Introduction
Retroperitoneal primary mucinous adenocarcinoma
(RPMA) is extremely rare and the histogenesis of this tumor remains
unknown. As with most retroperitoneal masses, RPMA causes clinical
symptoms or is perceived by patients only when the mass grows to a
sufficiently large size. Laboratory studies lack the appropriate
levels of specialization for this tumor and imaging methods merely
reveal cystic lesions, neither of which result in accurate
diagnosis. Surgical resection is standard for the treatment of
RPMA, whereas chemotherapy for this tumor has not been rendered an
efficacious treatment modality. This case study reports a
21-year-old woman with RPMA. Following laparotomy, combined
treatments were administered and the benefits thereof were
investigated.
Case report
A 21-year-old woman presented with chronic lower
back pain and weight loss for a period of 10 months. Her physical
examination did not present any irregularities. She had no
significant past medical histology or family history of disease.
Laboratory data showed high levels of the carcinoembryonic antigen
(CEA) (338.39 ng/ml) and carbohydrate antigen (CA) 19-9 (253.13
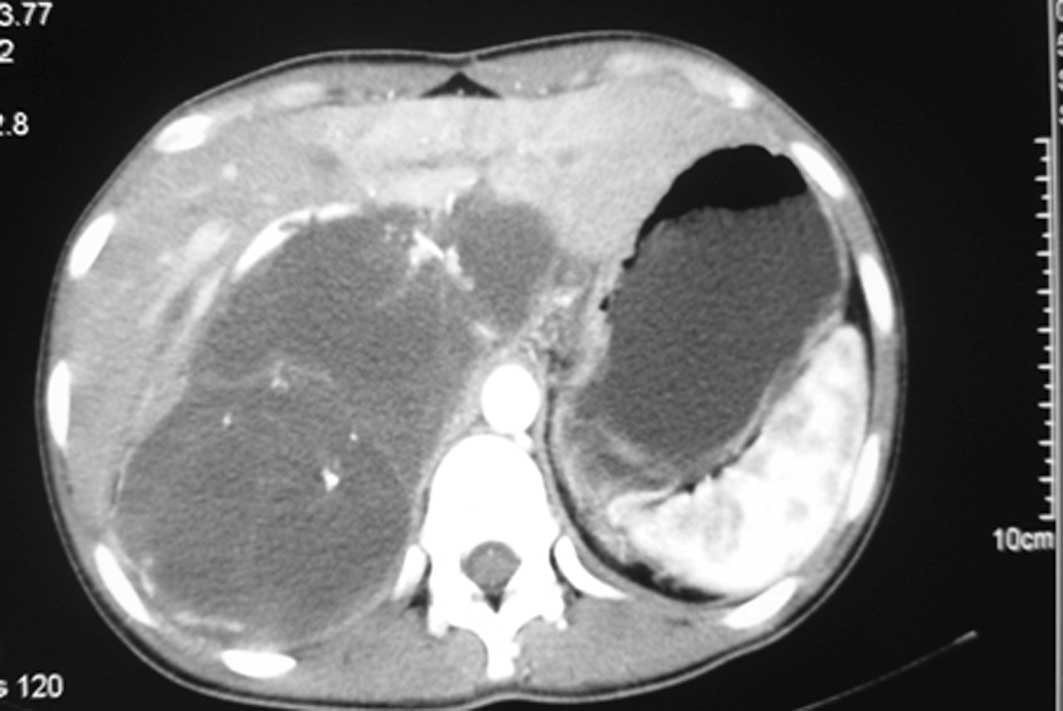
U/ml). A computed tomography (CT) scan revealed a mass measuring
approximately 14.6×7.7 cm in the abdominal cavity with enlarged
lymph nodes along the pancreas. There was a low-density area inside
the mass, which was slightly heterogeneously enhanced (Fig. 1). Following a laparotomy, an adult
