechanism for most tumors (10–12).
The intracellular signaling domain is derived from the cytoplasmic
part of a membrane-bound receptor to induce cellular activation,
e.g., CD3ζ chain, which appears to have the most potency and to be
sufficient for mediating T-cell activated signals (6). Tumor-specific cytotoxic T cells were
successfully generated by the introduction of a chimeric T-cell
antigen receptor gene, consisting of an extracellular scFv and an
intracellular part of a signaling molecule (CD3ζ) (13). Although engagement of ζ chains is
sufficient to induce tumoricidal activity, without adequate
co-stimulatory signals, it may not suffice to elicit substantial
lymphocyte activation (3).
Accumulated evidence has shown that T cells modified with chimeric
antigen receptors incorporating a CD28 signaling domain are much
more active when tested in vitro and in murine models
(6,10,12,14).
In the present study, a recombinant
anti-CD20scFvFc/CD28/CD3ζ gene was constructed that provided both
primary and costimulatory signals to T cells through the one
chimera. This recombinant gene comprises the extracellular scFv
linked to transmembrane and intracellular signaling domains of CD28
and CD3ζ in tandem. Our group (7)
has reported that engineered CD20-specific T cells, particularly
lysed CD20-positive target tumor cells, produced not only IFN-γ,
but also IL-2 cytokines after binding to their target cells.
Cytolysis of CD20-positive target cells by engineered CD20-specific
T cells is antigen-specific since CD20-negative target cells (K562)
were not lysed (7). Results of this
study showed that the treatment of Raji cells with engineered
CD20-specific T cells significantly inhibited IL-10 secretary.
Treatment of Raji cells with anti-CD20scFvFc/CD28/CD3ζ-transfected
T cells had a greater effect on the inhibition of IL-10 secretary
than treatment of Raji cells with anti-CD20scFv-transfected T
cells. Serum levels of IL-10 are elevated in a number of patients
with NHL and a high IL-10 is associated with a poor rate of
survival (15). Exogenous IL-10
significantly increases NHL tumor cell proliferation (16). As a protective factor, IL-10
enhances growth progression and aids in the pathogenesis of NHL
through autocrine/paracrine loops (16–18).
The MAP kinase signaling pathway is involved in the
regulation of IL-10 production in Burkitt lymphoma cell lines
(19,20). Elevated levels of activated MAP
kinase have been observed in a variety of solid tumors and
hematologic malignancies, and MAP kinases may be important in the
development of new therapeutic approaches (2,20). The
anti-CD20 monoclonal antibody rituximab triggers and modifies
various intracellular signaling pathways in NHL B-cell lines,
resulting in the induction of apoptosis and chemosensitization
(2,21,22).
Alas et al (18) reported
that rituximab down-regulated tumor-derived IL-10 transcription and
subsequently down-regulated Bcl-2 gene expression. Previous
findings suggest that IL-10 plays a role in the expression of the
anti-apoptotic Bcl-2 gene product via STAT3 activation (16,23).
Rituximab induces the inhibition of IL-10 transcription and
secretion. Rituximab also induces the inhibition of STAT3 activity
and Bcl-2 expression, as well as sensitization to the apoptotic
effects of various chemotherapeutic drugs, through the p38 MAPK
pathway (2). Thus, our study
examined p38 MAPK signaling pathways mediated by genetically
modified CD20-specific T cells in the Raji cell line. Engineered
CD20-specific T cells inhibited p-Lyn and p38 MAPK activities, and
decreased Sp1 and IL-10 levels in targeted Raji-cells. In addition,
genetically-modified T cells reduced NF-κB DNA-binding activities
and down-regulated p-STAT3 and Bcl-2 expression levels. These data
demonstrated the role of p38 MAPK and NF-κB signaling pathways in
the regulation of IL-10 transcription.
Vega et al (2) reported that the down-regulation of
NF-κB activity induced by rituximab was mediated through the p38
MAPK signaling pathway, and that phosphor-Lyn and p38 MAPK
activities were inhibited by rituximab, resulting in the inhibition
of IL-10 transcription via Sp1 (2).
