Introduction
The metastasis of malignant tumor cells from the
primary tumor to distant sites in the body is a complex process
that includes proliferation, adhesion, migration and invasion of
metastatic tumor cells (1–3). Focal adhesion kinase (FAK) mediates
numerous cell processes, including survival, migration and invasion
(4). Previously, it was reported
that Y925F-FAK-transfected (Y925F) melanoma cells impaired
metastatic ability compared to parental B16F10 cells or
vector-transfected (Vect) cells (5). Low levels of proteolipid protein 2
(PLP2) have been identified in Y925F cells by microarray analysis.
Additionally, it was reported that PLP2, a four-transmembrane
domain protein, has contributed to the experimental metastasis of
melanoma B16F10 cells (6). Using
biochemical methods, a ubiquitously expressed protein (PI3K) was
identified as an associated protein of PLP2, which activates Akt,
and passively enhances secretion of matrix metalloproteinase
(MMP)-2 and the metastatic function of PLP2. Although these data
validated the function of PLP2 in tumor metastasis, most of the
data were obtained by simulating the partly metastatic process from
tumor cells to distant tissues through the bloodstream. Therefore,
the role of PLP2 in the entire metastatic progress from primary
tumor to distant sites is unknown. Lymph node (LN) metastasis is
one of the earliest features of tumor cell dissemination, and
approximately 60% of metastasis is found in regional LNs in
malignant melanoma. Metastasis of cancer to the regional LNs
appears to reflect the biological aggressiveness of the primary
tumors. The mouse melanoma cell line B16BL6 provides a convenient
transplantable model, which is metastatic to LNs in the syngeneic
host, C57BL/6 mice (7).
Micro RNAs (miRNAs) are the recently discovered
non-coding, double-stranded RNAs that negatively regulate target
gene expression. A number of studies have successfully targeted
oncogenes using artificial synthetic miRNAs. Li et al
(8) reported that targeting FAK via
RNA interference suppressed tumor growth as well as metastasis into
lung and lymph nodes in melanoma B16F10 cells. The aim of this
study was to investigate the downregulation of PLP2 or FAK
expression with each artificial miRNA and confirm the suppression
of growth and popliteal LN metastasis of B16BL6 cells using this
miRNA in C57BL/6 mice.
Materials and methods
Cell culture
The melanoma cell line B16BL6, established by Fidler
(1), was obtained from Professor
Yoshikazu Sugimoto of Keio University and maintained in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine
serum (FBS).
Gene expression analysis using
GeneChip
A GeneChip analysis of empty Vect-transfected B16F10
cells and Y925F-FAK-transfected B16F10 was performed using
oligonucleotide arrays (GeneChip AceGene 30K-1, containing 30,000
mouse genes). Briefly, Cy3- or Cy5-labeled cDNAs generated from
these cells were hybridized to the DNA chips, and scanned with a
chip reader (Vertek, Canada). The scanned images were then
processed using the MicroArray Suite (Scanalytic, Rockville, MD,
USA). Data were normalized by the median of intensity of actin as a
housekeeping gene.
Immunoblotting and
immunoprecipitation
Immunoblotting was performed using anti-PLP2,
anti-PI3K, anti-phospho-Akt, Akt and anti-phosphotyrosine.
Immunoprecipitation was performed as previously described (6,9).
Animals
C57BL/6J mice (6–8 weeks old) were obtained from
Nippon Charles River (Tokyo, Japan). Throughout the experiments,
the mice were housed in plastic cages (at 21±2°C) with free access
to pelleted food and water, and exposed to a 12 h light-dark cycle.
Animal welfare and experimental procedures were performed strictly
in accordance with the Guide for the Care and Use of Laboratory
Animals and the related ethical regulations of the Keio University.
All efforts were made to minimize the suffering of the animals and
reduce the number of animals used.
Vector construction and cell
transfection
Three pre-miRNA sequences were designed to target
the region of the mouse PLP2 (Gene Bank accession no. NM019755) or
FAK (Gene Bank accession no. NM007982) mRNA using an online tool,
RNAi Designer (Invitrogen, Tokyo, Japan). Oligonucleotides encoding
three different miRNAs were commercially synthesized (Invitrogen).
Plasmids cloned into the pcDNA6.2-GW/EmGFPmiR parental vector of
the double-stranded DNA oligonucleotides corresponding to the three
different PLP2- or FAK-specific pre-miRNAs and a control sequence
were obtained from Invitrogen. Plasmids were transfected into
B16/BL6 melanoma cells using Lipofectamine 2000. Following an
overnight culture, the medium was exchanged to remove transfection
reagents and was further incubated for 72 h. Cells stably
expressing miRNA were selected using blastcidine (40 μg/ml;
InvivoGen, San Diego, CA, USA).
