Introduction
Gastric cancer accounts for a large proportion of
malignancies and gastric cancer-related deaths account for the
largest proportion of deaths from cancer (1). Disordered apoptosis has been linked to
cancer development, and repression of apoptosis has been observed
in gastric cancer (2). STATs and
Survivin, as significant apoptosis-regulated molecules, play a
pivotal role in oncogenesis (3–8).
The JAK/STAT-pathway was originally observed in
studies of interferon-unresponsive cells (9). This pathway is known to be involved in
two types of proteins. One of these types is the receptor
pre-associated tyrosine kinases, termed Janus kinases (JAKs); the
other is latent cytosolic transcription factors, termed signal
transducers and activators of transcription (STATs). The
dimerisation of cell surface receptors induces the mutual
phosphorylation of receptor-preassociated JAK proteins, after which
JAK proteins recruit and phosphorylate STAT proteins in the
cytoplasm. The phosphorylated STATs form dimers, migrate into the
nucleus, binding to specific DNA response elements in gene
promoters, and regulate gene transcription (10). The STAT protein family comprises at
least seven members, i.e. STAT1, STAT2, STAT3, STAT4, STAT5a,
STAT5b and STAT6 (11). STAT1 was
the first member of this family to be identified. As a tumor
surveillance gene, it plays a significant role in IFN-γ-induced
biological responses (4–8), the immune response (12,13)
and cell growth control (14,15).
It has been suggested that STAT1 serves as a tumor suppressor by
promoting the expression of p21waf, caspase 3 and
caspase 7 to activate pro-apoptotic pathways (16).
The inhibitor of the apoptosis (IAP) family of
proteins was originally named due to its physical ability to
inhibit caspases (17). IAPs
present an approximately 70 amino acid baculovirus IAP repeat (BIR)
(18). Survivin is a protein of 142
amino acids and is the smallest mammalian member of the IAP family
(18). It is an established cancer
gene, as it was found to be overexpressed in almost all human
tumors, whereas it is largely undetectable or minimally expressed
in normal mature tissues (19). Due
to its differential distribution of other IAPs, which are typically
found in normal tissues and occasionally up-regulated in cancer
(19), Survivin was regarded as one
of the most prominent cancer genes (20). Survivin blocks apoptosis induced by
various stimuli, including chemotherapeutic drugs (3,21),
FAS/CD95 (22) and irradiation
(23). Its ability to inhibit
apoptosis is believed to lie in its binding directly to
p21waf, caspase 3 and caspase 7 and preventing their
activation (22,24).
Little research has been conducted into the
functions of STAT1 and Survivin and their clinical characteristics
in gastric cancer. In previous studies, we observed that the
IFN-γ-STAT1 pathway, which adjusts p21waf and caspase 7
expression, was present in the SGC7901 gastric cancer cell line and
human tissues and that STAT1 initiates advanced gastric cancer
(25,26). Meanwhile, we observed that Survivin
inhibits p21waf and caspase 7 expression, whereas IFN-γ
inhibits Survivin expression in SGC7901 cells and initiates lymph
node metastasis of gastric cancer (26,27).
In addition, we noted that STAT1 protein expression was negatively
correlated with Survivin protein expression in human gastric cancer
tissues (26).
In the present study, we treated the SGC7901 cell
line with IFN-γ, STAT1 antisense oligonucleotides (ASONs) and
Survivin ASONs prior to performing immunocytochemistry and image
analysis to detect the expression regulation of STAT1 and Survivin
protein, and analyzed the antagonistic effect between STAT1 and
Survivin. We then performed immunohistochemistry to analyze the
expression of STAT1 and Survivin protein in 83 resected human
gastric cancer tissue samples, evaluated the clinicopathological
and prognostic significance of STAT1 and Survivin expression in
gastric cancer tissues, and analyzed the clinical characteristics
of the antagonistic effect between STAT1 and Survivin in gastric
cancer.
Materials and methods
Cell culture, IFN-γ treatment and ASON
treatment
The SGC7901 human gastric adenocarcinoma cell line,
obtained from the Chinese Academy of Medical Sciences Cell Center
of Basic Medicine (Beijing, China), was maintained in RPMI-1640
medium containing 10% fetal calf serum (FCS) at 37°C in a 5%
CO2 atmosphere.
For IFN-γ treatment, cells were plated as slides in
a 6-well plate for 24 h, then placed in RPMI-1640 medium containing
10% FCS and 1,000 U/ml concentration IFN-γ (28) (02CY27; Peprotech EC) for 24 h.
The phosphorothioate oligonucleotides used as
antisense for STAT1 and Survivin were 5′-CCACTGAGACATCCTGC CACC-3′
(29) and
5′-CCCAGCCTTCCAGCTCCTTG-3′ (30),
respectively. SGC7901 cells cultured on 6-well plates to reach a
confluence of 70–80% were incubated with STAT1 and Survivin ASONs
using Transfectin (TianGENE) at a charge ratio of 3:1
(Transfectin/ASON) in serum-free medium. At the end of a 6-h
incubation period, RPMI-1640 containing 10% FCS was added. A
combination of IFN-γ (1,000 U/ml) and STAT1 ASON (600 and 800 nM)
was administered to SGC7901 for 24 h. Survivin ASON (200 and 400
nM) was administered to SGC7901 for 24 h alone.
