Introduction
Head and neck squamous cell carcinomas (HNSCCs)
manifest various clinical behaviors according to their area of
origin, i.e., from various parts of the head and neck mucosa.
However, genetic and epigenetic factors involved in the
carcinogenesis of HNSCC in various tissues have yet to be
thoroughly studied and evaluated. Although there have been numerous
studies on the hypermethylation of the promoter regions of tumor
suppressor genes, particularly p16 (1–9),
differences in the frequencies of methylation among the cancer
sites in the head and neck region have yet to be elucidated, partly
due to the low number of samples. However, we have previously
pointed out differences in the frequencies of allelic loss of
certain areas of the genome between oral cancers and cancers of the
pharynx and larynx (10).
To elucidate the differences between oral cancer and
cancers of the pharynx and larynx, we investigated the genetic and
epigenetic changes in these tumors using molecular biology methods.
We analyzed the hypermethylation of the promoter region of the p16
tumor suppressor gene as an epigenetic change. We also examined
allelic loss of certain areas of the genome, i.e., 3p21, 9p21 and
17p13 (TP53) as genetic changes. Comparing the results of these two
approaches reveals novel findings regarding the differences between
oral cancer and cancers of the pharynx and larynx.
Materials and methods
Patients and tissue specimens
Primary tumors were removed surgically or obtained
as biopsies prior to treatment during the period between May 1994
and November 2000, following an approved protocol at the Miyagi
Cancer Center. The clinical features of the patients are summarized
in Table I.
 | Table IClinical features of the patients and
tumor location. |
Table I
Clinical features of the patients and
tumor location.
| Oral cavity | Pharynx | | |
|---|
|
|
| | |
|---|
| Overall | Tongue | FOM | BM | Gingiva | Overall | Meso | Hypo | Larynx | P-value |
|---|
| Age | | | | | | | | | | |
| 45> | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.021 (OC vs. P) |
| 45< | 41 | 28 | 6 | 4 | 3 | 39 | 14 | 25 | 33 | |
| Gender | | | | | | | | | | |
| Female | 14 | 9 | 0 | 4 | 1 | 2 | 0 | 1 | 1 | 0.0034 (OC vs.
P) |
| Male | 33 | 25 | 6 | 0 | 2 | 37 | 14 | 24 | 34 | 0.0018 (OC vs.
L) |
| Tumor
sizea,b | | | | | | | | | | |
| T1 | 8 | 6 | 2 | 0 | 0 | 1 | 0 | 1 | 8 | NS |
| T2 | 20 | 14 | 3 | 2 | 1 | 19 | 4 | 15 | 14 | |
| T3 | 13 | 10 | 1 | 2 | 0 | 9 | 6 | 3 | 6 | |
| T4 | 3 | 1 | 0 | 0 | 2 | 9 | 4 | 5 | 6 | |
| Lymph node
involvementa | | | | | | | | | | N0 vs. others |
| N0 | 28 | 20 | 4 | 2 | 2 | 10 | 2 | 8 | 29 | 0.0016 (OC vs.
P) |
| N1 | 8 | 6 | 1 | 1 | 0 | 7 | 3 | 4 | 1 | <0.0001 (P vs.
L) |
| N2 | 10 | 7 | 1 | 1 | 1 | 20 | 9 | 11 | 4 | |
| N3 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 1 | |
|
Stagea | | | | | | | | | | I and II vs. III
and IV |
| I | 8 | 7 | 1 | 0 | 0 | 1 | 0 | 1 | 7 | 0.0004 (OC vs.
P) |
| II | 17 | 12 | 3 | 1 | 1 | 5 | 0 | 5 | 11 | 0.0009 (P vs.
L) |
| III | 10 | 7 | 1 | 2 | 0 | 9 | 4 | 5 | 6 | |
| IV | 12 | 8 | 1 | 1 | 2 | 24 | 10 | 14 | 11 | |
| Total | 47 | 34 | 6 | 4 | 3 | 39 | 14 | 25 | 35 | |
This study was approved by the Institutional Review
Boards of Tohoku University and the Miyagi Cancer Center. Surgical
specimens were obtained under informed consent, and written
informed consent was obtained from all patients.