The most effective oligonucleotide sequences of the
engineered pre-miRNA and adjacent flanking regions used for plasmid
construction were: miRNA-FAK-3, forward: 5′-TGC
TGTACAAGAGAGACTTTCAGCTAGTTTTGGCCACT GACTGACTAGCTGAATCTCTCTTGTA-3′
and reverse: 5′-CCTGTACAAGAGAGATTCAGCTAGTCAGTCAGTGG
CCAAAACTAGCTGAAAGTCTCTCTTGTAC-3′; miRNA-PLP2-2, (forward):
5′-TGCTGATCTCTTGTACCA TAAGCACTGTTTTGGCCACTGACTGACAGTGCTTA
GTACAAGAGAT-3′ and reverse: 5′-CCTGATCTCTTGTAC
TAAGCACTGTCAGTCAGTGGCCAAAACAGTGCTTA TGGTACAAGAGATC-3′, negative
control, forward: 5′-TGC TGAATGTACTGCGCGTGGAGACGTTTTGGCCACTG
ACTGACGTCTCCACGCAGTAC-3′ and reverse: 5′-CCT
GAAATGTACTGCGTGGAGACGTCAGTCAGTGGCCAA AACGTCTCCACGCGC
AGTACATTTC-3′.
Cell proliferation
Cell proliferation was evaluated by the MTT
(tetrazolium salt) assay. Cells (2×103) were plated in
96 wells containing growth medium and assayed by MTT. A total of 10
μl MTT [5 mg/ml in phosphate-buffered saline (PBS)] was added to
each well and cells were incubated at 37°C in a CO2
incubator. Following 3 h, the cells were lysed using
isopropanol-HCl, and absorbance was measured at 570 nm. All cell
growth experiments were repeated at least three times.
RT-PCR
RNA was prepared from cells using a Qiagen Mini
RNA-Easy kit (Tokyo, Japan). Reverse transcription was performed
from 1 μg of RNA in a 20 μl reaction mixture according to the
manufacturer’s instructions (Takara RNA PCR kit, Ohtsu, Japan). PCR
amplification and analysis were achieved using real-time PCR (Prism
7000, Applied Biosystems, Foster City, CA, USA). Primer sequences
used were: PLP2, sense: 5′-GTGTGACCTGCACTCCAAGA-3′ and antisense,
5′-TCAGTG GGAGCTGCTGTATG-3′; FAK, sense;
5′-CGTGAAGCCTTTTCAAGGAG-3′; and antisense,
5′-ACTGGATCTCGGGCTAGGAT-3′; MMP-2 sense: 5′-CCT
GATGTCCAGCAAGTAGATG-3′ and antisense: 5′-GAGACT
TTGGTTCTCCAGCTTC-3′; MMP-9, sense: 5′-CCTGAT GTCCAGCAAGTAGATG-3′
and antisense: 5′-GAGACTTTG GTTCTCCAGCTTC-3′. GAPDH sense:
5′-ACTCCACTCACG GCAAATTC-3′ and antisense: 5′-CCTTCCACAATGCCAAA
GTT-3′.
Cell adhesion assay
For the cell adhesion assay, 96-well flat-bottomed
plates (ECM205 CytoMatrix screen kit, Chemicon, Temecula, CA, USA)
coated with fibronectin, vitronectin, laminin and collagen I and IV
were used. Cells were detached from the culture plates with a
dissociation buffer, seeded at a density of 6×104
cells/well in 100 μl medium and incubated at 37°C for 2 h. The
assay was terminated by washing the plates with PBS to remove
unbound cells. The cells that remained attached to the plates were
fixed and stained with 0.5% crystal violet/2% EtOH/0.1 M borate
buffer (pH 9.0). Following washing with PBS, 100 μl solubilization
buffer (1:1 mixture of 0.1 M NaH2PO4, pH 4.5
and EtOH) was added to the plates and measurements were taken with
an ELISA reader at 540 nm. The data were expressed as the mean
absorbance of triplicate wells ± standard deviation (SD).
Migration
Haptotaxis was assayed in triplicate using modified
Boyden chambers, and cell culture inserts (Falcon) with a
polyethylene terephthalate membrane (8-μm pore size, 13-mm
diameter) were used. Filters were precoated on the lower side with
a 5 μg/ml fibronectin solution as previously described (5). Following drying, the filters were
placed in the lower chamber containing 1% FBS-DMEM. Cells
(1×105) were then added to the upper chamber
supplemented with 1% FBS-DMEM and incubated at 37°C for 24 h. The
cells that had migrated to the underside of the insert membranes
were stained with Giemsa and counted. Five fields per insert were
scored, and the treatment was performed in triplicate.
