Introduction
Gastric cancer treated by surgical resection is
radical and shows a favorable prognosis; however, when cases are
inoperable due to the advanced stage, the prognosis is poor, with a
10% 5-year survival rate. Chemotherapies against gastric cancer
have been developed as combination chemotherapies since the 1980s.
In the 1990s, phase III randomized comparative studies between best
supportive care (BSC) and chemotherapies revealed a significant
improvement in overall survival rates (1–3).
Irinotecan hydrochloride (CPT-11), synthesized from
camptothecin contained in the Chinese tree Camptotheca
acuminate, inhibits type I topoisomerase and DNA synthesis, and
thus demonstrates antitumor effects. In Japan, CPT-11 has been
approved for various types of cancer, including small and non-small
cell lung cancer, uterine cervical cancer, ovarian cancer, gastric
cancer, colorectal cancer, breast cancer, squamous cell carcinoma
of the skin, and malignant lymphoma. The overall response rate (RR)
to CPT-11 monotherapy is reported to be 23.3% in late phase II
trials for advanced gastric cancer (4).
S-1 is an oral anticancer drug containing a
combination of tegafur (FT), a prodrug of 5-fluorouracil (5-FU),
5-chloro-2,4-dihydroxypyrimidine (CDHP) that inhibits the activity
of dihydropyrimidine dehydrogenase (DPD), and potassium oxonate
(Oxo), which reduces the gastrointestinal toxicity of 5-FU. The S-1
monotherapy for advanced gastric cancer revealed non-inferiority to
5-FU infusion in the JCOG9912 study. A subsequent study revealed
that a combination of S-1 and cisplatin (CDDP) is superior to the
S-1 monotherapy (5). Since these
trials, S-1 plus CDDP has been one of the standard chemotherapies
against advanced gastric cancer in Japan.
S-1 or S-1-containing regimens are used in adjuvant
chemotherapy following surgery or in first-line chemotherapy for
inoperable gastric cancer. One of the mechanisms of resistance
against S-1 is thought to be an increase of thymidylate synthase
(TS) activity, which is a target of 5-FU in tumor cells. A study
revealing that irinotecan downregulates intratumoral TS and makes
5-FU more effective in human colon cancer xenografts suggests the
possibility of overcoming S-1 resistance by adding irinotecan
(6). In this context, we planned a
feasibility study in which S-1-pretreated gastric cancer patients
were treated with a combination chemotherapy of S-1 and CPT-11 as a
feasibility test.
Materials and methods
Patient selection
Patients with histologically confirmed gastric
cancer with measureable or evaluable lesions were eligible for this
study. Patients were required to have been previously treated with
a first-line chemotherapy containing S-1, but not CPT-11. Other
eligibility criteria were: 20–75 years old, Eastern Cooperative
Oncology Group (ECOG) performance status (PS) of 0–2, capable of
oral intake, white blood cell count (WBC) of 3,500–12,000/μl,
neutrocyte count (Neu) >2,000/μl, platelet count (PLT)
>100,000/μl, hemoglobin (Hb) level >9.0 g/dl, serum total
bilirubin (T-bil) <1.5 mg/dl, serum aspartate aminotransferase
(AST) and serum alanine aminotransferase (ALT) <2 times the
normal limit, serum creatinine within the normal limit, creatinine
clearance calculated with the Cockcroft-Gault equation >50
ml/min, survival expectancy of at least 3 months, and written
informed consent for this study.
