Introduction
Chronic myelogenous leukemia (CML) has a typical
progressive course with transition from the chronic phase to the
terminal blast crisis phase. The mechanisms that lead to disease
progression have yet to be elucidated. Cytogenetic and genetic
changes occur in the majority of patients during disease
progression. Approximately 70–80% of patients with CML blast crisis
show additional chromosomal changes involving chromosomes 7, 8, 17,
19, 21 and 22, sometimes with duplication of the Ph chromosome
(1). Genetic changes occurring in
the progression to blast crisis include mutation of the p53
(20–30%), amplification of the c-myc (20%), deletion of the p16
(15%) and mutation of the Ras (6%) gene (2).
DNA methylation at CpG sites in promoter regions is
a frequent, acquired epigenetic event involved in the pathogenesis
of various types of human malignancies. Methylation in the promoter
region is capable of causing gene silencing, which may provide an
alternative pathway to gene inactivation, in addition to deletions
or mutations. The ABL1, calcitonin, ER and HIC1 genes were found to
be frequently methylated in CML (3). Moreover, methylation of the ABL1 gene
is associated with the progression of CML (4). These methylation phenotypes in CML
provided a rationale for using demethylating agents such as
5-azacytidine and decitabine in a clinical setting, and preliminary
clinical results were reported (3,5). To
determine the role of aberrant methylation in the progression of
CML, we analyzed DNA methylation patterns in CML blast crisis.
Materials and methods
Bone marrow cells were obtained from 16 patients who
developed blast crisis during the follow-up of CML. Genomic DNA was
extracted from low density mononuclear cells after the bone marrow
cells were centrifuged in the presence of TRIzol reagent (Life
Technologies Inc., Rockville, MD, USA). Control DNA was extracted
from the peripheral blood of 10 healthy individuals.
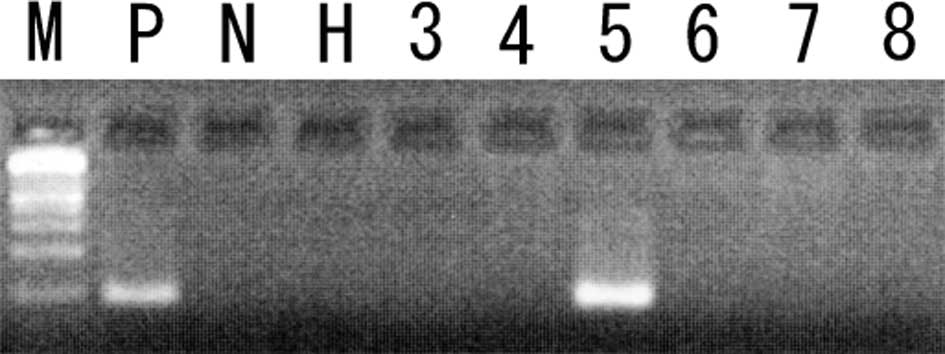
Methylation-specific PCR (MSP) was performed as previously
described (6,7). Briefly, 1.0 μg genomic DNA was
modified by bisulfite, and PCR was performed using specific primers
for each of the genes. Following amplification, 10 μl PCR product
was separated on 2% agarose gel containing 0.3 mg/ml ethidium
bromide. A total of 13 genes, including cell cycle regulating genes
(p14, p15, p16, Rb, APC and FHIT), DNA repair genes (hMLH1, hMSH2
and MGMT), apoptosis-related genes (DAPK and RIZ1), a
differentiation-associated gene (RARβ) and a cytokine signaling
gene (SOCS-1) were analyzed in this study.