Matrigel invasion assay
In vitro invasion assays were performed as
previously described, with some modifications (5). A Matrigel invasion chamber
(Becton-Dickinson, Franklin Lakes, NJ, USA) was used. A
chemoattractant, the conditioned medium of fibroblasts, was added
to the lower chamber to induce all invasion through the Matrigel.
Cells were added (1×105/well) to the inner chamber of
the cell culture insert and incubated at 37°C for 72 h. The filters
were fixed in 70% ethanol for 30 min and stained with Giemsa to
quantify invasion. Non-invading cells were removed from the upper
surface of the Matrigel by rubbing gently with a cotton-tipped
applicator. Cells that had passed through the membrane were counted
in five random microscopic fields of the lower filter surface.
Gelatin zymography
Cells (~70–80% confluent) were washed, replenished
with serum-free DMEM and incubated for 24 h. Serum-free conditioned
medium was combined with SDS buffer without heating or reduction
and applied to 10% polyacrylamide gels containing 1 mg/ml of
gelatin. Following electrophoresis, the gels were washed in 2.5%
(v/v) Triton X-100 for 2 h at room temperature to remove SDS,
rinsed with water, and incubated in protease buffer (50 mM
Tris-HCl, pH 7.5, 5 mM CaCl2, 1 mM ZnCl2 and
0.1 mM NaN3) for 40 h at 37°C. Gels were stained with
0.5% Coomassie blue R-250 in 10% 2-propanol and 10% acetic acid and
destained. Protease activity was visualized as a clear band.
In vivo metastasis assay
B16BL6 cells transfected with PLP2 or FAK miRNA in
the exponential growth phase were harvested by trypsinization and