DNA preparation
Genomic DNA was extracted from the frozen tissue
samples using proteinase K digestion and phenol extraction
according to standard methods (11,12).
Detection of hypermethylation in the p16
gene promoter region
The detection of gene hypermethylation was performed
using sodium bisulfite for DNA modification followed by
methylation-specific polymerase chain reaction (MSP). DNA
modification was performed using a CpGenome DNA Modification Kit
(Chemicon International, Temecula, CA, USA). The sequences of the
primers used were as follows: p16U forward, 5′-TTATT
AGAGGGTGGGGTGGATTGT-3′ and reverse, 5′-CAACCCC AAACCACAACCATAA-3′;
p16M forward, 5′-TTATTAGA GGGTGGGCGGATCGC-3′ and reverse,
5′-GACCCCGAACC GCGACCGTAA-3′. The annealing temperatures of the PCR
reactions for p16U and p16M were 60 and 69°C, respectively.
Purified PCR products were separated by electrophoresis on 3%
agarose gels containing 1 mg/ml ethidium bromide.
Detection of allelic loss
The polymorphic markers, D3S1067, IFNA, D3S171 and
TP53, were purchased from Invitrogen (Carlsbad, CA, USA) and each
forward primer of those markers was labeled using
[γ-32p]ATP and polynucleotide kinase (Takara Shuzo,
Kyoto, Japan). PCR was performed using a thermal cycler
(Perkin-Elmer Cetus, Boston, MA, USA) for 36 cycles under optimal
conditions, i.e., 1 min at 94°C, 1 min at 56°C and 1 min at 72°C,
except for the first denaturation, which was performed at 94°C for
5 min. Purified PCR products were separated by electrophoresis on
6% polyacrylamide gels containing 8.3 M urea (10).
Statistical analysis
Statistical analyses of the data were performed
using the χ2 test.
Results
Table I shows the
clinicopathological features of the patients and their tumors. The
ages of the patients with oral cancers were significantly lower
than those with cancers of the pharynx and larynx. As for gender,
the number of female patients was significantly higher among
patients with oral cancer than those with cancers of the pharynx