Results and Discussion
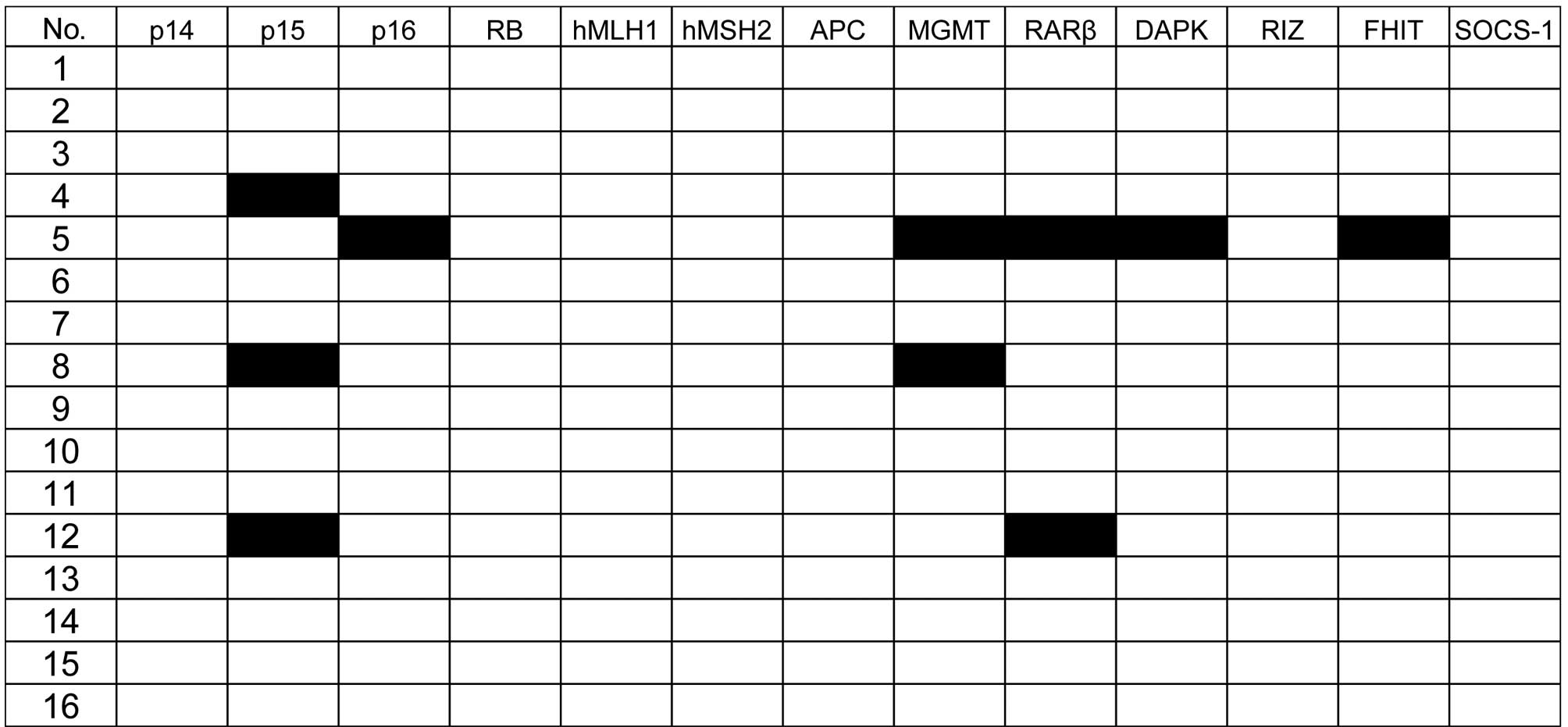
The methylation status of the 13 genes was analyzed
in 16 patients in the blast crisis phase of CML. The frequency of
samples with methylation in each of the following genes was: p15,
18%; MGMT, 12%; RARβ, 12%; p16, 6%; DAPK, 6%; FHIT, 6% (Figs. 1 and 2). The p14, Rb, hMLH1, hMSH2, APC, RIZ or
SOCS-1 genes were not methylated in any of the patients. Moreover,
none of these genes were methylated in white blood cell DNA from 10
healthy individuals.
In total, 4 (25%) of the 16 CML blast crisis
patients had methylation of at least one of these genes; 1 case
(6%) had methylation of five target genes; 2 (12%) had two genes
methylated, and the remaining case (6%) had methylation in one
gene. Although the number of cases that were analyzed is limited,
no significant correlation was found between methylation status and
the clinical characteristics of CML.