washed twice prior to injection. Cell vitality was >95% as
determined by trypan blue dye exclusion. B16BL6 cells
(2×105 cells in 50 μl PBS) were injected into the right
hind footpads of C57BL/6 mice (100% of injected mice formed
tumors). Tumor volumes were measured and calculated using the
formula: 0.5236 × L1 × (L2)2, where L1 is the long axis
and L2 is the short axis of the tumor (10). Twenty days later, mice were
sacrificed and images of the right footpads and popliteal LNs were
captured.
Statistical analysis
Data were expressed as the mean ± SD. Results of
in vitro experiments were analyzed using the Student’s t
test. For in vivo experiments, the Mann-Whitney U test was
used for comparing the two groups.
Results
Suppression of PLP2 or FAK expression
with each artificial miRNA
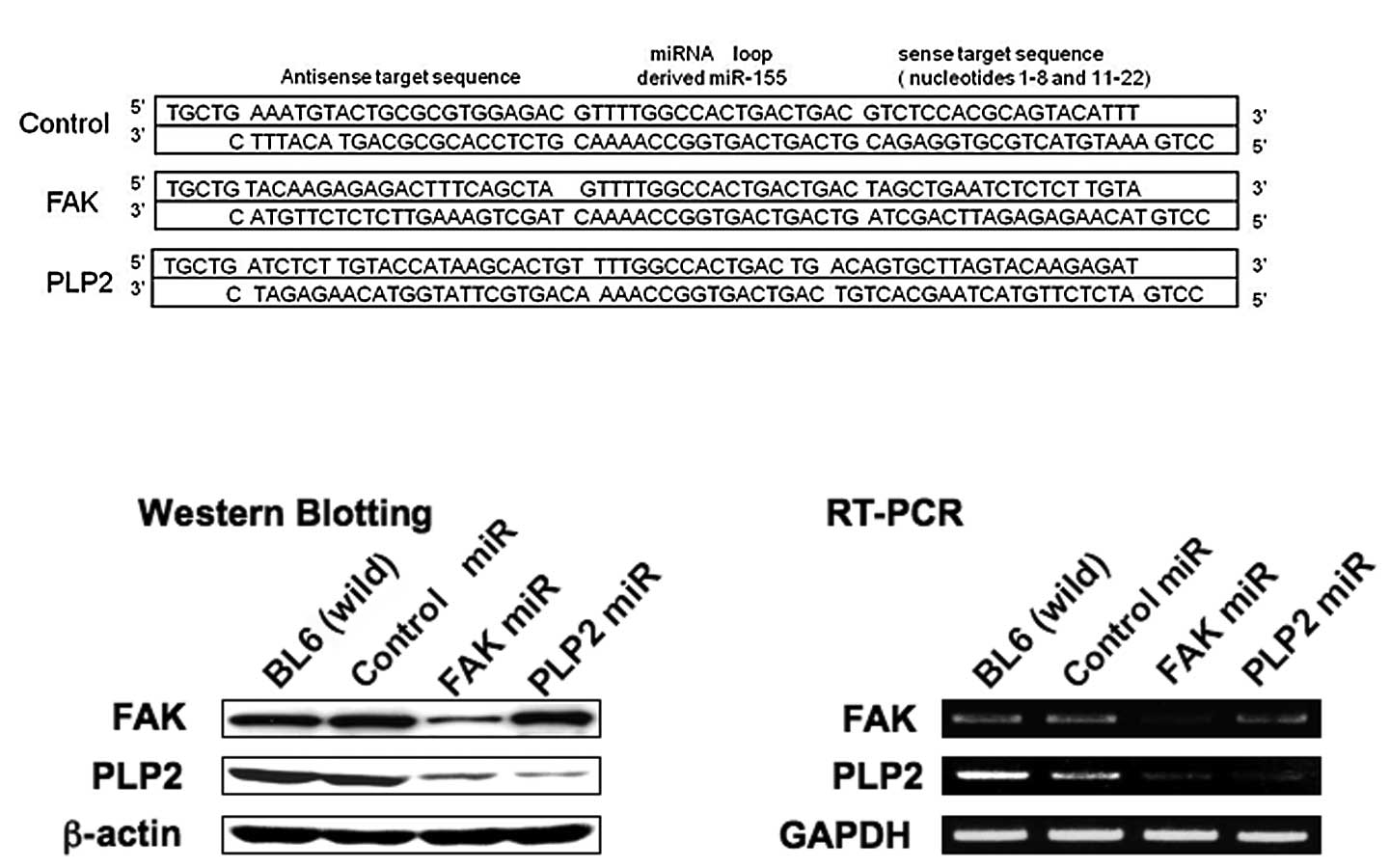
We designed three different artificial pre-miRNA
sequences that targeted the PLP2 or FAK mRNA. The pre-miRNAs were
cloned individually into the pcDNA6.2-GW/EmGFPmiR plasmid vector,
transfected to B16BL6 cells and selected using blasticidine. The
most effective sequence is shown in Fig. 1A. These cells were visualized by
green fluorescence (data not shown). Basal PLP2 or FAK expression
was suppressed in cells transfected with the
PLP2si-2/pcDNA6.2-GW/EmGFPmiR (PLP2miR) or the
FAKsi-3/pcDNA6.2-GW/EmGFPmiR (FAKmiR) plasmids individually. The
suppression of PLP2 or FAK gene expression was
evident compared to the cells with the control
miRNA/pcDNA6.2-GW/EmGFPmiR by Western blotting and RT-PCR (Fig. 1B and C).
Effect of artificial PLP2 or FAK miRNA on