Consequently, down-regulation of the autocrine/paracrine loop of
IL-10/IL-10R signaling partially inhibited p-STAT3 and Bcl-2
expression (2). Sp1 transcription
factor is activated by p38 MAPK and Sp1 is involved in the
regulation of IL-10 expression in a number of cell lines (24). Moreover, our study showed that the
treatment of Raji cells with engineered T cells expressing
anti-CD20scFvFc/CD28/CD3ζ led to a stronger inhibition of p38 MAPK
activity and down-regulation of Bcl-2 expression and IL-10
secretary than the treatment of Raji cells with engineered T cells
expressing anti-CD20scFvFc. These results demonstrate that CD3ζ and
CD28 co-stimulation signaling fusion with anti-CD20 immunoreceptor
in grafted T cells synergistically enhances target cytotoxicity,
inhibits p38 MAPK activity and decreases IL-10 secretary in the
target tumor cells.
In conclusion, our results showed that treatment of
Raji cells with engineered CD20-specific T cells inhibited p38 MAPK
and NF-κB DNA-binding activities, resulting in the inhibition of
IL-10 secretion, p-STAT3 and Bcl-2 expression. These results
indicated that modifications of the cellular p38 MAPK signaling
pathways in target cells by engineered CD20-specific T cells may be
crucial for the antitumor effect of adoptive T-cell therapy.
Findings of the abovementioned studies showed that combining
chimeric CD3ζ and CD28 with an anti-CD20 immunoreceptor in grafted
T cells led to improved activity in vitro, compared to
anti-CD20 immunoreceptor alone in grafted T cells. This process is
therefore expected to substantially enhance the antitumor efficacy
of genetically-modified T cells. However, it should be noted that
this study has examined only the non-Hodgkin’s lymphoma Raji cell
line, in vitro. Thus, further investigation of the activity
of these modified T cells in vivo is required.
Acknowledgements
The authors would like to thank Drs Daming Shan and
Hinrich Abken for kindly donating the pLNCX and pBULLET
vectors.
References
|
1
|
Vega MI, Huerta-Yepez S, Jazirehi AR,
Garban H and Bonavida B: Rituximab (chimeric anti-CD20) sensitizes
B-NHL cell lines to Fas-induced apoptosis. Oncogene. 24:8114–8127.
2005.PubMed/NCBI
|
|
2
|
Vega MI, Huerta-Yepaz S, Garban H,
Jazirehi A, Emmanouilides C and Bonavida B: Rituximab inhibits p38
MAPK activity in 2F7 B NHL and decreases IL-10 transcription:
pivotal role of p38 MAPK in drug resistance. Oncogene.
23:3530–3540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Till BG and Press OW: Treatment of
lymphoma with adoptively transferred T cells. Expert Opin Biol
Ther. 9:1407–1425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jensen MC, Cooper LJ, Wu AM, Forman SJ and
Raubitschek A: Engineered CD20-specific primary human cytotoxic T
lymphocytes for targeting B-cell malignancy. Cytotherapy.
5:131–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eshhar Z, Waks T, Gross G and Schindler
DG: Specific activation and targeting of cytotoxic lymphocytes
through chimeric single chains consisting of antibody-binding
domains and the gamma or zeta subunits of the immunoglobulin and T
cell receptors. Proc Natl Acad Sci USA. 90:720–724. 1993.
View Article : Google Scholar
|
|
6
|
Hombach A, Wieczarkowiecz A, Marquardt T,
et al: Tumor-specific T cell activation by recombinant
immunoreceptors: CD3 zeta signaling and CD28 costimulation are
simultaneously required for efficient IL-2 secretion and can be
integrated into one combined CD28/CD3 zeta signaling receptor
molecule. J Immunol. 167:6123–6131. 2001. View Article : Google Scholar
|
|
7
|
Yu K, Hu Y, Tan Y, et al: Immunotherapy of
lymphomas with T cells modified by anti-CD20 scFv/CD28/CD3zeta
recombinant gene. Leuk Lymphoma. 49:1368–1373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Y, Yu K, Du J, et al: Potential
therapeutic strategy for non-Hodgkin lymphoma by
anti-CD20scFvFc/CD28/CD3zeta gene transfected T cells. J Exp Clin
Cancer Res. 29:1212010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang L, Chen Y, Chan CY, et al:
Down-regulation of stathmin is required for TGF-beta inducible
early gene 1 induced growth inhibition of pancreatic cancer cells.
