Introduction
Central nervous system tumors are the most common
pediatric neoplasms, corresponding to approximately 20–23% of all
cases of childhood cancer, and less than 2% of adult tumors
(1). The prognosis for patients
with primary central nervous system tumors, such as glioma, remains
poor. The principal human tumor-suppressor gene TP53 encodes
a protein, p53, activated by stresses such as DNA damage, aberrant
growth signals and ultraviolet light. p53 acts as a nuclear
transcription factor, binds to particular DNA sequences and
activates the expression of adjacent genes, which directly or
indirectly results in cell death or inhibition of cell divisions
(2). Therefore, proper function of
tumor-suppressor genes including TP53 is highly correlated
with cancer risk. Approximately 25% of gliomas carry mutations in
the TP53 gene (3).
A number of polymorphisms have been identified
within the TP53 gene thus far, both in coding and non-coding
regions (4). These polymorphisms
include the serine 47 (5), the
codon 72 (C→G, Pro→Arg) (6), intron
3 (+16 bp) and intron 6 (G→C) (7).
Among these polymorphisms, the TP53 codon 72 polymorphism,
which is located in a proline-rich region in exon 4, is the most
frequently studied. The codon 72 polymorphism involves a guanine to
cytosine nucleotide exchange, leading to a non-conservative change
from arginine to proline (8,9). In a
cell-based study, an increase in apoptosis rate by up to 15-fold
was found for the Arg72 variant cells as compared to that in the
Pro72 variant (10). In addition,
an association between the Arg72 variant and increased risk of
epithelial cancer (11,12) was reported. Certain authors found
alternate correlations, i.e., an association between the Pro72
TP53 variant and increased cancer risk (13,14),
whereas other authors (40,41) failed to confirm the link between
TP53 codon 72 variants and the risk of cancer.
Studies exist regarding the association of the
TP53 codon 72 polymorphism with susceptibility to the
development of gliomas (15–21,42).
However, the results obtained thus far were inconsistent. For
example, Parhar et al (20)
suggested a possible association between the codon 72 polymorphism
and susceptibility to brain tumors, particularly high-grade
astrocytomas. El Hallani et al (15) suggested that the codon 72
polymorphism was associated with the age-related onset of grade IV
glioblastoma. Other investigators found that the codon 72
polymorphism is not correlated with susceptibility to glioma
development (18,19,21,42).
This discrepancy in the results may be due to different sample
sizes, ethnicities or qualities including the genotyping method and
study type among the various studies.
In this study, we performed a meta-analysis on the
most current published reports in the region, to obtain the most
precise estimation of the association between TP53 codon 72
polymorphism and the risk of human glioma.
Materials and methods
Eligibility of relevant studies
We searched the National Library of Medicine
(PubMed) database, using the terms ‘(p53 OR TP53) AND
((brain tumor) OR glioma) AND polymorphism’ (the latest search was
performed on June 3, 2011). In addition, we sent e-mails to the
corresponding authors of the studies to retrieve the original data.
The inclusion criteria were as follows: a) case-control studies
with non-related subjects; b) sufficient data to calculate the odds
ratio (OR); c) no deviation from the Hardy-Weinberg equilibrium
(HWE) for the genotype distribution of the controls; and d) English
articles. We excluded the following studies: a) studies that
contained overlapping data; b) studies in which the number of
wild-type genotypes could not be ascertained; and c) studies in
which family members were studied.
Data extraction
All abstracts were read and articles were screened
for suitability by two independent researchers (M.S. and R.H.).
These investigators also read the full texts to extract data and
reach a consensus on all of the eligible items: the first author,
year of publication, country of study population, genotyping
method, genotype frequency and source of the relevant control.
Meta- and statistical analysis
The meta-analysis evaluated the association between
glioma risk and TP53 codon 72 polymorphism, which included
the dominant model (G/G + G/C versus C/C), the recessive model (G/G
versus G/C + C/C) and the additive model (G allele versus C
allele). The strength of association was assessed by the OR with a
corresponding 95% confidence interval (95% CI). The heterogeneity
among these studies was checked by Q statistics, and was considered
statistically significant when Ph<0.10 (22). Study heterogeneity was quantified by
the I2 metric, which is independent of the number of
studies in the meta-analysis (I2<25% no
heterogeneity; 25≤I2≤50% moderate heterogeneity;
I2>50% extreme heterogeneity) (23). The combined OR of each study was
estimated by the fixed-effects (FE) model (Mantel-Haenszel) at
Ph≥0.10. Otherwise, the random-effects (RE) model
(DerSimonian and Laird) was applied (24). The studies were further stratified
by glioma grade (high-grade gliomas, WHO classification III and IV;
and low grade gliomas, WHO classification I and II) and the
high-grade gliomas were subgrouped by geographical locations
including Europe, America and Asia.