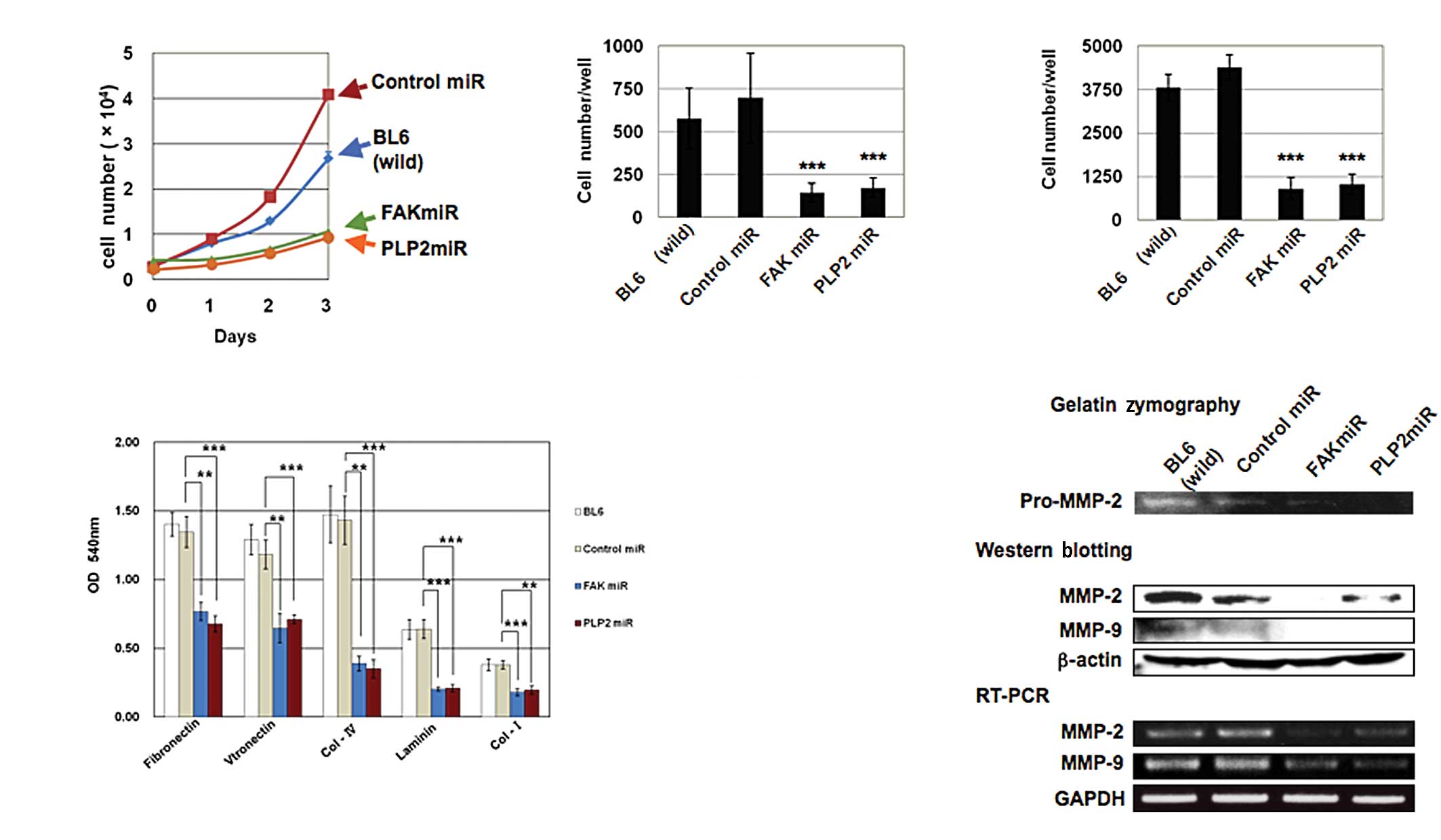
the proliferation of B16BL6 cells in vitro
We determined whether PLP2 or FAK downregulation
affected the tumor cell proliferation in vitro by counting
cell numbers for 3 days. The results shown in Fig. 2A indicate that PLP2 or FAK
downregulation markedly suppressed proliferation.
Inhibition of adhesion, migration and
invasion of B16BL6 cells by PLP2 or FAK miRNA
B16BL6 cells transfected with PLP2 or FAK miRNA were
investigated for their adhesion, migration and invasion abilities.
PLP2 or FAK miRNA-transfected cells exhibited a significant
decrease in adhesion ability to fibronectin, vitonectin, collagen
IV, laminin and collagen I by more than 50% (Fig. 2B). Cells migrating to the
undersurface in the migration assay were reduced by 21 and 25%
(Fig. 2C), and those traversing the
Matrigel in the invasion assay were reduced by 21 and 23% in B16BL6
cells transfected with PLP2 or FAK miRNA, respectively, compared to
cells transfected with negative control miRNA (Fig. 2D). We examined MMP activity as a
crucial step in the invasion, which involves the degradation of
extracellular matrix (ECM) components, and allows cells to
efficiently traverse the basement membranes. Gelatin zymography of
serum-free conditioned medium revealed that suppression of MMP
activity in cells transfected with PLP2 or FAK miRNA was evident
compared to cells transfected with negative control miRNA and
parental cells (Fig. 2E).
Suppression of MMP-2 and -9 was confirmed by Western blotting and
RT-PCR (Fig. 2E). Cells transfected
with PLP2 or FAK miRNA had decreased MMP-2 and -9 levels.