Study design
This study is a multicenter, non-randomized,
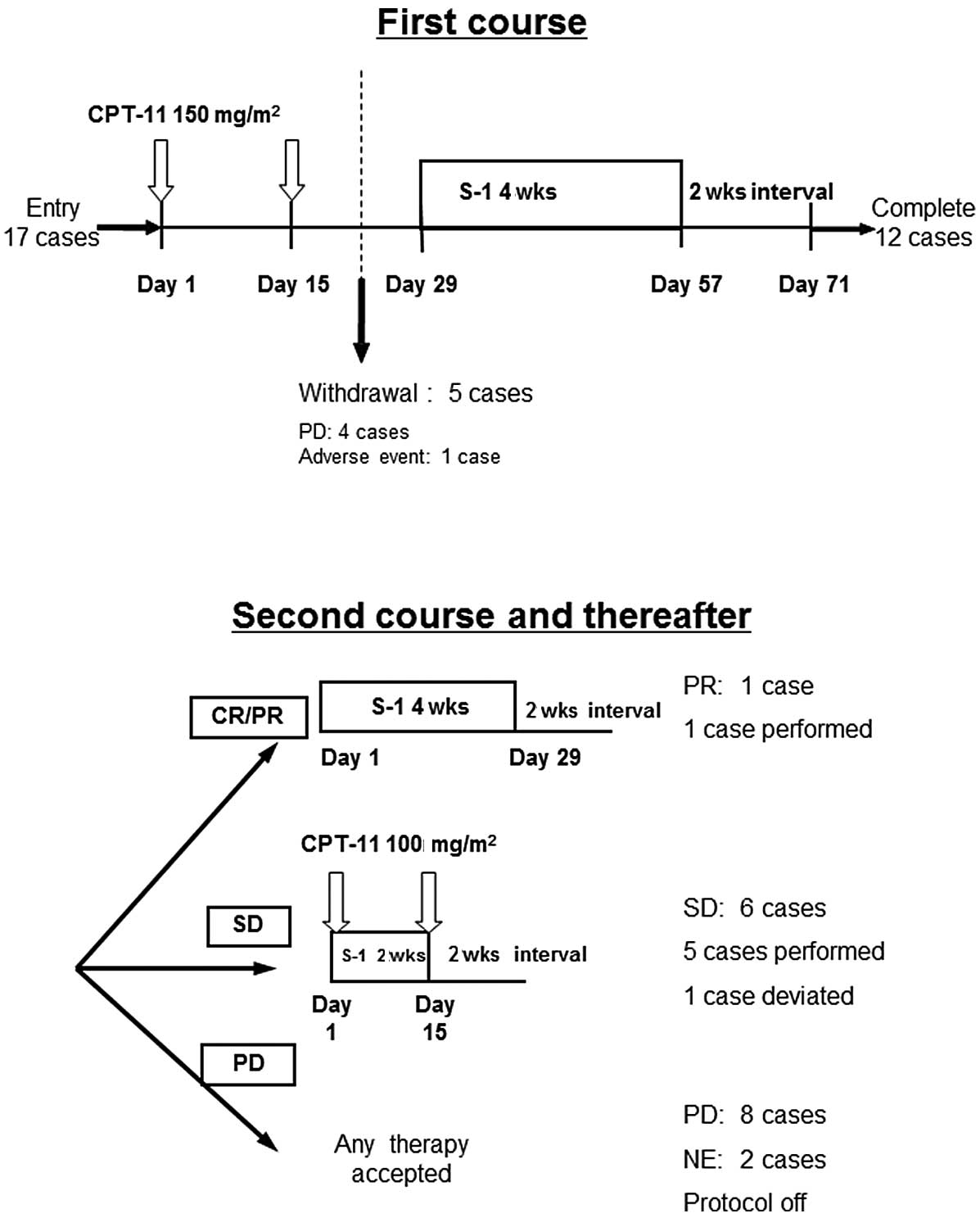
open-label feasibility study. An overview of the study is shown in
Fig. 1. In the first course, CPT-11
was administered intravenously at 150 mg/m2 on days 1
and 15. Subsequently, S-1 was administered orally for 4 weeks from
day 29 to 57, followed by a 2-week interval (sequential
S-1/CPT-11). The dosage of S-1 was based on body surface area
(BSA): 40 mg (BSA <1.25 m2), 50 mg (BSA ≥1.25 and
<1.5 m2) or 60 mg b.i.d. (BSA ≥1.5 m2). A
CT scan was performed during this 2-week interval to evaluate the
tumor response according to the Response Evaluation Criteria in
Solid Tumors (RECIST). When the tumor showed a complete response
(CR) or partial response (PR) in the first course, the same dose of
S-1 monotherapy was continued unless progressive disease (PD) was
observed. When the response was stable disease (SD), S-1 was
administered at the same dose for only 2 weeks (days 1–15), no drug
was administered for the following 2 weeks (4-week cycle) and
CPT-11 was administered intravenously at 100 mg/m2 on
days 1 and 15 (concurrent S-1/CPT-11) unless PD was observed. In
the case of PD, the study was terminated. There was no restriction
in third-line chemotherapy. Adverse events were evaluated using the
National Cancer Institute Common Terminology Criteria for Adverse
Events (NCI-CTCAE), version 3.0. The protocol was approved by the
Institutional Review Board of Hirosaki University School of
Medicine and other institutes. The primary endpoint of this study
was an antitumor effect and secondary endpoints were median
survival time (MST), progression-free survival (PFS),
time-to-treatment failure (TTF) and safety.