and larynx. Advanced stage (stage III and IV) patients were
significantly higher in patients with cancers of the pharynx and
larynx than those with oral cancer. This is due to the fact that
lymph node metastases were significantly more frequent in patients
with cancers of the pharynx and larynx than in those with oral
cancer.
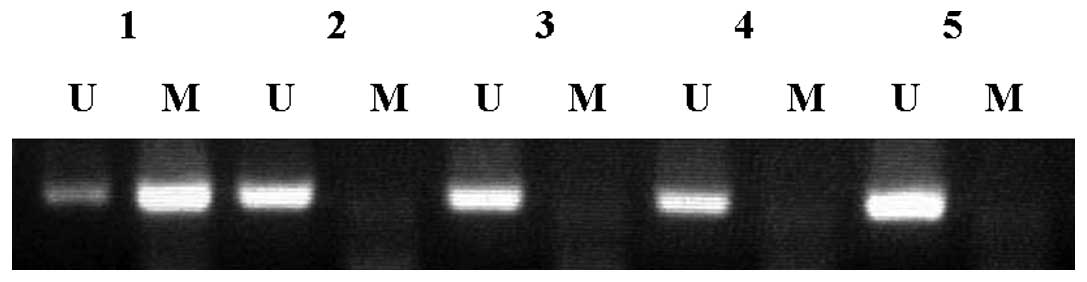
Hypermethylation of the promoter region of the p16
tumor suppressor gene was detected using the MSP method. We
examined 47 oral cancers, 39 pharyngeal cancers and 35 laryngeal
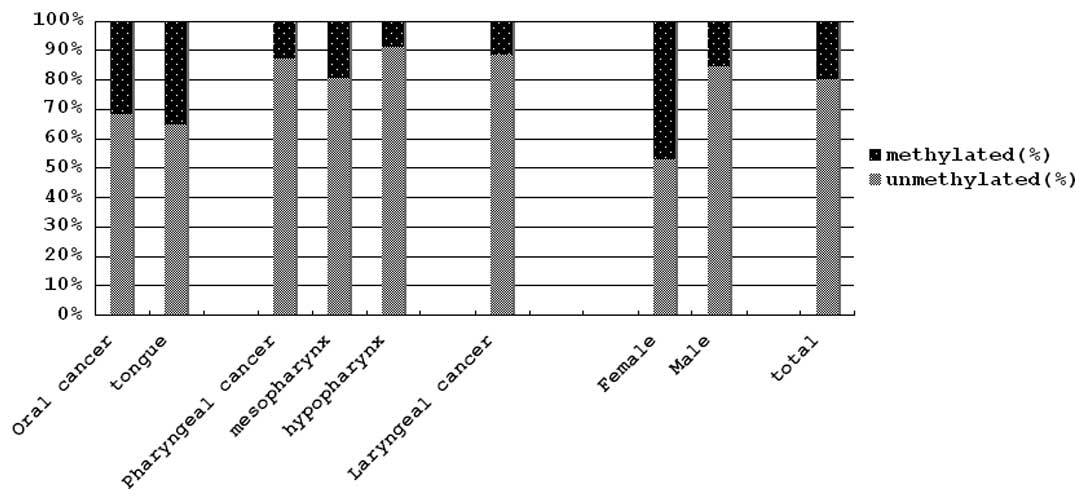
cancers. Representative results are shown in Fig. 1 and all results are summarized in
Table II and Fig. 2. The frequencies of methylation of
the promoter region of the p16 gene in oral and tongue cancers were
31.9 and 35.3%, respectively. On the contrary, those of pharyngeal
and laryngeal cancers were 12.8 and 11.4%, respectively, and those
of mesopharyngeal and hypopharyngeal cancers were 21.4 and 8.7%,
respectively. The frequency of methylation in oral cancers was
significantly higher than in hypopharyngeal cancers (p=0.047). The
frequency of methylation in tongue cancers was significantly higher
than in pharyngeal, hypopharyngeal and laryngeal cancers (p=0.046,
0.033 and 0.039, respectively). However, the frequency of
methylation in tumors of female patients (47.1%) was significantly
higher than in tumors of male patients (15.4%) (p=0.0067).
 | Table IIHypermethylation of the p16 promoter
region, tumor location and gender. |
Table II
Hypermethylation of the p16 promoter
region, tumor location and gender.
| Unmethylated | Methylated | Methylated (%) | P-value |
|---|
| Oral cancer | 32 | 15 | 31.9 | 0.047
(vs.HP)a |
| Tongue | 22 | 12 | 35.3 | 0.046
(vs.P)b |
| Floor of
mouth | 6 | 0 | | |
| Buccal mucosa | 1 | 3 | | |
| Gingiva | 3 | 0 | | |
| Pharyngeal
cancer | 34 | 5 | 12.8 | |
| Mesopharynx | 11 | 3 | 21.4 | |
| Hypopharynx | 23 | 2 | 8.7 | 0.033 (vs.
T)c |
| Laryngeal
cancer | 31 | 4 | 11.4 | 0.039 (vs.