To assess the stability of the results, sensitivity
and publication bias analysis were also performed. For the
sensitivity analysis, one study was omitted each time to reflect
the effect of the individual data-set to the pooled OR (25). Publication bias was investigated by
Begg’s test (P<0.05 was considered to indicate statistical
significance) (26) and Egger’s
test (P<0.05 was considered to be statistically significant)
(27). Deviations from HWE for
controls were analyzed by the Chi-square goodness of fit test.
Statistical analyses were performed using the Review Manager 5.0
software (The Cochrane Collaboration, Oxford, England) and STATA
version 11 (STATA Corporation, College Station, TX, USA).
Results
Characteristics of the retrieved
studies
The search terms used for the TP53 codon 72
polymorphism resulted in 268 articles, 22 of which were relevant
upon further review. Three articles contained overlapping data
(28–30), eight were no-control studies
(31–37), and one was a non-glioma brain tumor
study (38). In addition, deviation
from HWE was found in one publication (39) and the glioma data could not be
extracted from another (Fig. 1)
(40). Therefore, eight studies
published from 2004 to 2010, including 2,260 glioma cases and 3,506
controls, were eligible for the inclusion criteria in the
meta-analysis. Table I shows the
main characteristics of these studies, including the Jha study
(39). The sample size in each
study varies from 84 to 636. In addition, the original data of two
studies were retrieved from the corresponding authors (18,41).
 | Table ICharacteristics of studies included
in the meta-analysis. |
Table I
Characteristics of studies included
in the meta-analysis.
| References | Geographical
location | Genotyping
method | Number of cases
(Age range, mean) | Number of controls
(Age range, mean) | Source of
controls | Matching |
|---|
| El Hallani et
al (2009) | France | TaqMan | 254 (19.2–83.6,
56.5) | 238 (16–75,
NA) | NA | Not matched |
| Idbaih et al
(2007) | France | TaqMan | 293 (16–84,
43) | 175 (NA, NA) | NA | NA |
| Lima-Ramos et
al (2008) | Europe | PCR-RFLP | 171 (NA, 49.5) | 526 (NA, 38.1) | Hospital-based | Gender |
| Malmer et al
(2007) | Nordic-UK | TaqMan | 636 (18–69,
45) | 1461 (19–70,
50) |
Population-based | Age, gender,
geographical location |
| Parhar et al
(2005) | USA | PCR-RFLP | 135 (0–79, NA) | 117 (NA, NA) | NA | Not matched |
| Pinto et al
(2008) | Southeast
Brazil | PCR-RFLP | 94 (1–75, 45) | 100 (18–72 45) | NA | Age, gender |
| Rajaraman et
al (2007) | USA | TaqMan | 386 (>18,
NA) | 547 (>18,
NA) | Hospital-based | Age, gender,
ethnicity, residential proximity to the hospital |
| Wang et al
(2004) | USA | PCR-RFLP | 309 (20–60,
NA) | 342 (20–60,
NA) | Hospital-based | Age, gender,
ethnicity |
| Jha et al
(2010) | North India | Sequence
analysis | 84 (NA, NA) | 112 (NA, NA) | NA | NA |
Gliomas from the eight eligible studies were
classified according to the WHO classification criteria. Among
them, six studies provided data on high-grade glioma genotype
distribution, while the other two studies provided data on
low-grade glioma genotype distribution (Table II). Therefore, these studies were
treated as a mixed study at first, and then analyzed for high-grade
and low-grade gliomas. The eight studies were conducted in
different populations of various geographical locations: four were
European populations (15,16,17,21),
four were American (18,19,20,42)
and only one was Asian (39). A
stratified analysis for different geographical locations in
high-grade gliomas was also conducted. However, such an analysis
for geographical locations other than Europe was not performed due
to insufficient data.
 | Table IIDistribution of TP53 codon 72
genotype and allele frequencies. |
Table II
Distribution of TP53 codon 72
genotype and allele frequencies.