Statistical analysis
For prognostic values, the MST, PFS and TTF were
calculated using the Kaplan-Meier method from the date of
registration. The estimated RR and threshold RR were set at 15 and
5%, respectively, and the calculated minimum sample size was
estimated to be 43, with an α value of 0.05 and a β value of 0.20.
We estimated that the number of possible patient exclusions or
dropouts would be 2, and the sample size was increased to 45. In
the planned interim analysis, when the number of response cases was
≤1 out of 12 enrolled cases, the study would be terminated since
the estimated RR would be <5%.
Results
Patient characteristics
Patient characteristics are shown in Table I. A total of 17 patients (14 males
and 3 females), were enrolled in this study between November 2004
and June 2006. The median age was 63 years, and a PS of 0/1 was
observed in 14/3 patients, respectively. Histological results
revealed that 8 patients had intestinal and 9 had diffuse cancer
types. Eleven patients had primary lesions and 5 did not, and no
information was available in 1 case. Prior to enrollment in this
study, 7 patients underwent surgical treatment and 10 patients did
not. Previous chemotherapy regimens were S-1 monotherapy (n=9),
S-1/CDDP (n=7) and S-1/taxotere (TXT) (n=2). None of the patients
had received radiotherapy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Patient no. |
|---|
| Gender (M/F) | 14/3 |
| Age
(mean/median) | 59.6/63 |
| Performance status
(0/1) | 14/3 |
|
Untreated/recurrence | 10/7 |
| Histology
(intestinal/diffuse) | 8/9 |
| Primary lesion
(+/−/unknown) | 11/5/1 |
| Metastasis |
| Lung | 2 |
| Liver | 8 |
| Bone | 1 |
| Abdominal lymph
node | 11 |
| Other | 5 |
| Radiotherapy
(+/−) | 0/17 |
| Surgical operation
(+/−) | 7/10 |
| Prior chemotherapy
regimen |
| S-1 | 8 |
| S-1/CDDP | 7 |
| S-1/taxotere | 2 |
| Detail of S-1
resistance |
| Unresectable and
formerly effective | 5 |
| Unresectable and
formerly resistant | 5 |
| Recurrence during
adjuvant chemotherapy | 5 |
| Recurrence after
adjuvant chemotherapy | 2 |
Tumor response and survival rate
Five out of 17 patients ceased chemotherapy halfway
through the first course of treatment due to PD in 4 patients and
an adverse event in 1 patient. Twelve patients completed the first
course. However, 5 patients out of 12 dropped out due to PD
following completion of the first course. The tumor response