Cancer Lett. 274:101–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Wei H, Zhang R, et al: Genetically
targeted T cells eradicate established breast cancer in syngeneic
mice. Clin Cancer Res. 15:943–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng MW, Kershaw MH, Moeller M, Smyth MJ
and Darcy PK: Immunotherapy of cancer using systemically delivered
gene-modified human T lymphocytes. Hum Gene Ther. 15:699–708. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haynes NM, Trapani JA, Teng MW, et al:
Single-chain antigen recognition receptors that costimulate potent
rejection of established experimental tumors. Blood. 100:3155–3163.
2002. View Article : Google Scholar
|
|
13
|
Rossig C and Brenner MK: Genetic
modification of T lymphocytes for adoptive immunotherapy. Mol Ther.
10:5–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Finney HM, Akbar AN and Lawson AD:
Activation of resting human primary T cells with chimeric
receptors: costimulation from CD28, inducible costimulator, CD134,
and CD137 in series with signals from the TCR zeta chain. J
Immunol. 172:104–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Far M, Fouda M, Yahya R and el-Baz H:
Serum IL-10 and IL-6 levels at diagnosis as independent predictors
of outcome in non-Hodgkin’s lymphoma. J Physiol Biochem.
60:253–258. 2004.PubMed/NCBI
|
|
16
|
Voorzanger N, Touitou R, Garcia E, et al:
Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s
lymphoma cells and act as cooperative growth factors. Cancer Res.
56:5499–5505. 1996.PubMed/NCBI
|
|
17
|
Benjamin D, Park CD and Sharma V: Human B
cell interleukin 10. Leuk Lymphoma. 12:205–210. 1994. View Article : Google Scholar
|
|
18
|
Alas S, Emmanouilides C and Bonavida B:
Inhibition of interleukin 10 by rituximab results in
down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin’s
lymphoma to apoptosis. Clin Cancer Res. 7:709–723. 2001.PubMed/NCBI
|
|
19
|
Vockerodt M, Haier B, Buttgereit P, Tesch
H and Kube D: The Epstein-Barr virus latent membrane protein 1
induces interleukin-10 in Burkitt’s lymphoma cells but not in
Hodgkin’s cells involving the p38/SAPK2 pathway. Virology.
280:183–198. 2001.
|
|
20
|
Platanias LC: Map kinase signaling
pathways and hematologic malignancies. Blood. 101:4667–4679. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pedersen IM, Buhl AM, Klausen P, Geisler
CH and Jurlander J: The chimeric anti-CD20 antibody rituximab
induces apoptosis in B-cell chronic lymphocytic leukemia cells
through a p38 mitogen activated protein-kinase-dependent mechanism.
Blood. 99:1314–1319. 2002. View Article : Google Scholar
|
|
22
|
Jazirehi AR, Gan XH, De Vos S,
Emmanouilides C and Bonavida B: Rituximab (anti-CD20) selectively
modifies Bcl-xL and apoptosis protease activating factor-1 (Apaf-1)
expression and sensitizes human non-Hodgkin’s lymphoma B cell lines
to paclitaxel-induced apoptosis. Mol Cancer Ther. 2:1183–1193.
2003.PubMed/NCBI
|
|
23
|
Alas S and Bonavida B: Rituximab
inactivates signal transducer and activation of transcription 3
(STAT3) activity in B-non-Hodgkin’s lymphoma through inhibition of
the interleukin 10 autocrine/paracrine loop and results in
down-regulation of Bcl-2 and sensitization to cytotoxic drugs.
Cancer Res. 61:5137–5144. 2001.PubMed/NCBI
|
|
24
|
Ma W, Lim W, Gee K, et al: The p38
mitogen-activated kinase pathway regulates the human interleukin-10
promoter via the activation of Sp1 transcription factor in
lipopolysaccharide-stimulated human macrophages. J Biol Chem.
276:13664–13674. 2001.
|