fist-sized well-defined tumor was observed in the right
retroperitoneum, which was covered with intact peritoneum. No
ascites were noted, and the liver and kidneys appeared normal and
were medially displaced. During surgical resection the mass, which
consisted of multiloculated cyst with abundant intracytoplasmic
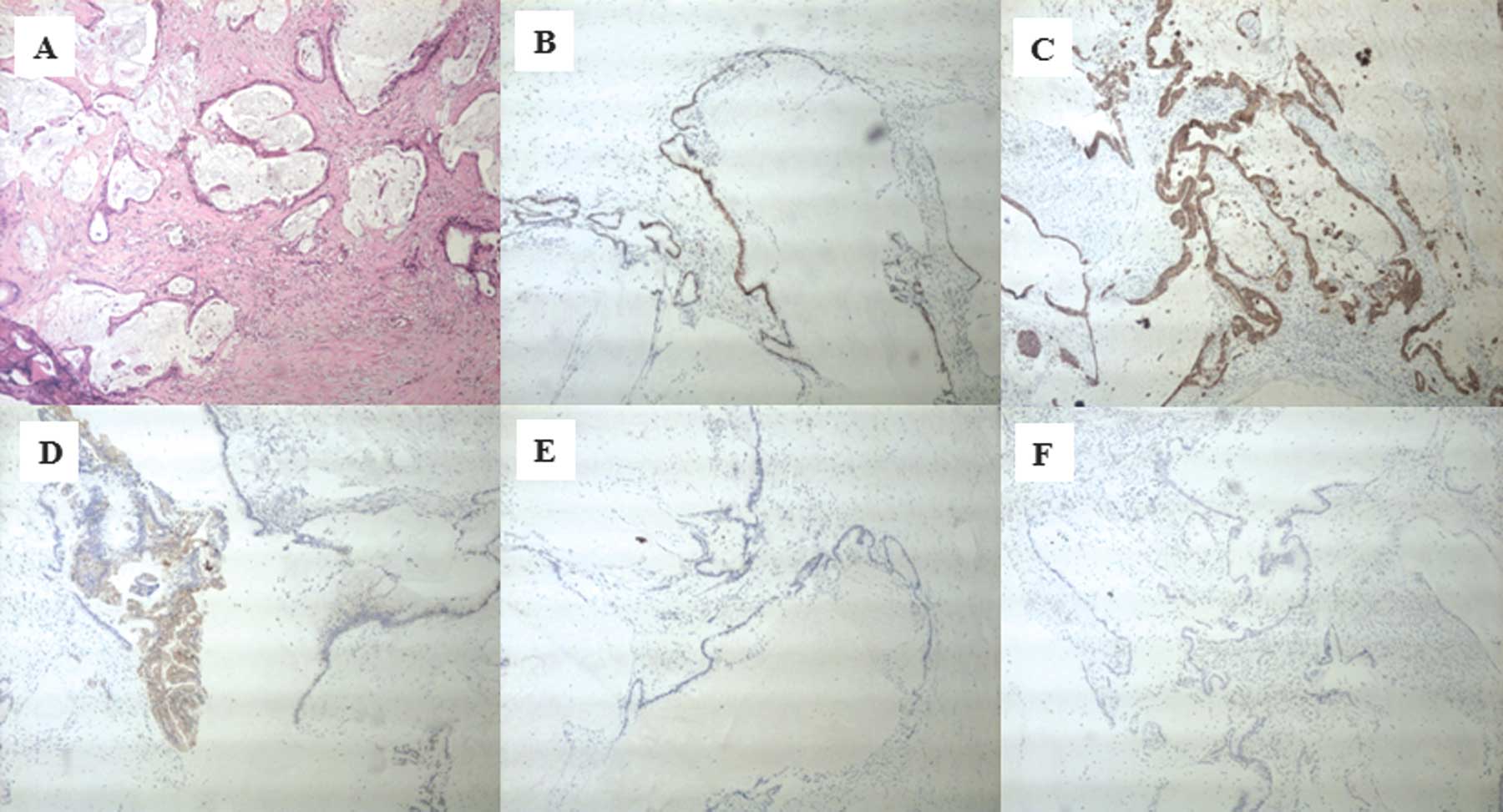
mucin was ruptured. The microscopic examination confirmed a
mucinous adenocarcinoma (Fig. 2A)
and tumor cells were positive for caudal-related homeodomain
protein 2 (CDX2) (Fig. 2B),
cytokeratin 20 (Fig. 2C) and
cytokeratin 19 (Fig. 2D), but
negative for CA125 (Fig. 2E), and
the estrogen and progesterone receptors (ER/PR) (Fig. 2F). The patient returned 4 months
after the operation with elevated levels of CEA (970 ng/ml) and
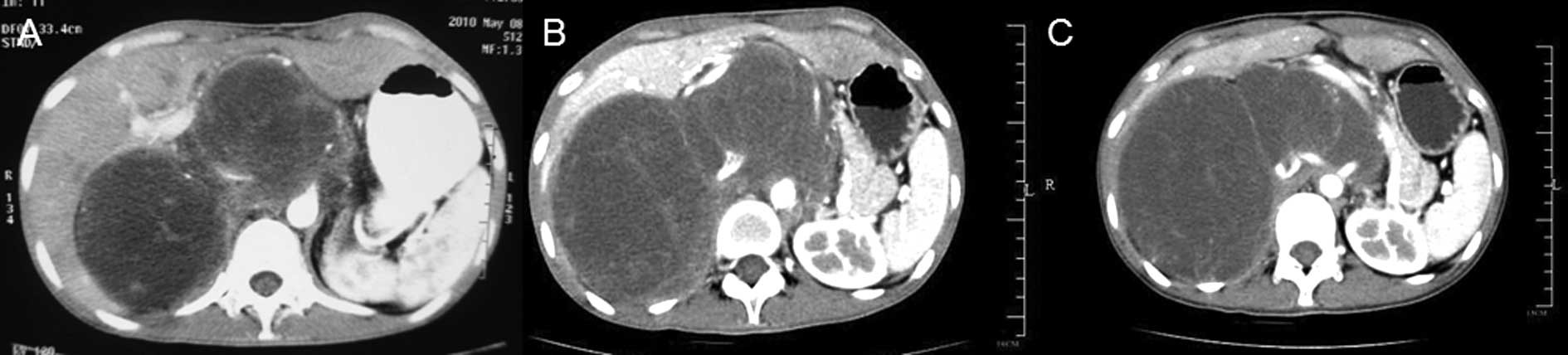
CA19-9 (1762 U/ml). A CT scan revealed local recurrences in the
retroperitoneum (Fig. 3A). After
receiving intravenous oxaliplatin and 5-fluorouracil (5-FU) for 3
cycles, no change was evident in the CEA and CA19-9 levels, and the
CT scans revealed a slightly larger tumor (Fig. 3B). The regimen was then switched to
5-FU and paclitaxel. Following 4 cycles of 5-FU and paclitaxel, the
CEA and CA19-9 levels decreased to 313 ng/ml and 272.5 U/ml,
respectively. During the regular follow-up, the tumor remained
stable (Fig. 3C).
Discussion
RPMA is a rare phenomenon, as indicated by the few