following the first cycle is shown in Table II. Only 1 patient showed PR
following the first course (RR, 5.9%; 95% CI, 0.1–28.7). The PR
patient received 2 cycles of S-1 monotherapy and the 5 SD patients
received a median of 2 cycles (mean 3.6 cycles; range 1–6) of
concurrent S-1/CPT-11. One of the 5 SD patients showed PR during
the S-1/CPT-11 chemotherapy. Therefore, the most positive tumor
response rate was 2 PR, 5 SD, 8 PD and 2 not evaluable (NE) (RR,
11.8%; 95% CI, 1.5–31.4) (Table
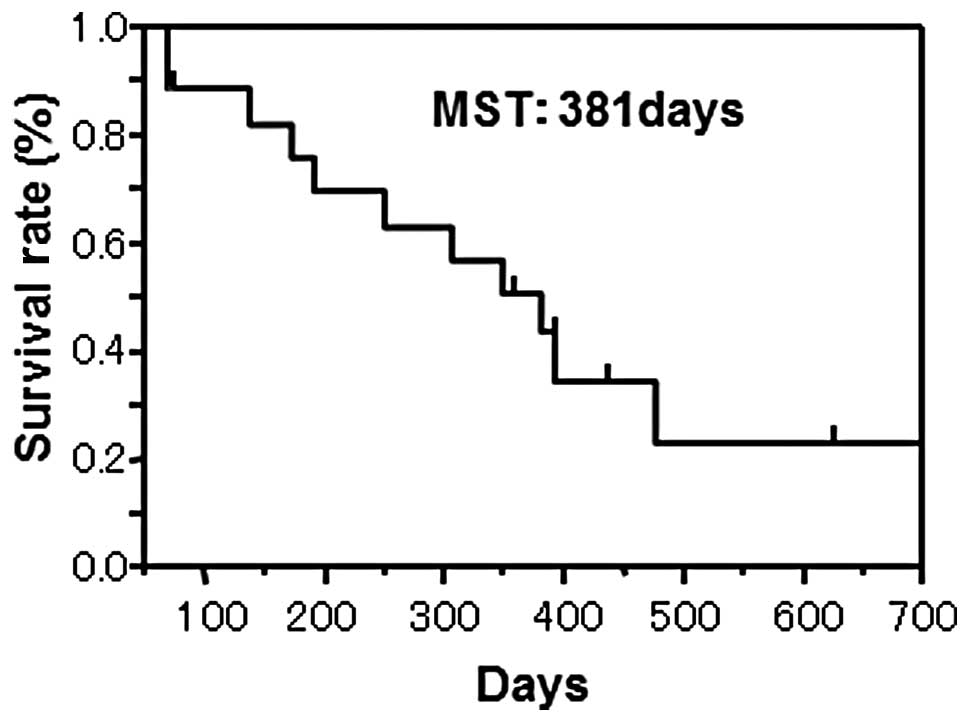
II). An overall survival curve is shown in Fig. 2. Regarding the prognostic analysis
by the log-rank test, the MST, median TTF and PFS were 381, 69 and
71 days, respectively.
 | Table IITumor response. |
Table II
Tumor response.
A, After the first
course.
|
| n | CR | PR | SD | PD | NE | RR |
| 17 | 0 | 1 | 6 | 8 | 2 | 5.9% |
|
| B, Overall. |
|
| n | CR | PR | SD | PD | NE | RR |
| 17 | 0 | 2 | 5 | 8 | 2 | 11.8% |
Retrospectively, we performed a subset analysis
using stratification by different patient background of S-1
resistance. We compared the S-1-sensitive group (for which the
first-line chemotherapy was effective or 6-month adjuvant
chemotherapy was completed) and the S-1-resistant group (for which
the first-line chemotherapy was not effective or adjuvant
chemotherapy was not completed). However, no significant difference
in MST, TTF or PFS was observed in the subset analysis.
After 17 cases had enrolled in this study, an
interim analysis was performed according to the initial schedule.
Since the RR following the first course was only 5.9% and 10 cases
received only the first course of chemotherapy, this protocol was
evaluated as being unfeasible.
Toxicity
Observed toxicities associated with this
chemotherapy protocol are shown in Table III. The most common toxicities
were leukocytopenia and neutropenia in both the first and
subsequent courses (58.8 and 66.7%, respectively). Nausea and
vomiting were also common. One patient had G4 neutropenia and a
further patient had G4 anorexia. Diarrhea, a common adverse event
of CPT-11 and S-1, was observed more frequently in concurrent
S-1/CPT-11 chemotherapy, but not in the sequential regimen.
Chemotherapy-related mortality was not observed.
 | Table IIIAdverse events during the course of
the study. |
Table III
Adverse events during the course of
the study.