T)d |
| Gender |
| Female | 9 | 8 | 47.1 | 0.0067 |
| Male | 88 | 16 | 15.4 | |
| Total | 97 | 24 | 19.8 | |
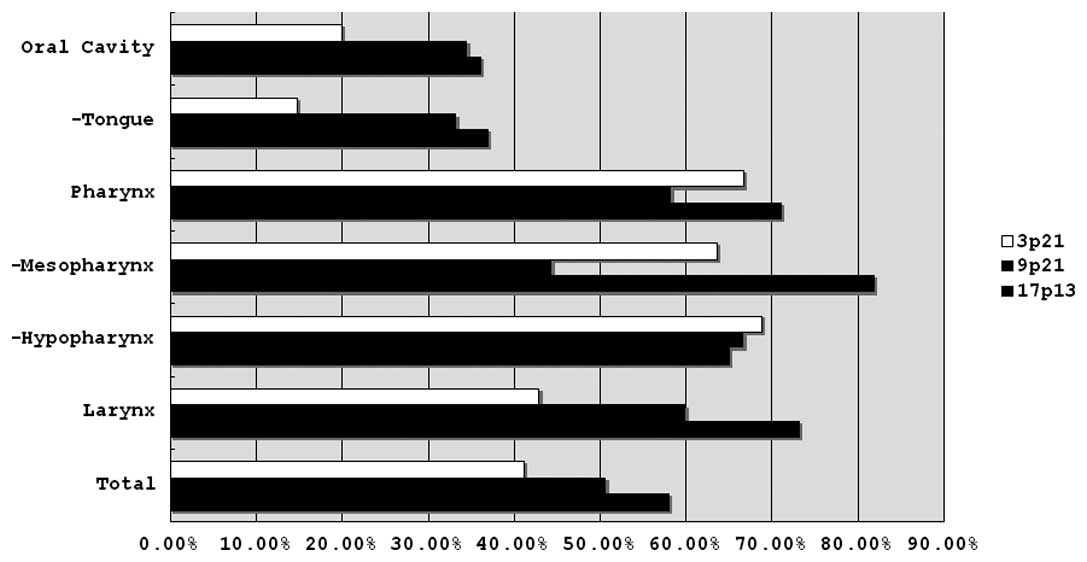
Specimens of 47 oral, 39 pharyngeal and 35 laryngeal
squamous cell carcinomas were characterized for allelic loss of
certain areas of the genome, i.e., 3p22, 9p21 and 17p13 (TP53). The
frequency of loss of heterozygosity (LOH) at 3p21 in pharyngeal
cancers (66.7%) was significantly higher than in oral and tongue
cancers (20.0 and 14.8%) (p=0.0006 and 0.0003, respectively)
(Table IIIA). The frequencies of
LOH at 3p21 in mesopharyngeal and hypopharyngeal cancers (63.6 and
68.8%) were also significantly higher than in oral cancers (p=0.018
and p=0.002, respectively). The frequencies of LOH at 3p21 in
mesopharyngeal and hypopharyngeal cancers were also significantly
higher than in tongue cancers (p=0.009 and p=0.001,
respectively).
 | Table IIILOH at 3p21, 9p21 and TP53 and tumor
location. |
Table III
LOH at 3p21, 9p21 and TP53 and tumor
location.
| A, LOH at 3p21 and
location of tumors. |
|---|
|
|---|
| 3p21 |
|---|
|
|
|---|
| Location | N | LOH(+) | LOH(−) | P-value |
|---|
| Oral cavity | 35 | 7 | 28 | p=0.018
(MP)a, p=0.002 (HP)b |
| Tongue | 27 | 4 | 23 | p=0.009
(MP)c, p=0.001 (HP)d |
| Floor of
mouth | 4 | 1 | 3 | |
| Buccal mucosa | 2 | 1 | 1 | |
| Gingiva | 2 | 1 | 1 | |
| Pharynx | 27 | 18 | 9 | p=0.0006
(OC)e, p=0.0003 (T)f |
| Mesopharynx | 11 | 7 | 4 | |
| Hypopharynx | 16 | 11 | 5 | |
| Larynx | 28 | 12 | 16 | |
| Total | 90 | 37 | 53 | |
| B, LOH at 9p21 and
location of tumors. |
|---|
|
|---|
| 9p21 |
|---|
|
|
|---|
| Location | N | LOH(+) | LOH(−) | P-value |
|---|
| Oral cavity | 26 | 9 | 17 | - |
| Tongue | 18 | 6 | 12 | - |
| Floor of
mouth | 4 | 2 | 2 | - |
| Buccal mucosa | 3 | 0 | 3 | - |
| Gingiva | 1 | 1 | 0 | - |
| Pharynx | 24 | 14 | 10 | - |
| Mesopharynx | 9 | 4 | 5 | - |
| Hypopharynx | 15 | 10 | 5 | - |
| Larynx | 25 | 15 | 10 | - |
| Total | 75 | 38 | 37 | - |
| C, LOH at TP53 and
location of tumors. |
|---|
|
|---|
| TP53 |
|---|
|
|
|---|
| Location | N | LOH(+) | LOH(−) | P-value |
|---|
| Oral cavity | 36 | 13 | 23 | p=0.02 (MP)a, p=0.009 (P)b |
| Tongue | 27 | 10 | 17 | p=0.03 (MP)c, p=0.02 (P)d |
| Floor of
mouth | 4 | 1 | 3 | |
| Buccal mucosa | 3 | 0 | 3 | |
| Gingiva | 2 | 2 | 0 | |
| Pharynx | 31 | 22 | 9 | |
| Mesopharynx | 11 | 9 | 2 | |
| Hypopharynx | 20 | 13 | 7 | |
| Larynx | 26 | 19 | 7 | p=0.009
(OC)e, p=0.018 (T)f |
| Total | 93 | 54 | 39 | |
Although the frequencies of LOH at 9p21 in
pharyngeal (58.3%) and laryngeal cancers (60.0%) were higher than
in oral cancers (34.6%), significant differences were not observed.
The frequencies of LOH at 9p21 in mesopharyngeal (44.4%) and
hypopharyngeal cancers (66.7%) were also higher than in tongue