| References | Genotype | Allele | HWE (P) |
|---|
|
|
| |
|---|
| Cases (n) gliomas
(high/low) | Controls (n) | Cases (n) gliomas
(high/low) | Controls (n) | |
|---|
|
|
| |
|---|
| GG | GC | CC | GG | GC | CC | G | C | G | C | |
|---|
| El Hallani et
al (2009) | 140 (140/NA) | 92 (92/NA) | 22 (22/NA) | 142 | 82 | 14 | 372 (372/NA) | 136 (136/NA) | 366 | 110 | 0.637956 |
| Idbaih et al
(2007) | 149 (61/88) | 108 (45/63) | 18 (10/8) | 107 | 57 | 11 | 431 (178/253) | 155 (72/83) | 271 | 79 | 0.367309 |
| Lima-Ramos et
al (2008) | 101 (47/NA) | 56 (24/NA) | 14 (4/NA) | 298 | 197 | 31 | 258 (118/NA) | 84 (32/NA) | 793 | 259 | 0.835726 |
| Malmer et al
(2007) | 361 (159/NA) | 241 (194/NA) | 34 (10/NA) | 801 | 556 | 104 | 309 (214/NA) | 963 (512/NA) | 764 | 2158 | 0.576667 |
| Parhar et al
(2005) | 38 (8/NA) | 94 (55/NA) | 3 (1/NA) | 72 | 42 | 3 | 170 (71/NA) | 100 (57/NA) | 186 | 48 | 0.275542 |
| Pinto et al
(2008) | 53 (33/20) | 34 (14/20) | 7 (5/2) | 48 | 42 | 10 | 140 (80/60) | 48 (24/24) | 138 | 62 | 0.855325 |
| Rajaraman et
al (2007) | 213 (NA/NA) | 146 (NA/NA) | 27 (NA/NA) | 300 | 209 | 38 | 572 (NA/NA) | 200 (NA/NA) | 809 | 285 | 0.84566 |
| Wang et al
(2004) | 165 (NA/NA) | 126 (NA/NA) | 18 (NA/NA) | 194 | 128 | 20 | 456 (NA/NA) | 162 (NA/NA) | 516 | 168 | 0.853764 |
| Jha et al
(2010) | 33 (13/NA) | 27 (17/NA) | 24 (13/NA) | 15 | 70 | 27 | 93 (43/NA) | 75 (43/NA) | 100 | 124 | 0.00512 |
Meta-analysis results
Primary meta-analysis results are shown in Table III. We adopted the random-effects
model to test the association between TP53 codon 72 C allele
and glioma risk. The overall OR for the C-allele was 1.10, its 95%
CI was 0.93-1.30. Therefore, C allele was considered as a high-risk
allele in this literature review of the data. The pooled estimates
across all eight studies showed no association between TP53
codon 72 polymorphism and glioma risk within the three genotype
models: [C/C + G/C vs. G/G: OR=1.17, 95% CI (0.91, 1.50), P=0.23,
Ph<0.0001], [C/C vs. G/C+ G/G: OR=0.97, 95% CI (0.77,
1.21), P=0.77, Ph=0.64], [C allele vs. G allele:
OR=1.10, 95% CI (0.93, 1.30), P=0.27, Ph=0.002].
 | Table IIIThe odds ratios (ORs) of TP53
codon 72 polymorphism, glioma subtype and geographical location
status with glioma. |
Table III
The odds ratios (ORs) of TP53
codon 72 polymorphism, glioma subtype and geographical location
status with glioma.
| Allele and
genotype | Outcome or
subgroup | Studies | Cases/controls | Statistical
method | Effect
estimate | P | P
(Heterogeneity) |
I2(%) |
|---|
| C/C + G/C vs. G/G
(dominant model) |
| Gliomas | 8 | 2260/3506 | Odds ratio (M-H,
Random, 95% CI) | 1.17 [0.91,
1.50] | 0.23 | <0.0001 | 78 |
| High-grade
gliomas | 6 | 924/2617 | Odds ratio (M-H,
Random, 95% CI) | 1.45 [0.88,
2.39] | 0.15 | <0.00001 | 87 |
| Low-grade
gliomas | 2 | 201/275 | Odds ratio (M-H,
Fixed, 95% CI) | 1.20 [0.82,
1.74] | 0.35 | 0.60 | 0 |
| High-grade gliomas
in Europeans | 4 | 808/2400 | Odds ratio (M-H,
Fixed, 95% CI) | 1.35 [1.14,
1.59] | 0.0005 | 0.13 | 47 |
| C/C vs. G/C+ G/G
(recessive model) |
| Gliomas | 8 | 2260/3506 | Odds ratio (M-H,
Fixed, 95% CI) | 0.97 [0.77,
1.21] | 0.77 | 0.64 | 0 |
| High-grade
gliomas | 6 | 924/2617 | Odds ratio (M-H,
Random, 95% CI) | 0.88 [0.50,
1.56] | 0.67 | 0.07 | 52 |
| Low-grade
gliomas | 2 | 201/275 | Odds ratio (M-H,
Fixed, 95% CI) | 0.67 [0.30,
1.47] | 0.32 | 0.54 | 0 |
| High-grade gliomas
in Europeans | 4 | 808/2400 | Odds ratio (M-H,
Random, 95% CI) | 0.90 [0.43,
1.90] | 0.78 | 0.02 | 71 |
| C allele vs. G
allele (an additive model) |
| Gliomas | 8 | 2260/3506 | Odds ratio (M-H,
Random, 95% CI) | 1.10 [0.93,
1.30] | 0.27 | 0.002 | 68 |
| High-grade
gliomas | 6 | 924/2617 | Odds ratio (M-H,
Random, 95% CI) | 1.23 [0.90,
1.66] | 0.19 | 0.0003 | 79 |
| Low-grade
gliomas | 2 | 201/275 | Odds ratio (M-H,
Fixed, 95% CI) | 1.00 [0.74,
1.35] | 0.98 | 0.62 | 0 |
| High-grade gliomas
in Europeans | 4 | 808/2400 | Odds ratio (M-H,
Fixed, 95% CI) | 1.16 [1.02,
1.33] | 0.03 | 0.37 | 5 |
In the stratified analysis for glioma grade, no
association was found between high-grade and low-grade gliomas.