| A, First course. |
|---|
|
|---|
| n=17 | G1 | G2 | G3 | G4 | All grades (%) | >G3 (%) |
|---|
| Leukocytopenia | 3 | 5 | 2 | 0 | 58.8 | 11.8 |
| Neutropenia | 1 | 3 | 5 | 1 | 58.8 | 35.3 |
| Anemia | 1 | 3 | 2 | 0 | 35.3 | 11.8 |
| Thrombocytopenia | 1 | 0 | 0 | 0 | 5.9 | 0.0 |
| Nausea/vomiting | 4 | 1 | 3 | 0 | 47.1 | 17.6 |
| Stomatitis | 0 | 1 | 0 | 0 | 5.9 | 0.0 |
| Diarrhea | 1 | 2 | 1 | 0 | 23.5 | 5.9 |
| Exanthema | 0 | 0 | 1 | 0 | 5.9 | 5.9 |
| Alopecia | 3 | 3 | 0 | 0 | 35.3 | 0.0 |
| Anorexia | 1 | 0 | 2 | 1 | 23.5 | 17.6 |
| General malaise | 2 | 1 | 3 | 0 | 35.3 | 17.6 |
| Abdominal pain | 0 | 1 | 0 | 0 | 5.9 | 0.0 |
| Abdominal
distension | 0 | 1 | 0 | 0 | 5.9 | 0.1 |
|
| B, After the first
course. |
|
| n=6 | G1 | G2 | G3 | G4 | All grades (%) | >G3 (%) |
|
| Leukocytopenia | 1 | 3 | 0 | 0 | 66.7 | 0.0 |
| Neutropenia | 1 | 0 | 2 | 1 | 66.7 | 50.0 |
| Anemia | 0 | 0 | 1 | 0 | 16.7 | 11.8 |
|
Nausea/vomiting | 3 | 0 | 0 | 0 | 50.0 | 17.6 |
| Stomatitis | 1 | 0 | 0 | 0 | 16.7 | 0.0 |
| Diarrhea | 0 | 2 | 1 | 0 | 50.0 | 5.9 |
| Alopecia | 1 | 1 | 0 | 0 | 33.3 | 0.0 |
| Anorexia | 2 | 1 | 1 | 0 | 66.6 | 17.6 |
| General
malaise | 1 | 1 | 0 | 0 | 33.3 | 17.6 |
| Skin
pigmentation | 1 | 0 | 0 | 0 | 16.7 | 0.1 |
Discussion
This trial tested the feasibility of sequential
CPT-11 and S-1 in S-1-refractory gastric cancer patients. The RR
following the first course was only 5.9% and the most positive RR
was 11.8% (Table II). The MST was
381 days (Fig. 2) and the median
TTF and PFS were 69 and 71 days, respectively. Therapy-related
mortality was not observed, although leukocytopenia and neutropenia
were observed in over half of the patients (Table III), indicating moderate toxicity
of this protocol. Since the RR following the first course was lower
than that estimated in an interim analysis, the trial was
terminated.
The rationale of this study was that a preceding
administration of CPT-11 against S-1-resistant tumors may affect
the downregulation of TS, resulting in recovered sensitivity to S-1
(6). We chose the sequential,
rather than concurrent, administration of CPT-11 and S-1, for two
reasons. The first was that the downregulation of TS requires a
certain period of time to take effect following CPT-11
administration. The second was that avoidance of concurrent
administration of these drugs may alleviate adverse effects and
achieve a longer continuity period of chemotherapy.
Practical uses of the S-1/CPT-11 combination have
been reported (7) as a second-line
therapy. It was reported that the S-1 monotherapy, following
failure of preceding S-1-containing regimens, was worthy of testing
in larger-scale clinical trials. However, a recent study negatively
evaluated this re-use of S-1 (8).
This strongly suggests that continuation of S-1 administration
following failure is pointless.
Consequently, the reversal of S-1 resistance
indicates a potential way to reuse this drug. DPD, TS and CYP2A6
are involved in fluorouracil drug resistance, and modulators of
these enzymes are candidates for concurrent or sequential usage. It
is reported that inter-individual deviation in the gene expression
and activity of these enzymes are associated with the ability to
predict the effects of the chemotherapy (9–12).
This suggests that tailor-made chemotherapy using intratumoral TS
activity is possible. Using a xenograft model Fukushima et
al reported that CPT-11 reversed chemo-resistance against 5-FU
(6). The concept of our study
originated from this application to clinical use.
As a result, the overall RR was 11.8% and the TTF,
PFS and MST were 69, 71 and 381 days, respectively. The TTF and PFS
were relatively short but the MST was long. However, with regard to
our main aim of testing, the reversal of S-1 resistance by a
preceding treatment of CPT-11 was unfeasible since the RR following
the first cycle was lower than estimated.