Pathological examination
The 83 human gastric cancer tissue samples, which
were histological and clinically verified between 1998 and 2003,
were collected at the Jiangda Pathology Institution, China. For the
use of these clinical materials for research purposes, prior
patient consent and approval from the Institute Research Ethics
Committee were obtained. Of the 83 patients included in this study,
60 patients were male and 23 were female, age range 26–82 years
(mean 58). Routine pathological examination was performed to
establish the depth of invasion and histological classification of
gastric cancer. All lymph nodes were found using the clearing fat
method (>15/case) (31). Depth
of invasion and lymph node metastasis were staged according to the
standards of the WHO, sixth edition. Histological classification
was divided into two types according to the standards of the WHO,
sixth edition. The well-differentiated type included
well-differentiated adenocarcinoma. The poorly differentiated type
included poorly differentiated adenocarcinoma, mucinous
adenocarcinoma and signet-ring cell carcinoma. Tumor size was
calculated using the largest diameter of tumor. Heterogeneity
defines a tumor that has more than two histological types.
Clinicopathological characteristics were recorded for all cancer
patients (Table I). Pathological
status was classified according to the sixth edition of the TNM
classification of the WHO (2003). None of the patients received
chemotherapy or radiation therapy prior to surgery. No patients
were lost prior to follow-up. Median time of follow-up was 28.8
months (range 1–159).
 | Table ICorrelation between STAT1 and Survivin
expression and clinicopathological factors of gastric cancer. |
Table I
Correlation between STAT1 and Survivin
expression and clinicopathological factors of gastric cancer.
| Variables | STAT1 protein
expression | P-value | Survivin protein
expression | P-value |
|---|
|
| |
| |
|---|
| Negative (n=51) | Positive (n=32) | | Negative (n=40) | Positive (n=43) | |
|---|
| Gender |
| Male | 40 | 20 | 0.12 | 28 | 32 | 0.660 |
| Female | 11 | 12 | | 12 | 11 | |
| Age |
| ≤60 | 29 | 18 | 0.96 | 22 | 25 | 0.780 |
| >60 | 22 | 14 | | 18 | 18 | |
| Size (diameter) |
| <5 cm | 27 | 17 | 0.99 | 20 | 24 | 0.600 |
| ≥5 cm | 24 | 15 | | 20 | 19 | |
| Depth of
invasion |
| T1 | 2 | 2 | 0.01a | 3 | 1 | 0.530 |
| T2 | 3 | 6 | | 3 | 6 | |
| T3 | 17 | 14 | | 17 | 14 | |
| T4 | 29 | 10 | | 17 | 22 | |
| Histological
type |
|
Well-differentiated | 23 | 8 | 0.07 | 11 | 20 | 0.080 |
| Poorly
differentiated | 28 | 24 | | 29 | 23 | |
| Heterogeneity |
| Yes | 34 | 17 | 0.22 | 25 | 26 | 0.850 |
| No | 17 | 15 | | 15 | 17 | |
| Lymph node
metastasis |
| N0 | 7 | 6 | 0.49 | 3 | 10 | 0.002a |
| N1 | 16 | 3 | | 7 | 12 | |
| N2 | 12 | 12 | | 11 | 13 | |
| N3 | 16 | 11 | | 19 | 8 | |
Immunohistochemistry, immunocytochemistry
and image analysis
The primary monoclonal antibody for STAT1 P84/P91
(C-136; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was
purchased from Beijing ZhongShan Ltd. (China). The primary
polyclonal antibody for Survivin (RAB-0536; NeoMarkers) and SP kit
was purchased from Fujian Maxin Ltd. (China). The
immunohistochemical and immunocytochemical staining was performed
according to the manufacturer’s instructions. Diaminobenzedin (DAB)
was used for color development. The positive results of STAT1 were
present in the cytoplasm or/and nuclei of tumor cells exhibiting
brown coloration. The positive result of Survivin were present in
cytoplasm of tumor cells exhibiting brown coloration. In the
immunocytochemical staining process, all stainings were carried out
under identical conditions and simultaneously, using image analysis
software (Motic) to collect immunocytochemical staining images and
detect their average optical density (OD) values.
Statistical analysis
The statistical software package SPSS 12.0 was used.
The average OD values of image were analyzed by the Student’s
t-test analysis. The correlations of STAT1 and Survivin expression
and clinicopathological factors were analyzed by the Spearman’s
rank correlation analysis and the χ2 test. Survival
curves were plotted according to the Kaplan-Meier method, and the
generalized log-rank test was applied to compare the survival
curve. Multivariate survival analysis was performed on all of the
parameters that were found to be significant on the univariate
analysis using Cox’s regression model. The statistical significance
of differences was determined by one-way analysis of variance.
P<0.05 was considered to indicate statistical significance.