However, heterogeneity was detected in high-grade gliomas [C/C +
G/C vs. G/G: OR=1.45, 95% CI (0.88, 2.39), P=0.15,
Ph<0.00001], [C allele vs. G allele: OR=1.23, 95% CI
(0.90, 1.66), P=0.19, Ph=0.0003]. Therefore, we further
sub-grouped the high-grade glioma group by geographical locations.
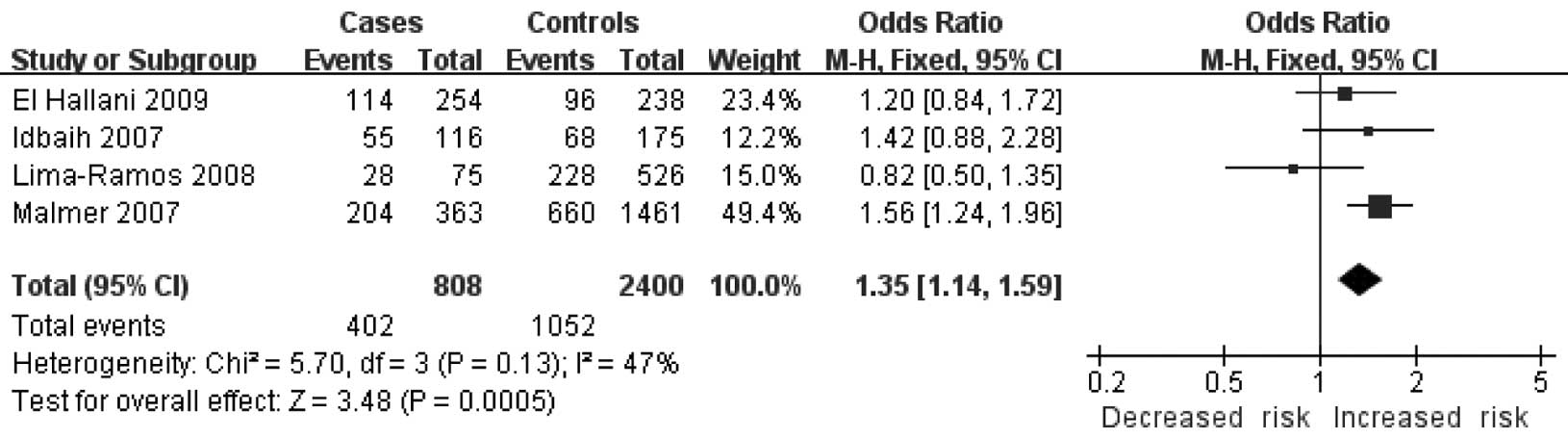
Homogeneity and significant associations were found in two models:
the dominant model [C/C + G/C vs. G/G: OR=1.35, 95% CI (1.14,
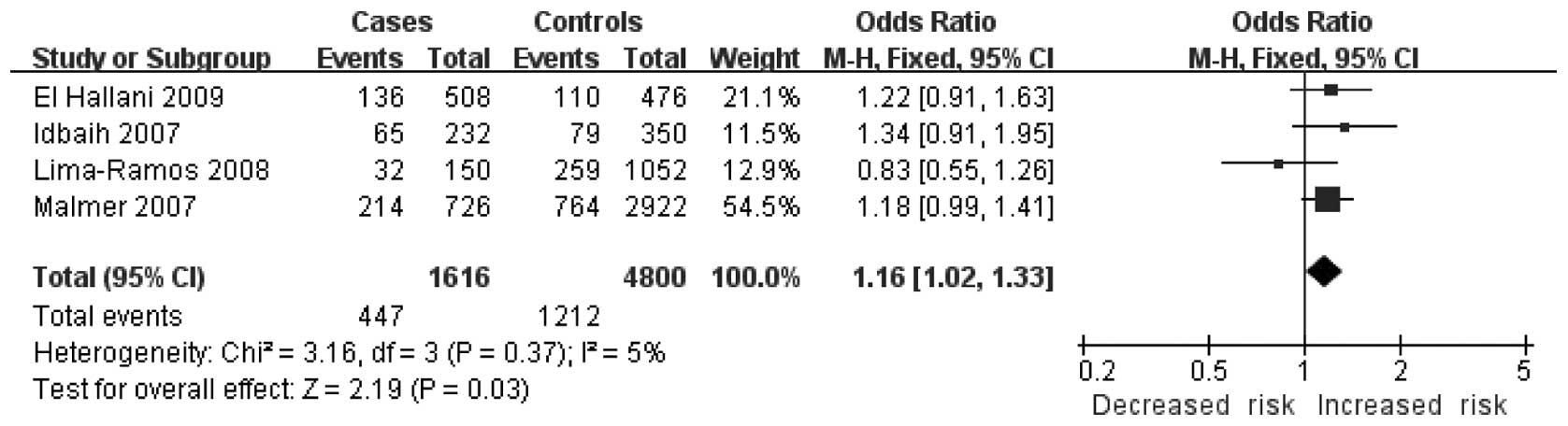
1.59), P=0.0005, Ph=0.13] (Fig. 2) and the additive model [C allele
vs. G allele: OR=1.16, 95% CI (1.02, 1.33), P=0.03,
Ph=0.37] (Fig. 3). No
other significant association was found.