We investigated whether the time point of S-1
resistance acquisition affected the RR of this study by additional
subset analyses. The previously resistant group tended to
demonstrate a poor response, although the difference was not
significant. This may be due to the small sample size.
Three patients withdrew from the study due to
adverse events, rather than PD. A number of ≥G3 non-hematological
adverse events were observed including 3 cases of nausea and
vomiting, 1 of diarrhea, 1 of exanthema, 2 of anorexia, 3 of
general malaise, and one ≥G4 hematological adverse event in the
first course. Nine G3 hematological adverse events also occurred.
Intensity of CPT-11 administration was found to be over the
recommended dose, although we did not determine the
uridine-diphosphate glucuronosyltransferase (UGT) 1A1 polymorphism
(13).
A randomized, multicenter phase II/III study, JACCRO
GC-05, comparing CPT-11/S-1 and CPT-11 monotherapy as a second-line
therapy in S-1-resistant patients with advanced gastric cancer is
currently under way. Future findings may thus reveal whether CPT-11
provides any favorable effects on S-1 resistance, which was not
shown in the present study.
In conclusion, S-1/CPT-11 sequential therapy based
on the reversal of S-1 drug resistance by CPT-11 was tested,
although the interim analysis revealed a lower response rate than
expected. This protocol was concluded as being unfeasible.
References
|
1
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glimelius B, Hoffman K, Haglund U, Nyrén O
and Sjödén PO: Initial or delayed chemotherapy with best supportive
care in advanced gastric cancer. Ann Oncol. 5:189–190.
1994.PubMed/NCBI
|
|
3
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomized comparison of 5-fluorouracil, epirubicin, and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Brit J
Cancer. 71:587–591. 1995.PubMed/NCBI
|
|
4
|
Futatsuki K, Wakui A, Nakao I, et al: Late
phase II study of irinotecan hydrochloride (CPT-11) in advanced
gastric cancer. CPT-11 Gastrointestinal Cancer Study Group. Gan To
Kagaku Ryoho. 21:1033–1038. 1994.PubMed/NCBI
|
|
5
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukushima M, Sakamoto K, Ohshimo H,
Nakagawa F and Taguchi T: Irinotecan overcomes the resistance to
5-fluorouracil in human colon cancer xenografts by down-regulation
of intratumoral thymidylate synthase. Oncol Rep. 24:835–842. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takiuchi H: Combination therapy with S-1
and irinotecan (CPT-11) for advanced or recurrent gastric cancer.
Gastric Cancer. 12:55–59. 2009. View Article : Google Scholar
|
|
8
|
Ono A, Boku N, Onozawa Y, et al: Activity
of S-1 in advanced or recurrent gastric cancer patients after
failure of prior chemotherapy, including irinotecan + cisplatin or
fluorouracil (except S-1). Jpn J Clin Oncol. 39:332–335. 2009.
|
|
9
|
Miyamoto S, Ochiai A, Boku N, et al:
Discrepancies between the gene expression, protein expression, and
enzymatic activity of thymidylate synthase and dihydropyrimidine
dehydrogenase in human gastrointestinal cancers and adjacent normal
mucosa. Int J Oncol. 18:705–713. 2001.
|
|
10
|
Fukushima M, Morita M, Ikeda K and
Nagayama S: Population study of expression of thymidylate synthase
and dihydropyrimidine dehydrogenase in patients with solid tumors.
Int J Mol Med. 12:839–844. 2003.PubMed/NCBI
|
|
11
|
Ichikawa W, Takahashi T, Suto K, et al:
Thymidylate synthase predictive power is overcome by irinotecan
combination therapy with S-1 for gastric cancer. Br J Cancer.
91:1245–1250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takiuchi H, Kawabe S, Gotoh M and Katsu K:
Thymidylate synthase gene expression in primary tumors predicts
activity of S-1-based chemotherapy for advanced gastric cancer.
Gastrointest Cancer Res. 1:171–176. 2007.PubMed/NCBI
|
|
13
|
Innocenti F, Undevia SD, Iyer L, et al:
Genetic variants in the UDP-glucuronosyltransferase 1A1 gene
predict the risk of severe neutropenia of irinotecan. J Clin Oncol.
22:1382–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|