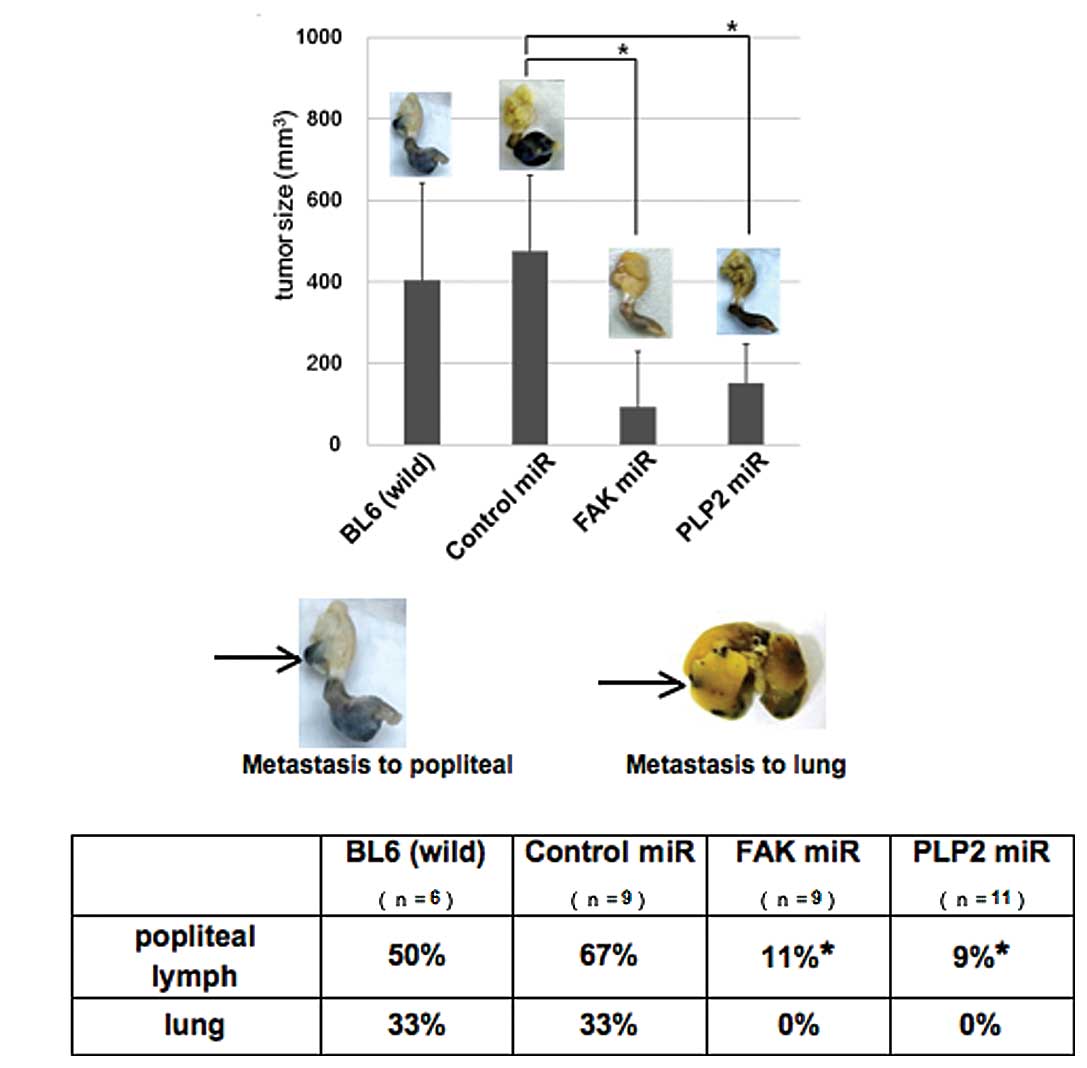
Inhibition of tumor growth and
spontaneous metastasis by PLP2 or FAK miRNA in vivo
We then investigated whether PLP2 or FAK play a
critical role in tumor formation in vivo. B16BL6 cells
transfected with the PLP2 or FAK miRNA expression plasmids were
subcutaneously injected into the footpads of C57BL/6J mice. PLP2 or
FAK miRNA significantly suppressed tumor growth in mice, whereas
control siRNA did not affect tumor growth (Fig. 3A). The mice were sacrificed and
their footpads, inoculated with B16BL6 cells, were collected and
images were captured. Notably, the popliteal LNs from the control
miRNA-treated groups showed high ratios of visible metastases, with
approximately 67% for parent and control siRNA groups. By contrast,
metastasis was decreased to 11% in the popliteal LNs of PLP2 or FAK
siRNA groups (Fig. 3B). Detectable
metastases were observed in the lungs of the parental and control
groups, but not in the PLP2 or FAK siRNA groups.