cancers (33.3%). However, significant differences were also not
observed (Table IIIB).
The frequencies of LOH at 17p13 in pharyngeal
(71.0%) and laryngeal cancers (73.1%) were significantly higher
than in oral cancers (36.1%) (p=0.009 and p=0.009, respectively).
The frequencies of LOH at 17p13 in pharyngeal and laryngeal cancers
were significantly higher than in tongue cancers (37.0%) (p=0.02
and p=0.018, respectively). The frequency of LOH at 17p13 in
mesopharyngeal cancers (81.8%) was significantly higher than in
oral and tongue cancers (p=0.02 and p=0.03, respectively) (Table IIIC). These results are summarized
in Fig. 3.
Discussion
We focused on differences in HNSCCs at various
regions of origin with respect to the genetic and epigenetic
aspects of the tumors. Although we have previously reported
differences in the frequencies of allelic loss in several regions
of head and neck cancers (10), in
the present study our results indicate that there are marked
differences in the frequencies of hypermethylation of genes between
oral cancers and cancers of the pharynx and larynx, as well as
allelic loss.
There have been numerous studies on the
hypermethylation status of the promoter region of the p16 tumor
suppressor gene. The frequency of methylation was found to be
approximately 30% in head and neck squamous cell carcinoma
specimens obtained from surgically removed tumors (1–9).
Although several reports have described the hypermethylation of the
promoter region of the p16 tumor suppressor gene in oral cancer and
in cancers of the pharynx and larynx, there have not been any
reports which point out differences in the frequencies of the
methylation among those cancers. This may have been largely due to
the low number of samples.
Our results indicate that the hypermethylation of
the promoter region of the p16 tumor suppressor gene is related to
gender, i.e., female patients tend to have more frequent
hypermethylation of the p16 gene. El-Naggar et al also
reported that there was no significant correlation between p16
alterations and the clinicopathological factors of the patients,
with the exception of gender (3).
Although their methods of characterization were different from ours
and the detection of the hypermethylation itself was carried out
using a method different from ours, their results are compatible
with ours. Oral cancer patients included significantly more females
than patients with cancers of the pharynx and larynx. We assumed
that this tendency resulted in the significant differences of the
frequencies of hypermethylation between oral cancer and cancers of
the pharynx and larynx. This indicates that the hypermethylation of
the promoter region of the p16 tumor suppressor gene may be a
crucial factor in the carcinogenesis of squamous cell carcinomas of
the head and neck in female patients.