cases reported since it was first described in 1977 by Roth
(1). No other reported cases of
RPMA in Chinese women are available in the English literature.
Due to its rarity, the histogenesis of RPMA remains
to be determined and four main hypotheses have been proposed to
explain the histogenic origin of the tumor. One hypothesis suggests
that the tumor arises from a teratoma with predominant mucinous
epithelium (2), whereas other
authors postulate that it is caused by intestinal duplication, also
known as enterogenous genesis (3).
The intestinal-like epithelioma surrounding the cystic tumors in
our case potentially support this hypothesis. The third hypothesis
supports that the tumor arises from heterotopic ovarian tissue.
However, no records exist pertaining to ovarian tissue in RPMA
(4,5), and in our case the ovaries were
normal. Previously, a fourth hypothesis became widely accepted,
which suggests that tumors arise from invagination of the
peritoneal epithelium and undergo metaplasia during embryonic
growth (6).
RPMA occurs almost exclusively in women, with the
exception of 4 male cases reported in the literature (7–10).
RPMA is usually observed in middle-aged individuals, although
patient ages have ranged from 17 to 86 years. In the latter cases,
the mass was usually large, ranging from 10 to more than 20 cm in
diameter. According to the literature, RPMA symptoms are
non-specific, with the most common ones being abdominal discomfort
and palpable asymptomatic mass.