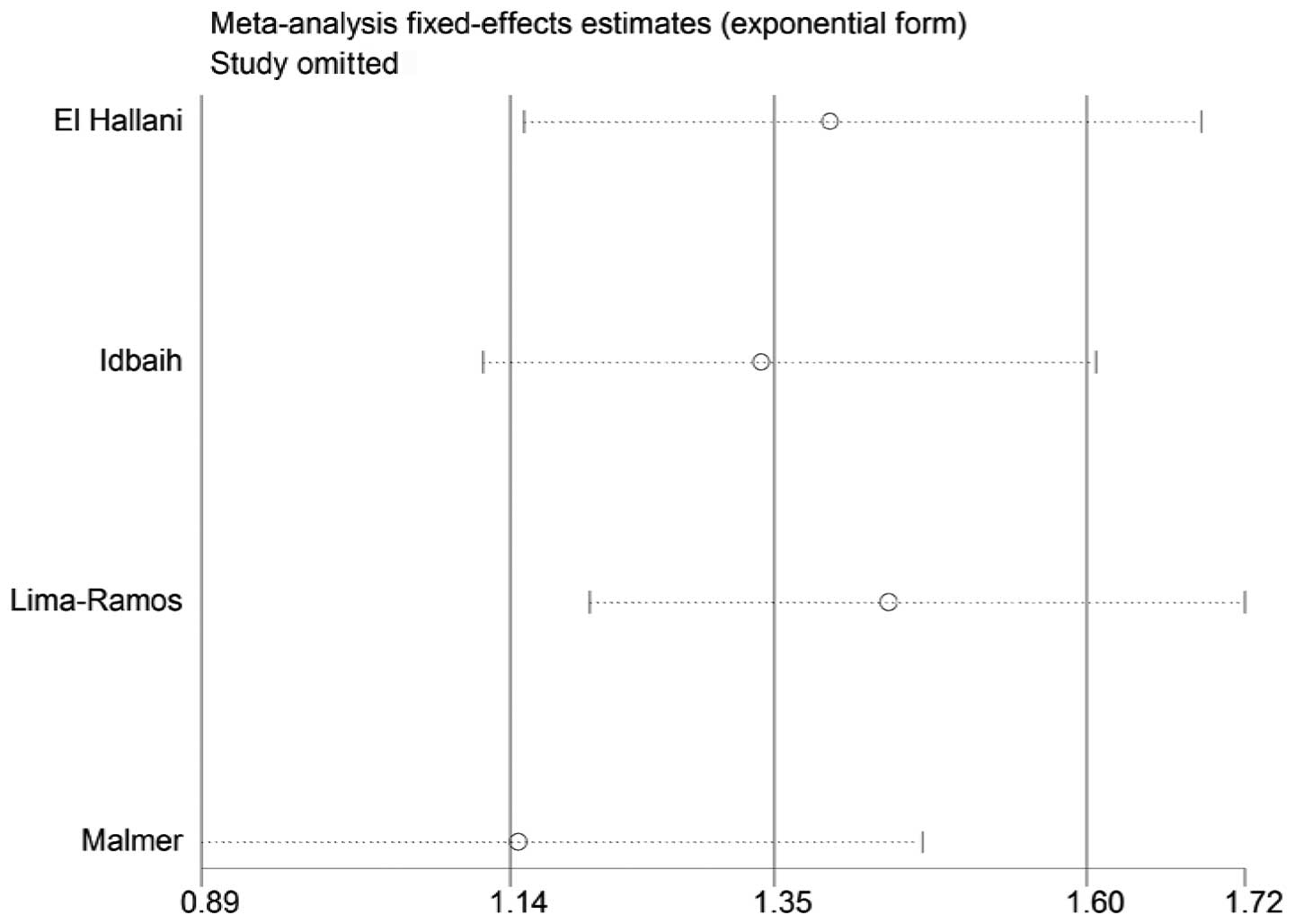
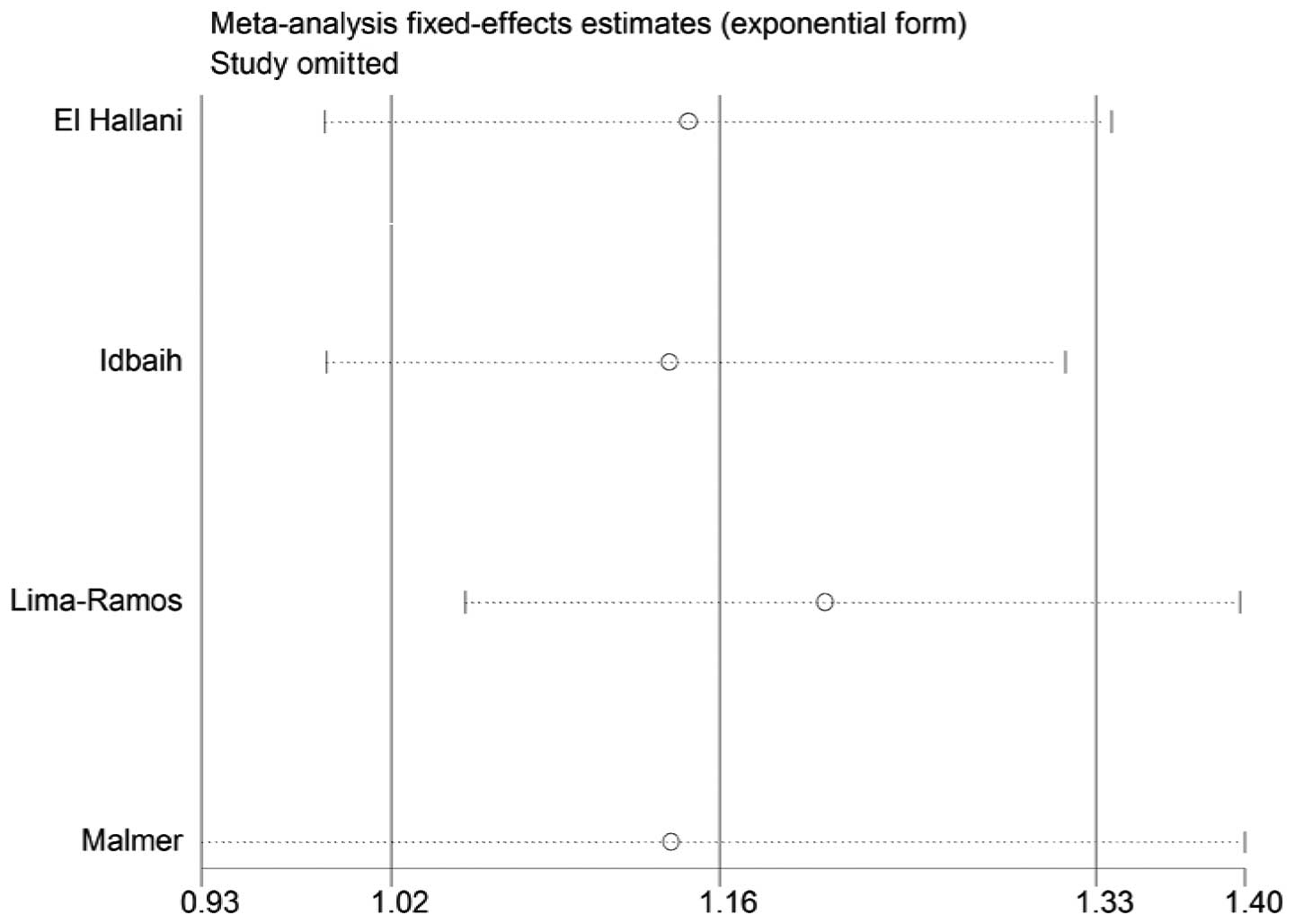
Sensitivity analysis
A single study in the meta-analysis was deleted to
test for the effect of that individual data set on the pooled ORs.
The corresponding pooled ORs were not significantly altered in the
sensitivity analysis (Figs. 4 and
5).