Results
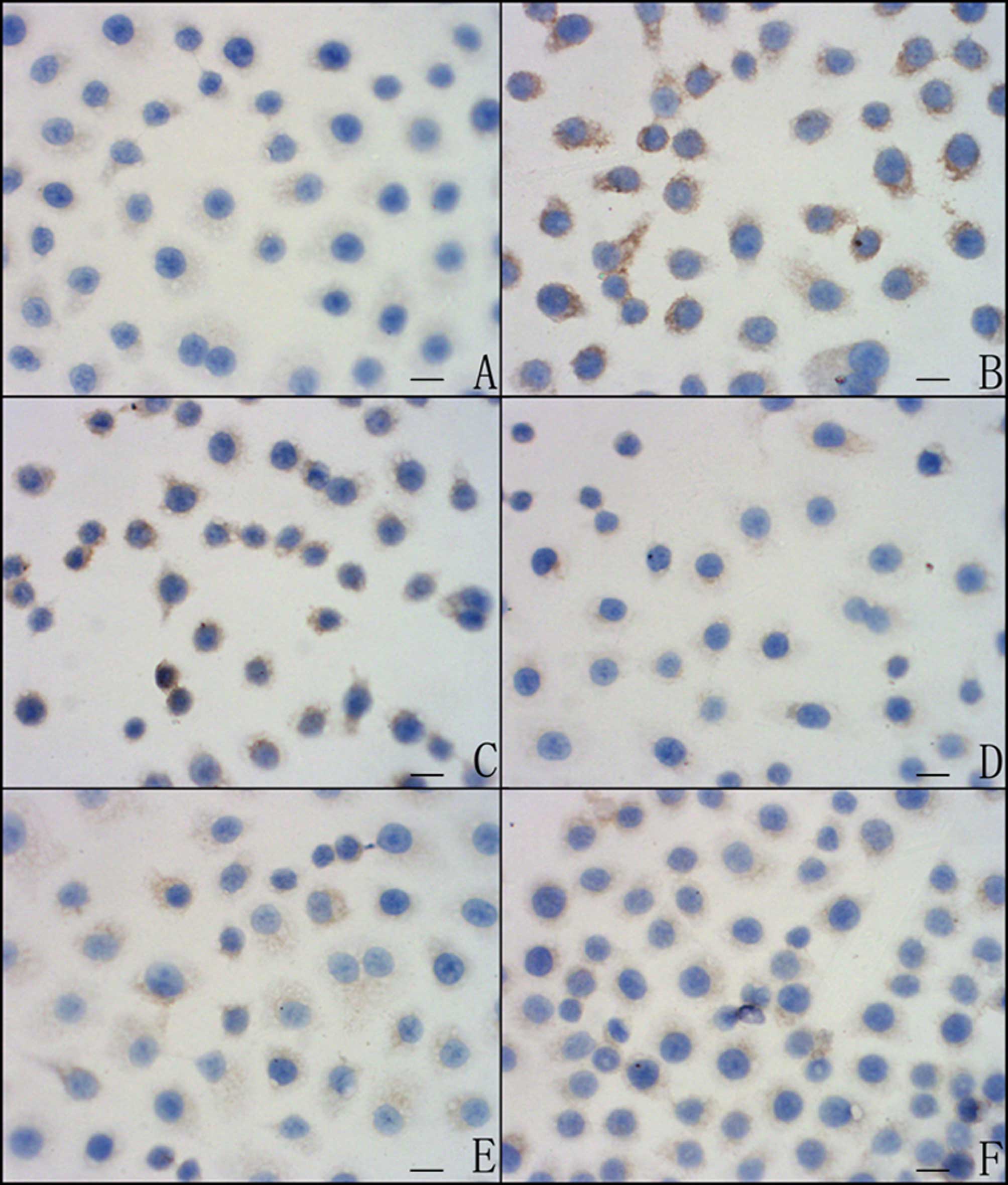
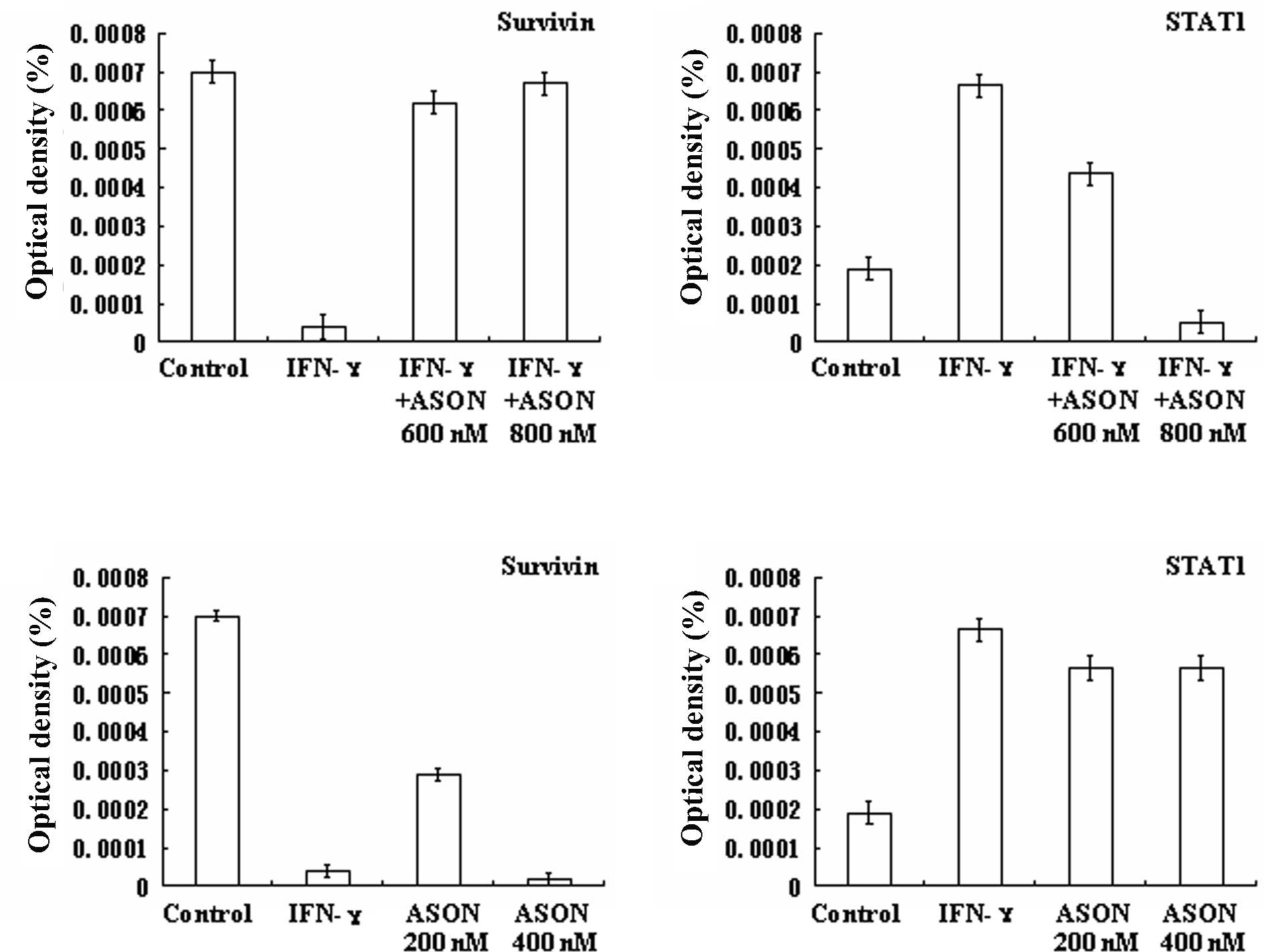
IFN-γ inhibits Survivin protein
expression by promoting STAT1 protein expression in SGC7901
cells
STAT1 and Survivin expression was examined in cells
treated with IFN-γ (1,000 U/ml) for 24 h. We observed that STAT1
protein expression was increased and Survivin protein expression
was decreased (Figs. 1–3, Tables
II and III). Cells that had
been treated with IFN-γ (1,000 U/ml) and STAT1 ASON (600 and 800
nM) for 24 h were selected to confirm whether IFN-γ inhibited
Survivin protein expression by promoting STAT1 protein expression.
We noted that when SGC7901 cells were treated with IFN-γ (1,000
U/ml) and the concentration of STAT1 ASON ranged from 600 to 800
nM, STAT1 protein expression was gradually decreased.
Simultaneously, Survivin protein expression was gradually increased
(Figs. 1–3, Tables
II and III).
 | Table IIIFN-γ and ASONs induced STAT1 protein
expression changes in SGC7901 cells. |
Table II
IFN-γ and ASONs induced STAT1 protein
expression changes in SGC7901 cells.
| IFN-γ | IFN-γ + STAT1 ASON
600 nM | IFN-γ + STAT1 ASON
800 nM | Survivin ASON 200
nM | Survivin ASON 400
nM |
|---|
|
|
|---|
| P-value | P-value | P-value | P-value | P-value |
|---|
| Control | 0.04a | 0.18 | 0.200 | 0.030a | 0.0100a |
| IFN-γ | - | 0.28 | 0.009a | 0.500 | 0.6000 |
| IFN-γ + STAT1 ASON
600 nM | - | - | 0.040a | 0.400 | 0.3000 |
| IFN-γ + STAT1 ASON
800 nM | - | - | - | 0.001a | <0.0001a |
| Survivin ASON 200
nM | - | - | - | - | 0.5000 |
 | Table IIIIFN-γ and ASONs induced Survivin
protein expression changes in SGC7901 cells. |
Table III
IFN-γ and ASONs induced Survivin
protein expression changes in SGC7901 cells.