Preoperative diagnosis of RPMA is difficult, as
tumor markers such as CA-125, CEA and CA19-9 may not increase and
may lack specificity, thereby making the exact origin of the lesion
from other tumors, such as ovarian cyst, cystic mesothelioma,
cystic lymphangioma, non-pancreatic pseudocyst and renal cyst,
difficult to pinpoint. However, Tangjitgamol et al
hypothesized that tumor markers may help in determining a recurrent
tumor, such as colon cancer (11).
In our case, CEA and CA19-9 reached levels of 970 ng/ml and 1762
U/ml, respectively, 4 months after sugery and a CT scan confirmed
local recurrences in the retroperitoneum. After receiving 5-FU and
paclitaxel, the serum CEA and CA19-9 levels in the patient
decreased. Ultrasonography, CT and magnetic resonance imaging are
often used to localize the tumor. However, these methods cannot
easily differentiate between a benign and a malignant neoplasm
(12). Yang et al suggested
that when encountering a cystic lesion with the characteristic of
displacing the colon, kidney or ureter medially, surgeons should
include RPMA in the preoperative diagnosis (13). Needle biopsy may also be an
unreliable method with which to diagnose this tumor, since it is
not effective in determining malignancy in cystic tumors.
Laparotomy is necessary to facilitate accurate
decision-making and treatment. Investigators are in agreement
regarding the complete removal of the lesion. However, how
extensive the surgery should be remains controversial. Given the
assumption that RPMA occurs in heterotopic ovarian tissue, Lee
et al recommended total hysterectomy as well as oophorectomy
(14). On the other hand, Kessler
et al suggested that hysterectomy and salpingo-oophorectomy
are not suitable for the treatment of RPMA if the uterus and
ovaries are macro- scopically normal (4). Moreover, Law et al advocated
laparoscopic excision of the tumor, thereby sparing fertility in
these women (15). In our case, we
resected the tumor, since the uterus and ovaries were relatively
normal in appearance, and the patient hoped to remain fertile.
Chemotherapy for RPMA is not well established as the
benefits of adjuvant chemotherapy have yet to be established. Lee
et al (16) reported 5
patients who were administered with adjuvant chemotherapy following
resection. Of these patients, 1 developed paraovarian recurrence
despite undergoing cytoxan chemotherapy for 21 months, while 2
succumbed to widespread metastasis 4 and 18 months after surgery
(16). Certain authors have
suggested that since RPMA had similar mechanisms in its
histogenesis to the ovarian mucinous tumor, chemotherapy should be
administered, as in the case of this latter tumor. Paclitaxel with
cisplatin or carboplatin combination chemotherapy may also be
effective. Tenti et al reported 2 cases of RPMA with cystic
rupture; the patient who underwent adjuvant chemotherapy was free
of tumors for 33 months postoperatively (17). Kessler et al recommended that
chemotherapy should be performed in the cases whose tumor was
ruptured during surgery or had invaded to adjacent structures
(4,15). In our case, the tumor was ruptured
during surgery and the patient experienced a recurrence 4 months
after operation. The patient received chemotherapy with intravenous
oxaliplatin and 5-FU for 3 cycles based on the intestinal-like
epithelioma surrounding the cystic tumors. The CEA and CA19-9 serum
levels stopped increasing, whereas the tumor increased slightly in
size. To obtain a greater efficacy, the regimen was switched to
5-FU and paclitaxel for 4 cycles, resulting in decreased serum
levels of CEA (313 ng/ml) and CA19-9 (272.5 U/ml) and a stable
tumor. Thus, our clinical experience indicated that 5-FU and
paclitaxel may be effective for RPMA.
In conclusion, RPMA is a rare tumor and usually
presents with an asymptomatic abdominal mass. Preoperative
diagnosis of RPMA remains difficult and surgeons should be aware of
this tumor when encountering a large retroperitoneal cystic mass.
Treatment of RPMA remains controversial. Extirpative surgery is
currently the standard treatment, since the role of chemotherapy
for the treatment of RPMA has yet to be determined. Further studies
are therefore required to establish optimal treatment protocols for
this rare neoplasm.