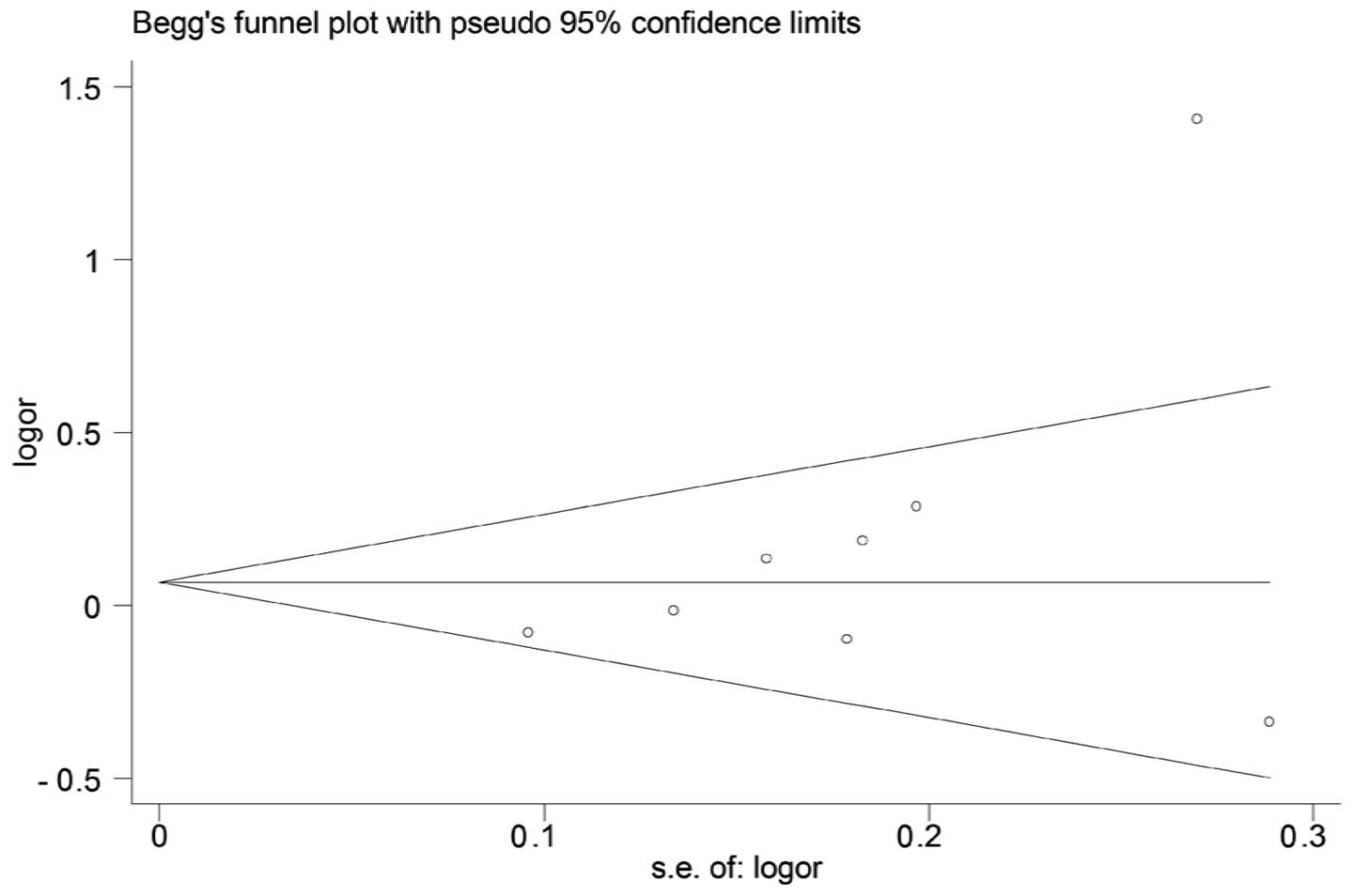
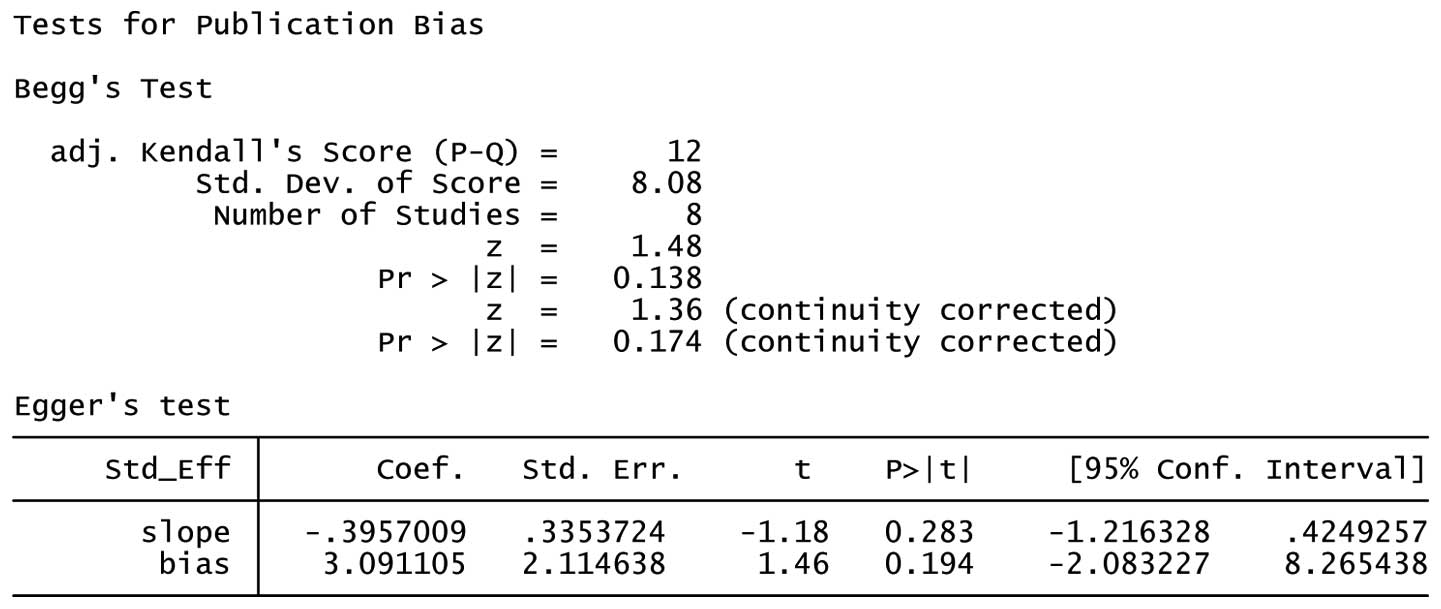
Publication bias
Both Begg’s and Egger’s test were performed to
assess the publication bias. The results suggested no evidence of
publication bias (C/C + G/C vs. G/G: Begg’s test P=0.174, Egger’s
test P=0.194) (Figs. 6 and 7).
Discussion
The TP53 codon 72 polymorphism was
intensively studied and reported to affect the functions of the
TP53 network, which is central to the development of
gliomas, particularly high-grade gliomas. The previously published
studies presented conflicting results over the association between
TP53 codon 72 polymorphism and the risk of glioma.
Therefore, we conducted this meta-analysis on data collected from
the most up-to-date publications to evaluate this putative
association.
The tumor suppressor gene TP53 is a core gene
in the TP53 signaling pathway and is significant in tumor
suppression. The mutation of the TP53 gene was recently
defined as one of the most crucial factors in the development of
malignant gliomas (43). Parhar
et al (20) found a
significant association between the G/C genotype and an increased
risk for high-grade astrocytomas. However, the present results did
not show any association between the type of single-nucleotide
polymorphism (SNP) and the risk of high-grade gliomas. This
inconsistency may be due to a number of reasons. First, our
analysis stratified the data into high-grade and low-grade gliomas,
whereas Parhar et al stratified the data into high-grade
astrocytomas and non-astrocytomas. Since astrocytoma is a subtype
of glioma, non-astrocytomas include other types of high-grade
gliomas. Therefore, the tumor classification is different between
the two studies. Second, the sample sizes were different. Parhar
et al only included 252 subjects, whereas we included 2,145
subjects. The relatively small sample size in the study by Parhar
et al may contribute to their conclusion. Our analysis,
which combines data from all eight studies that included 2,145