| IFN-γ | IFN-γ + STAT1 ASON
600 nM | IFN-γ + STAT1 ASON
800 nM | Survivin ASON 200
nM | Survivin ASON 400
nM |
|---|
|
|
|---|
| P-value | P-value | P-value | P-value | P-value |
|---|
| Control | <0.0001a | 0.0050a | 0.1200 | 0.0005a | <0.0001a |
| IFN-γ | - | <0.0001a | <0.0001a | <0.0001a | 0.2100 |
| IFN-γ + STAT1 ASON
600 nM | - | - | 0.0400a | <0.0001a | <0.0001a |
| IFN-γ + STAT1 ASON
800 nM | - | - | - | <0.0001a | <0.0001a |
| Survivin ASON 200
nM | - | - | - | - | 0.0005a |
Survivin inhibits STAT1 protein
expression in SGC7901 cells
Survivin protein expression was at a high level in
the SGC7901 cell line (35). Cells
that had been treated with Survivin ASON (200 and 400 nM) for 24 h
were selected to confirm whether or not Survivin protein expression
inhibits STAT1 protein expression. We noted that when SGC7901 cells
were treated with a concentration of Survivin ASON ranging from 200
to 400 nM, Survivin protein expression was gradually/decreased.
Simultaneously, STAT1 protein expression was gradually increased
(Figs. 1–3, Tables
II and III).
STAT1 exhibited a negative correlation with depth of
invasion in Survivin protein-negative gastric cancer tissues.
Additionally, exhibited a negative correlation with N stage in
STAT1 protein-negative tissues.
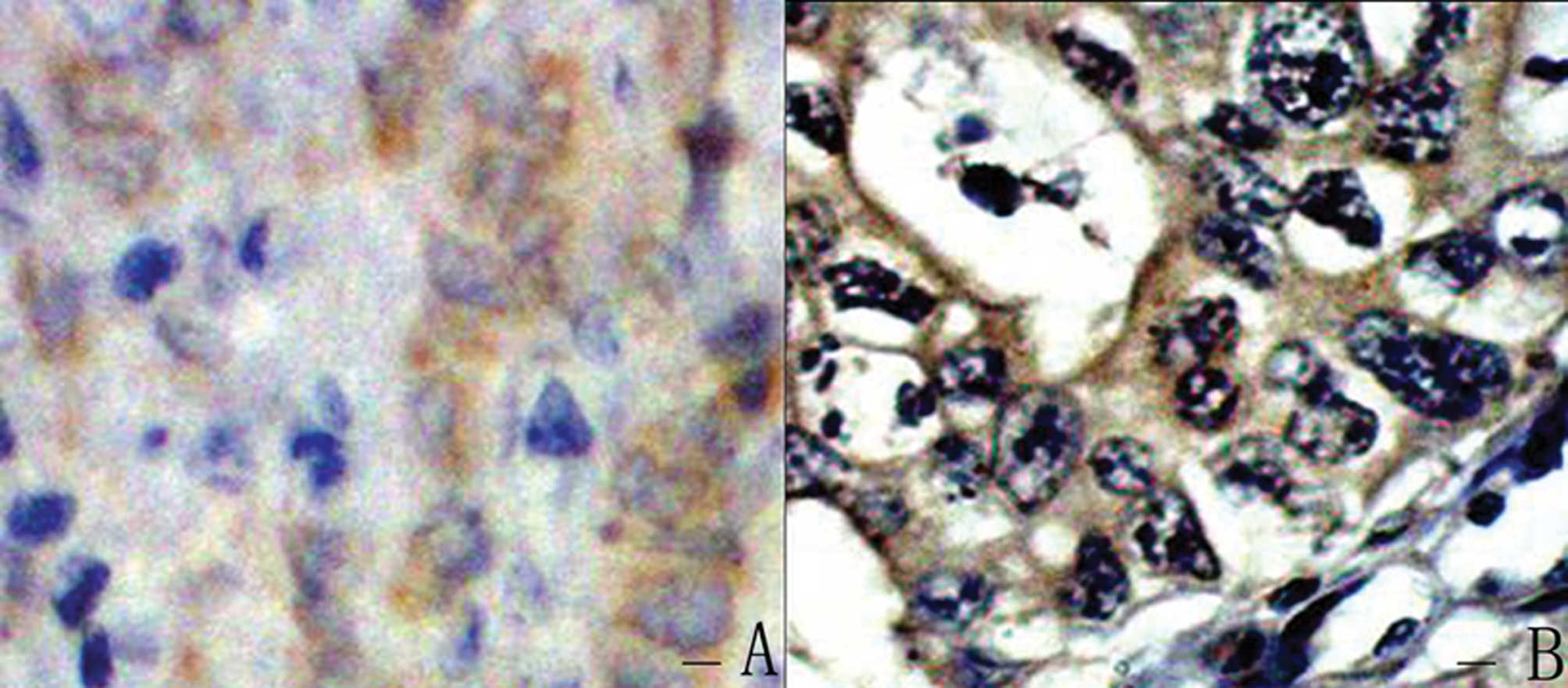
In 83 human gastric cancer tissues, the positive
rate of STAT1 protein expression was 38.6% (32/83) (Fig. 4A) and that of Survivin protein
expression was 51.8% (43/83) (Fig.