References
|
1
|
Roth LM and Ehrlich CE: Mucinous
cystadenocarcinoma of the retroperitoneum. Obstet Gynecol.
49:486–488. 1977.PubMed/NCBI
|
|
2
|
Papadogiannakis N, Gad A and Ehliar B:
Primary retroperitoneal mucinous tumor of low malignant potential:
histogenetic aspects and review of the literature. Acta Pathol
Microbiol Immunol Scand Suppl. 105:483–486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorbeck VC, Gustein D, Salvi M and Plata
J: Retroperitoneal enteroid cystadenocarcinoma (possible intestinal
origin). Rev Esp Enferm Apar Dig (In Spanish). 66:329–334.
1984.PubMed/NCBI
|
|
4
|
Kessler TM, Kessler W, Neuweiler J and
Nachbur BH: Treatment of a case of primary retroperitoneal mucinous
cyst-adenocarcinoma: is adjuvant hysterectomy and bilateral
salpingo-oophorectomy justified? Am J Obstet Gynecol. 187:227–232.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De León DC, Pérez-Montiel D,
Chanona-Vilchis J, Dueñas-González A, Villavicencio-Valencia V and
Zavala-Casas G: Primary retroperitoneal mucinous
cystadenocarcinoma: report of two cases. World J Surg Oncol.
5:52007.PubMed/NCBI
|
|
6
|
Subramony C, Habibpour S and Hashimoto LA:
Retroperitoneal mucinous cystadenoma. Arch Pathol Lab Med.
125:691–694. 2001.
|
|
7
|
Green JM, Bruner BC, Tang WW and Orihuela
E: Retroperitoneal mucinous cystadenocarcinoma in a man: case
report and review of the literature. Urol Oncol. 25:53–55. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thamboo TP, Sim R, Tan SY and Yap WM:
Primary retroperitoneal mucinous cystadenocarcinoma in a male
patient. J Clin Pathol. 59:655–657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motoyama T, Chida T, Fujiwara T and
Watanabe H: Mucinous cystic tumor of the retroperitoneum: a report
of two cases. Acta Cytol. 38:261–266. 1994.PubMed/NCBI
|
|
10
|
Hrora A, Reggoug S, Jallal H, Sabbah F,
Benamer A, Alaoui M, Raiss M and Ahallat M: Primary retroperitoneal
mucinous cyst-adenocarcinoma in a male patient: a case report.
Cases Journal. 2:71962009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tangjitgamol S, Manusirivithaya S,
Sheanakul C, Leelahakorn S, Thawaramsara T and Kaewpila N:
Retroperitoneal mucinous cystadenocarcinoma: A case report and
review of literature. Int J Gynecol Cancer. 12:403–408. 2002.
View Article : Google Scholar
|
|
12
|
Matsubara M, Shiozawa T, Tachibana R,
Hondo T, Osasda K, Kawaguchi K, Kimura K and Konishi I: Primary
retroperitoneal mucinous cystadenoma of borderline malignancy: a
case report and review of the literature. Int J Gynecol Pathol.
24:218–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang DM, Jung DH, Kim H, Kang JH, Kim SH,
Kim JH and Hwang HY: Retroperitoneal cystic masses: CT, clinical,
and pathological findings and literature review. Radiographics.
24:1353–1365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee IW, Ching KC, Pang M and Ho TH: Two
cases of primary retroperitoneal mucinous cystadenocarcinoma.
Gynecol Oncol. 63:145–150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Law KS, Chang TM and Tung JN:
Fertility-sparing treatment of a primary retroperitoneal mucinous
cystadenocarcinoma. BJOG. 113:612–614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SA, Bae SH, Ryoo HM, Jung HY, Jang SB
and Kum YS: Primary retroperitoneal mucinous cystadenocarcinoma: a
case report and review of the literature. Korean J Intern Med.
22:287–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tenti P, Carnevali L, Tateo S and Durola
R: Primary mucinous cystadeno-carcinoma of retroperitoneum: Two
cases. Gynecol Oncol. 55:308–312. 1994. View Article : Google Scholar
|