In HNSCC, allelic loss on chromosome 9p occurs most
frequently at 9p21 (1,13–15)
and it occurs as an early event in the process of carcinogenesis in
head and neck cancers (13). This
region of 9p21 includes the tumor suppressor and cell cycle
regulator gene, p16 (INK4a, also known as CDKN2 or MTS1) (16–19).
Although homozygous deletions, monosomy and point mutations of p16
occur frequently in HNSCC cell lines, corresponding evidence of the
same alterations in primary cancer specimens is less common
(1,14,20,21).
However, primary HNSCC shows frequent methylation of the promoter
region of the p16 gene (22,23)
and LOH at 9p21. It appears that these two types of gene alteration
are the main mechanism underlying the inactivation of the p16 gene
as a tumor suppressor. In our study, LOH at 9p21 was observed in
approximately half of the specimens, and it was more frequent in
cancers of the pharynx and larynx than in oral cancer. In contrast,
hypermethylation of the p16 gene was more frequent in oral cancer
than in cancers of the pharynx and larynx. These results indicate
that the type of inactivation of the p16 gene is different in
various sites of head and neck squamous cell carcinomas. It should
also be noted that allelic loss at 3p and 17p is significantly more
frequent in cancers of the pharynx and larynx than in oral
cancers.
In conclusion, although these tumors were diagnosed
as squamous cell carcinomas, the process of carcinogenesis may be
different in tumors located in different parts of the head and
neck. Loss of function of tumor suppressor genes by allelic loss is
mainly active and give rise to tumors in the pharynx and larynx,
while loss of function due to methylation of the promoter region of
the genes is related to carcinogenesis in the oral cavity, which
may place additional burden on allelic loss.
Acknowledgements
We are grateful to Ms. Elizabeth Hearing for her
editorial work in the preparation of this manuscript.
References
|
1
|
Reed AL, Califano J, Cairns P, Westra WH,
Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H, Barterk J
and Sidransky D: High frequency of p16 (CDKN2/MTS-1/INK4A)
inactivation in head and neck squamous cell carcinoma. Cancer Res.
56:3630–3633. 1996.PubMed/NCBI
|
|
2
|
Gonzalez MV, Pello MF, Lopez-Larrea C,
Suarez C, Menendez MJ and Coto E: Deletion and methylation of the
tumor suppressor gene p16/CDKN2 in primary head and neck squamous
cell carcinoma. J Clin Pathol. 50:509–512. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Naggar AK, Lai S, Clayman G, Lee J-KJ,
Luna MA, Goepfert H and Batsakis JG: Methylation, a major mechanism
of p16/CDKN2 gene inactivation in head and neck squamous cell
carcinoma. Am J Pathol. 151:1767–1774. 1997.PubMed/NCBI
|
|
4
|
Sanchez-Cespedes M, Esteller M, Wu L,
Nawroz-Danish H, Yoo GH, Koch WM, Jen J, Herman JG and Sidransky D:
Gene promoter hypermethylation in tumors and serum of head and neck
cancer patients. Cancer Res. 60:892–895. 2000.PubMed/NCBI
|
|
5
|
Hasegawa M, Nelson HH, Peters E, Ringstrom
E, Posner M and Kelsey KT: Patterns of gene promoter methylation in
squamous cell cancer of the head and neck. Oncogene. 21:4231–4236.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weber A, Wittekind C and Tannapfel A:
Genetic and epigenetic alterations of 9p21 gene products in benign
and malignant tumors of the head and neck. Pathol Res Pract.