4B). STAT1 expression exhibited a negative correlation with
depth of invasion (P=0.01, r=−0.27). Survivin expression exhibited
a negative correlation with N stage (P=0.002, r=−0.34). A
significant negative correlation was observed between STAT1
expression and Survivin expression (P=0.04, r=−0.23).
To confirm whether or not the antagonistic effect
between STAT1 and Survivin was capable of affecting their
correlations with clinicopathological characteristics, a positive
and negative expression of STAT1 and Survivin proteins was used to
divide 83 human gastric cancer tissues into four groups. We found
that STAT1 exhibited a negative correlation with depth of invasion
in Survivin protein-negative tissues (P=0.04, r=−0.30) and no
correlation in Survivin protein-positive tissues (P=0.16, r=−0.22).
Survivin exhibited a negative correlation with N stage in STAT1
protein-negative tissues (P=0.009, r=−0.36), and no correlation
with N stage in STAT1 protein-positive tissues (P=0.18,
r=−0.24).
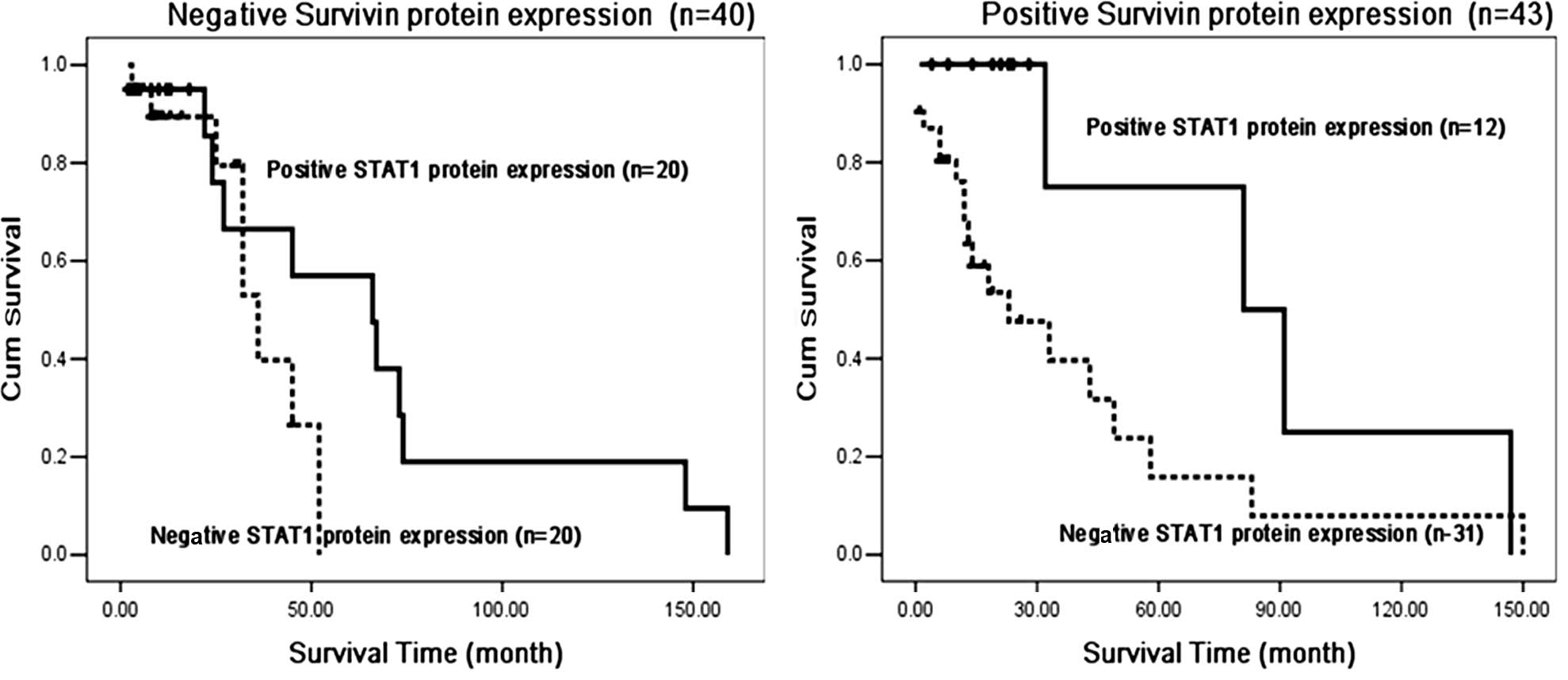
STAT1 protein expression is an
independent prognostic factor in Survivin protein-negative gastric
cancer tissues
Univariate and multivariate analyses indicated that
STAT1 protein expression (P=0.008, χ2=6.98), depth of
invasion (P=0.014, χ2=6.00) and N stage (P=0.026,
χ2=4.99) were independent prognostic factors of survival
for gastric cancer patients.