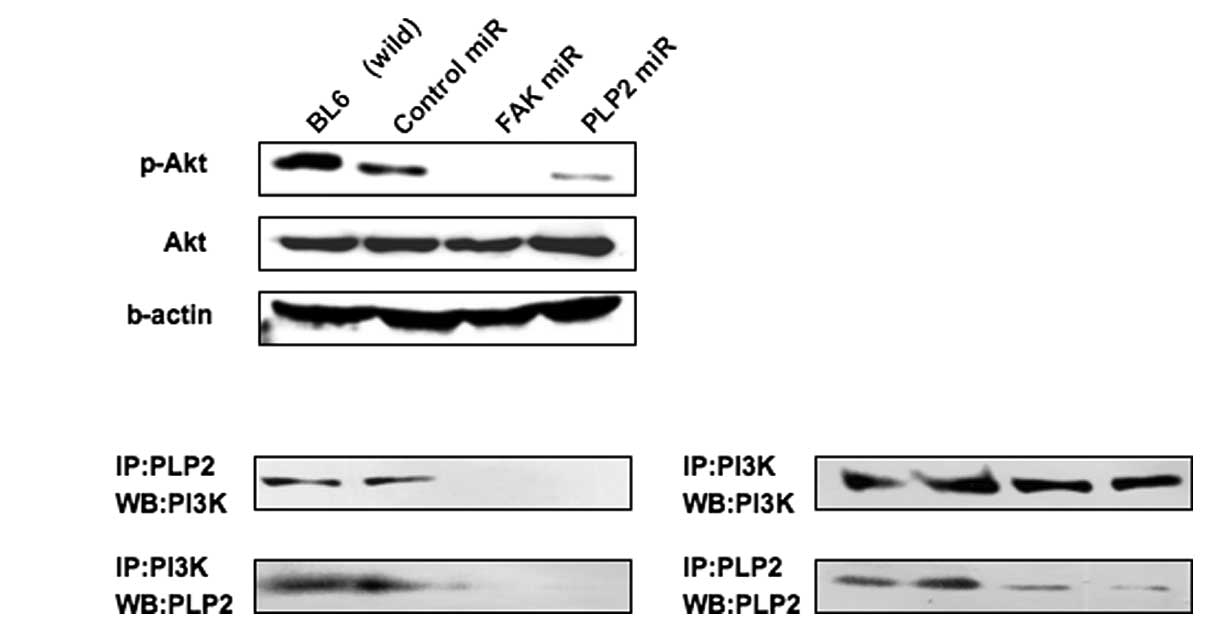
Blockade of Akt signaling by the PLP2 or
FAK miRNA
Previous investigations have demonstrated that PLP2
associates with PI3K to activate Akt in B16F10 cells (6). Western blotting was performed for Akt
phosphorylation to verify that the FAK-PI3K-Akt pathway was blocked
due to suppression of PLP2 or FAK expression with the artificial
miRNAs. The results revealed that Akt phosphorylation was detected
in B16BL6 cells (Fig. 4A).
Conversely, Akt phosphorylation was not detected in PLP2 or FAK
miRNA-transfected cells. Furthermore, the association of PLP2 with
PI3K was confirmed. It was determined that PLP2 interacted with
PI3K (Fig. 4B). The
immunoprecipitation data demonstrated that this association was not
detectable in the cells transfected with the miPLP2 or miFAK
expression plasmids.
Discussion
We used B16BL6 cells to establish a spontaneous
metastatic model and investigated its characteristics against
vector-based miRNA, which was used to directly knock down the
endogenous expression of PLP2 or FAK in B16BL6 cells. Tumor
metastasis is a complex process that includes the detachment of
cancer cells from a primary tumor, entry into the circulatory
system, and adherence and migration of the tumor cells to distant
sites. The first stage of tumor cell detachment from the original
site depends on adhesiveness to the ECM and migration to blood
capillaries and lymphatic vessels. Therefore, we examined these
abilities in miPLP2- or miFAK-transfected B16BL6 cells. PLP2 or FAK
knockdown by specific miRNAs suppressed B16BL6 cell proliferation
in vitro, inhibited the adhesive ability of B16BL6 cells to
the ECM, such as fibronectin, and inhibited migration and invasion
ability in the transwell-based assay. The adhesion, migration and
invasion ability of tumor cells may be regulated by multiple
signaling cascades, including integrin-mediated signaling,
mitogen-activated protein kinase signaling and cytoskeletal
reorganization. This hypothesis is substantiated by the observation
that PLP2 directly interacted with PI3K and activated the Akt
signaling pathway. These findings further confirm that PLP2 and/or
FAK play a key role in the acquisition of metastatic potential by
tumor cells.