199:391–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ai L, Stephenson KK, Ling W, Zuo C,
Mukunyadzi P, Suen JY, Hanna E and Fan C-Y: The p16 (CDKN2a/INK4a)
tumor- suppressor gene in head and neck squamous cell carcinoma: a
promoter methylation and protein expression study in 100 cases. Mod
Pathol. 16:944–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maruya S, Issa J-PJ, Weber RS, Rosenthal
DI, Haviland JC, Lotan R and El-Naggar AK: Differential methylation
status of tumor-associated genes in head and neck squamous cell
carcinoma: incidence and potential implications. Clin Cancer Res.
10:3825–3830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puri SK, Si L, Fan C-Y and Hanna E:
Aberrant promoter hypermethylation of multiple genes in head and
neck squamous cell carcinoma. Am J Otolaryngol. 26:12–17. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiga K, Matsuura K, Tateda M, Saijo S,
Ogawa T, Miyagi T and Kobayashi T: Allelic loss correlated with
tissue specificity in head and neck squamous cell carcinomas and
the clinical features of patients. Tohoku J Exp Med. 204:163–172.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiga K, Yamamoto H and Okamoto H:
Isolation and characterization of the human homologue of rig
and its pseudogenes: The functional gene has features
characteristic of housekeeping genes. Proc Natl Acad Sci USA.
87:3594–3598. 1990.PubMed/NCBI
|
|
12
|
Shiga K, Shiga C, Sasano H, Miyazaki S,
Yamamoto T, Yamamoto M, Hayashi N, Nishihira T and Mori S:
Expression of c-erbB-2 in human esophageal carcinoma cells:
overexpression correlated with gene amplification or with GATA-3
transcription factor expression. Anticancer Res. 13:1293–1302.
1993.PubMed/NCBI
|
|
13
|
Van der Riet P, Nawroz H, Hruban RH, Corio
R, Tokino K, Koch W and Sidransky D: Frequent loss of chromosome
9p21-22 early in head and neck cancer progression. Cancer Res.
54:1156–1158. 1994.PubMed/NCBI
|
|
14
|
Zhang S-Y, Klein-Szanto AJP, Sauter ER,
Shafarenko M, Mitsunaga S, Nobori T, Carson DA, Ridge JA and
Goodrow TL: Higher frequency of alterations in the p16/CDKN2 gene
in squamous cell carcinoma cell lines than in primary tumors of the
head and neck. Cancer Res. 54:5050–5053. 1994.PubMed/NCBI
|
|
15
|
Nawroz H, van der Riet P, Hruban RH, Koch
W, Ruppert JM and Sidransky D: Allotype of head and neck squamous
cell carcinoma. Cancer Res. 54:1152–1155. 1994.PubMed/NCBI
|
|
16
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serrano M, Lee H-W, Chin L, Cordon-Cardo
C, Beach D and Depinho RA: Role of the INK4a locus in tumor
suppression and cell mortality. Cell. 85:27–37. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamb A, Gruis NA, Weaver-Fejdhaus J, Lin
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cairns P, Polascik TJ, Eby Y, Tokino K,
Califano J, Merlo A, Mao L, Herath J, Jenkins R, Westra W, et al:
Frequency of homozygous deletion at p16/CDK2 in primary human
tumors. Nat Genet. 11:210–212. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lydiatt WM, Murty VV, Davidson BJ, Xu L,
Dyomina K, Sacks PG, Schantz SP and Chaganti RS: Homozygous
deletions and loss of expression of the CDKN2 gene occur frequently
in head and neck squamous cell carcinoma cell lines but
infrequently in primary tumors. Genes Chromosomes Cancer. 13:94–98.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herman JG, Merlo A, Mao L, Lapidus RG,
Issa J-PJ, Davidson NE, Sidransky D and Baylin SB: Inactivation of
the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA
methylation in all common human cancers. Cancer Res. 55:4525–4530.
1995.PubMed/NCBI
|
|
23
|
Merlo A, Herman JG, Mao L, Lee DJ,
Gabrielson E, Burger PC, Baylin SB and Sidransky D: 5′ CpG island
methylation is associated with transcriptional silencing of the
tumor suppressor p16/CDKN2/MTS1 in human cancers. Nat Med.
1:686–692. 1995.
|