We also observed that STAT1 protein expression was
an independent prognostic factor in the negative Survivin protein
expression group (P=0.033, χ2=4.55), and had no
correlation with survival in the positive Survivin protein
expression group (P=0.17, χ2=1.92) (Fig. 5).
Discussion
Survivin is expressed in almost all human
malignancies as well as embryonic and fetal tissues, but is almost
undetectable in adult tissues (32). Overexpression of Survivin in cancer
invariably provides a survival advantage in tumor cells. Therefore,
lack of Survivin or disruption of the Survivin function causes cell
death, such as apoptosis and mitotic catastrophe (33). Limited studies have focused on the
mechanisms by which Survivin protein expression is regulated in
gastric cancer. Findings of a recent study showed that IFN-γ was
capable of down-regulating Survivin protein expression in gastric
cancer cells (27). STAT1 is an
important molecule in the IFN-γ-JAK/STAT-pathway. STAT1 and
Survivin regulate apoptosis, but their biological effects are
adverse. As a transcription factor, STAT1 up-regulates the
expression of caspases 3 and 7 simultaneously with the enzymatic
substrate of caspases 3 and 7. Survivin is capable of binding with
caspases 3 and 7 to inhibit their activity. The relationship of
STAT1 and Survivin in gastric cancer, and whether or not they
adjust the expression of one another, has yet to be elucidated. We
confirmed that IFN-γ down-regulates Survivin protein expression and
simultaneously up-regulates STAT1 protein expression in gastric
cancer cells. When IFN-γ and STAT1 ASON were administered to the
cell line together, we observed that STAT1 protein expression was
gradually increased and that Survivin protein expression was
gradually decreased in a concentration-dependent manner. These
results indicate that IFN-γ inhibits Survivin protein expression
via the IFN-γ-STAT1 signal pathway in gastric cancer.
Recently, another STATs family member, STAT3
protein, has been shown to correlate with Survivin and to have a
clear bearing on gastric cancer progression, although the detailed
mechanism for this relationship has yet to be clarified (34). The SGC7901 cell line that we used in
this study was derived from poorly differentiated and metastatic
gastric adenocarcinoma from the Chinese population, and exhibited a
high Survivin expression in the protein level (35). Our results showed that when the
SGC7901 cell line was treated with Survivin ASON, Survivin protein
expression was gradually increased and STAT1 protein expression was
gradually decreased in a manner dependent on the concentration of
Survivin ASON. These results indicate that Survivin may also
inhibit STAT1 protein expression in gastric cancer cells, and that
there is an antagonistic effect between STAT1 and Survivin in
gastric cancer cells.
STAT1 and Survivin are important apoptosis
regulators and have important clinical significance in gastric
cancer. STAT1 is a molecular marker involved in the prediction of
advanced gastric cancer and Survivin is a molecular marker of lymph
node metastasis in gastric cancer (26). In this study, STAT1 was found to be
negatively correlated with depth of invasion in Survivin
protein-negative tissues, and Survivin exhibited a negative
correlation with N stage in STAT1 protein-negative tissues. In
addition, STAT1 was an independent survival factor only in Survivin
protein-positive tissues. These results confirmed that there is
antagonistic effect between STAT1 and Survivin in gastric cancer
tissues, and that this antagonistic effect had clinical
significances in gastric cancer.
In conclusion, our study indicates that there is an
antagonistic effect between STAT1 and Survivin in gastric cancer,
and that this effect is of clinical significance. Thus, STAT1 and
Survivin may be potential molecular targets for cancer therapy,
which may allow for more individualized treatments of gastric
cancer patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China grant no. 81000884 (H. Deng), the Wuhan
Young Chenguang Foundation grant no. 20045006071-7 (H. Deng) and
the Hubei Natural Science Foundation grant no. 2006AB191 (H. Deng).
It was also supported by the National Natural Science Foundation of
China grant no. 0870981 (L.J. Liu).
References
|
1
|
Liu T, Wang XY, Song WJ, Zu CZ and Li Y:
Incidence of gastric malignant tumors during the past 20 years in
Tianjin. Shijie Huaren Xiaohua ZaZhi. 12:20–22. 2004.
|
|
2
|
Lanwers GY, Scoot GV and Karpeh MS:
Immunohistochemical evaluation of bcl-2 protein expression in
gastric adenocarcinoma. Cancer. 75:2209–2213. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambrosini G, Adida C and Altieri D: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nature Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaplan DH, Shankaran V, Dighe AS, Stockert
E, Aguet M, Old LJ and Schreiber RD: Demonstration of an
interferon-γ-dependent tumor surveillance system in immunocompetent
mice. Proc Natl Acad Sci USA. 95:7556–7561. 1998.
|
|
6
|
Lee CK, Rao DT, Gertner R, Gimeno R, Frey
AB and Levy DE: Distinct requirements for IFNs and STAT1 in NK cell
function. J Immunol. 165:3571–3577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee CK, Smith E, Gimeno R, Gertner R and
Levy DE: STAT1 affects lymphocyte survival and proliferation
partially independent of its role downstream of IFN-γ. J Immunol.