Experimentally passive models, which mimic the
metastatic events after tumor cells enter the circulatory system by
injecting tumor cells to tail veins, have been used to investigate
the function of PLP2 in vivo, but the role of PLP2 in cancer
cell detachment from the original site, such as adhesion to the
ECM, digestion of the matrix and intravasation, has not been
explored. A previous study indicated that PLP2 transfectants attach
well to fibronectin, a common matrix of adhesion and migration
assays (6). The present study
reconfirmed those observations and showed that PLP2 or FAK miRNA
may inhibit the adhesion, migration and invasion ability of B16BL6
cells in vitro. Additionally, PLP2 or FAK miRNA treatment
markedly reduced the metastatic ability of B16BL6 cells to migrate
to popliteal LNs, and no visible metastatic sites were detected in
that group. These results suggest that the in vivo
proliferation of B16BL6 cells at the primary tumor site and the
development of macroscopic metastases were highly dependent on the
PLP2 or FAK expression level. Untreated B16BL6 cells, with normal
endogenous PLP2 expression, showed more apparent metastasis to
popliteal LNs than that of PLP2 miRNA-treated cells, whereas 33% of
metastatic sites were visible in the lung.
The data show the substantial role that PLP2 and FAK
play in spontaneous metastasis in vivo. Further studies are
necessary to reveal whether PLP2 regulates the expression of these
factors in the process of popliteal LN metastasis. B16BL6 cells
treated with PLP2 miRNA as well as FAK miRNA showed slow growth
compared with those treated with negative miRNA in primary tumors
in vivo.
In conclusion, the present study reproduced the role
of PLP2 or FAK in the entire process of spontaneous metastasis from
primary tumor to regional LN. We also found that specifically
reduced PLP2 expression inhibited the growth of B16BL6 cells in
vivo and prevented detectable metastasis from primary tumors by
decreasing adhesive ability in the ECM, such as fibronectin and
laminin, and by reducing the migratory ability of B16BL6 cells. We
found that PLP2 or FAK played a more pivotal role in tumor
formation and metastasis in B16BL6 cells. The current results
extend potential therapeutic applications of PLP2 or FAK miRNA to
the clinical treatment of malignant tumors.
Acknowledgements
We thank Erika Suzuki and Saori Kato for their
technical assistance. This study was supported in part by a grant
(no. 20590069) from the Ministry of Education, Culture, Sports,
Science and Technology of Japan.
References
|
1
|
Fidler IJ: The pathogenesis of cancer
metastasis the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
2
|
MacDonald IC, Groom AC and Chambers AF:
Cancer spread and micrometastasis development: quantitative
approaches for in vivo models. Bioessays. 24:885–893. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hendrix MJ, Seftor EA, Seftor RE,
Kasemeier-Kulesa J, Kulesa PM and Postovit LM: Reprogramming
metastatic tumour cells with embryonic microenvironments. Nat Rev
Cancer. 7:246–255. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanks SK, Ryzhova L, Shin NY and Brábek J:
Focal adhesion kinase signaling activities and their implications
in the control of cell survival and motility. Front Biosci.
8:982–996. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaneda T, Sonoda Y, Ando K, Suzuki T,
Sasaki Y, Oshio T, Tago M and Kasahara T: Mutation of Y925F in
focal adhesion kinase (FAK) suppresses melanoma cell proliferation
and metastasis. Cancer Lett. 270:354–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sonoda Y, Warita M, Suzuki T, Ozawa H,
Fukuda Y, Funakoshi-Tago M and Kasahara T: Proteolipid protein 2 is
associated with melanoma metastasis. Oncol Rep. 23:371–6.
2010.PubMed/NCBI
|
|
7
|
Raz A, Bucana C, McLellan W and Fidler IJ:
Distribution of membrane anionic sites on B16 melanoma variants
with differing lung colonising potential. Nature. 284:363–364.
1980. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Dong W, Zong Y, Yin W, Jin G, Hu Q,
Huang X, Jiang W and Hua ZC: Polyethylenimine-colexed plasmid
particles targeting focal adhesion kinase function as melanoma
tumor therapeutics. Mol Ther. 15:515–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonoda Y, Watanabe S, Matsumoto Y,
Aizu-Yokota E and Kasahara T: FAK is the upstream signal protein of
the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen
peroxide-induced apoptosis of a human glioblastoma cell line. J
Biol Chem. 274:10566–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian F, Li YP, Sheng X, Zhang ZC, Song R,
Dong W, Cao SX, Hua ZC and Xu Q: PRL-3 siRNA inhibits the
metastasis of B16-BL6 mouse melanoma cells in vitro and
in vivo. Mol Med. 13:151–9. 2007.PubMed/NCBI
|