The most frequently methylated gene was p15 (3 of 16
patients: 18%). This is in accordance with previous reports
(8,9). The other cell cycle control gene, p16,
was methylated in another patient. Taken together, 4 (25%) of the
16 patients had methylation in cell cycle control genes, suggesting
that inactivation of cell cycle control genes by promoter
hypermethylation plays a significant role in the progression of
CML.
Microsatellite instability (MSI) is caused by
defects of the DNA mismatch repair system, and inactivation of the
hMLH1 and hMSH2 genes by promoter hypermethylation is frequently
associated with MSI (10). In this
study, none of the 16 cases showed hypermethylation of the hMLH1
and hMSH2 genes. This is in accordance with our previous findings
that MSI is infrequent in CML blast crisis patients (11). Taken together, the deficiency of the
DNA mismatch repair system does not contribute to the disease
progression of CML. We conclude that hypermethylation of the cell
cycle control genes, and not DNA mismatch repair genes, plays a
significant role in the progression of CML.
Acknowledgements
This study was supported in part by grants from the
Uehara Memorial Foundation and a grant-in-aid from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
References
|
1
|
Shet AS, Jahagirdar BN and Verfaillie CM:
Chronic myelogenous leukemia: mechanisms underlying disease
progression. Leukemia. 16:1402–1411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Randhawa GS, Cui H, Barletta JA, et al:
Loss of imprinting in disease progression in chronic myelogenous
leukemia. Blood. 91:3144–3147. 1998.PubMed/NCBI
|
|
3
|
Santini V, Kantarjian HM and Issa JP:
Changes in DNA methylation in neoplasia: pathophysiology and
therapeutic implications. Ann Intern Med. 134:573–86. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asimakopoulos FA, Shteper PJ, Krichevsky
S, et al: ABL1 methylation is a distinct molecular event associated
with clonal evolution of chronic myeloid leukemia. Blood.
94:2452–2460. 1999.PubMed/NCBI
|
|
5
|
Sacchi S, Kantarjian HM, O'Brien S, et al:
Chronic myelogenous leukemia in nonlymphoid blastic phase: analysis
of the results of first salvage therapy with three different
treatment approaches for 162 patients. Cancer. 86:2632–2641. 1999.
View Article : Google Scholar
|
|
6
|
Hofmann WK, Tsukasaki K, Takeuchi N,
Takeuchi S and Koeffler HP: Methylation analysis of cell cycle
control genes in adult T-cell leukemia/lymphoma. Leuk Lymphoma.
42:1107–1109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uehara E, Takeuchi S, Tasaka T, et al:
Aberrant methylation in promoter-associated CpG islands of multiple
genes in therapy-related leukemia. Int J Oncol. 23:693–696.
2003.PubMed/NCBI
|
|
8
|
Nguyen TT, Mohrbacher AF, Tsai YC, et al:
Quantitative measure of c-abl and p15 methylation in chronic
myelogenous leukemia: biological implications. Blood. 95:2990–2992.
2000.PubMed/NCBI
|
|
9
|
Kusy S, Cividin M, Sorel N, et al: p14ARF,
p15INK4b, and p16INK4a methylation status in chronic myelogenous
leukemia. Blood. 101:374–375. 2003. View Article : Google Scholar
|
|
10
|
Sheikhha MH, Tobal K and Liu Yin JA: High
level of microsatellite instability but not hypermethylation of
mismatch repair genes in therapy-related and secondary acute
myeloid leukaemia and myelodysplastic syndrome. Br J Haematol.
117:359–365. 2002. View Article : Google Scholar
|
|
11
|
Mori N, Takeuchi S, Tasaka T, et al:
Absence of microsatellite instability during the progression of
chronic myelocytic leukemia. Leukemia. 11:151–152. 1997. View Article : Google Scholar : PubMed/NCBI
|