164:1286–1292. 2000.PubMed/NCBI
|
|
8
|
Shankaran V, Ikeda H, Bruce AT, White JM,
Swanson PE, Old LJ and Schreiber RD: IFNgamma and lymphocytes
prevent primary tumour development and shape tumour immunogenicity.
Nature. 410:1107–1111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu KD, Gaffen SL and Goldsmith MA:
JAK/STAT signaling by cytokine receptors. Curr Opin Immunol.
10:271–278. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schindler C and Darnell JE Jr:
Transcriptional responses to polypeptide ligands: the JAK-STAT
pathway. Annu Rev Biochem. 64:621–651. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dupuis S, Dargemont C, Fieschi C,
Thomassin N, Rosenzweig S, Harris J, Holland SM, Schreiber RD and
Casanova JL: Impairment of mycobacterial but not viral immunity by
a germline human STAT1 mutation. Science. 293:300–303. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Durbin JE, Hackenmiller R, Simon MC and
Levy DE: Targeted disruption of the mouse Stat1 gene results in
compromised innate immunity to viral disease. Cell. 84:443–450.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouchi T, Lee SW, Ouchi M, Aaronson SA and
Horvath CM: Collaboration of signal transducer and activator of
transcription 1 (STAT1) and BRCA1 in differential regulation of
IFN-γ target genes. Proc Natl Acad Sci USA. 97:5208–5213.
2000.PubMed/NCBI
|
|
16
|
Chin YE, Kitagawa M, Kuida K, Flavell RA
and Fu XY: Activation of the STAT signaling pathway can cause
expression of caspase 1 and apoptosis. Mol Cell Biol. 17:5328–5337.
1997.PubMed/NCBI
|
|
17
|
Salvesen GS and Duckett CS: Apoptosis: IAP
proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol.
3:401–410. 2002.
|
|
18
|
Srinivasula SM and Ashwell JD: IAPs:
what’s in a name? Mol Cell. 30:123–135. 2008.
|
|
19
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandele A, Prasad V, Jagtap JC, Shukla R
and Shastry PR: Upregulation of survivin in G2/M cells and
inhibition of caspase 9 activity enhances resistance in
staurosporine-induced apoptosis. Neoplasia. 6:29–40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
Caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
23
|
Lu B, Mu Y, Cao C, Zeng F, Schneider S,
Tan J, Price J, Chen J, Freeman M and Hallahan DE: Survivin as a
therapeutic target for radiation sensitization in lung cancer.
Cancer Res. 64:2840–2845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC,
Hwang JI, Chung CW, Jung YK and Oh BH: An anti-apoptotic protein
human survivin is a direct inhibitor of caspase-3 and -7. Biochem.
40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng H, Zhen HY, Zhou HY, Chen QX and Liu
LJ: Role of IFN-γ-STAT1 pathway in human gastric adenocarcinoma.
World Chin J Digestol. 17:1103–1107. 2009.
|
|
26
|
Deng H, Wu RL, Chen Y and Liu LJ: STAT1
and Survivin expression in full lymph node examined gastric cancer
by using tissue microarray technique. Chin Ger J Clin Oncol.
5:249–252. 2006. View Article : Google Scholar
|
|
27
|
Deng H, Huang X, Gao YJ, Zhen HY and Liu
LJ: Regulatory effect of IFN-γ on the survivin signaling pathway in
gastric adenocarcinoma. World Chin J Digestol. 18:3249–3253.
2010.
|
|
28
|
Beppu K, Morisaki T, Matsunaga H, Uchiyama
A, Ihara E, Hirano K, Kanaide H, Tanaka M and Katano M: Inhibition
of interferon-c-activated nuclear factor-κB by cyclosporin A: a
possible mechanism for synergistic induction of apoptosis. Biochem
Biophys Res Commun. 305:797–805. 2003.
|
|
29
|
Grandis JR, Drenning SD, Chakraborty A,
Zhou MY, Zeng Q, Pitt AS and Tweardy DJ: Requirement of Stat3 but
not Stat1 activation for epidermal growth factor receptor-mediated
cell growth in vitro. J Clin Invest. 102:1385–1392. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olie RA, Simões-Wüst AP, Baumann B, Leech
SH, Fabbro D, Stahel RA and Zangemeister-Wittke U: A novel
antisense oligonucleotide targeting survivin expression induces
apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer
Res. 60:2805–2809. 2000.PubMed/NCBI
|
|
31
|
Liu LJ and Zhang YT: The clinical research
of lymph node metastasis in gastric cancer. Chin J Exper Surgery.
12:91–92. 1995.
|
|
32
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar
|
|
33
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee J, Kang WK, Park JO, et al: Expression
of activated signal transducer and activator of transcription 3
predicts poor clinical outcome in gastric adenocarcinoma. Acta
Pathol Microbiol Immunol Scand Suppl. 117:598–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Han J, Wang LF, Lin SX, Yao LB, Yu Q
and Liu XP: Characteristics of anoikis resistance of human gastric
cancer cell lines. J Fourth Mil Med Univ. 24:485–488. 2003.
|