subjects, should minimize the random error.
The genesis of glioma is closely associated with the
interaction between environmental factors and genetic background.
It was reported that the allele distribution at codon 72 of
TP53 varies depending on geographical locations (44,45).
Therefore, to clarify the association between codon 72 polymorphism
and the glioma risk in different genetic backgrounds and remove
heterogeneity, we analyzed the collected data by subgrouping the
high-grade glioma group according to the geographical locations.
The results have shown that the respective SNPs at codon 72 of
TP53 are associated with an increased risk of high-grade
gliomas in Europeans.
Based on the literature review of the available
data, our results suggest that TP53 codon 72 C carriers
(Pro) are associated with an increased risk of high-grade glioma in
Europeans. Thomas et al (46) indicated that the Pro72 variant
induced slower kinetics of apoptosis and suppressed transformation
less efficiently than the Arg72 variant. In their study, Dumont
et al (10) reported that
the Pro72 variant bears only 1/15 apoptosis-inducing ability
compared to the Arg72 variant in cells with endogenous p53, as well
as in cell lines containing inducible alleles encoding the Pro72 or
Arg72. Thus, our results are consistent with the data describing
the biological functions of p53.
However, there are a number of limitations in our
analysis. Although we carefully selected studies by performing a
careful search, using strict study inclusion criteria, precise data
extraction and statistical analysis, significant heterogeneity
between studies still exists. E-mails were forwarded to the
corresponding authors of each publication. However, only two of the
original sets of data were retrieved; thus, we failed to adjust our
meta-analysis by age and gender. Future studies should include
other co-variants, such as age, gender, ethnicity, environmental
factors and lifestyle for a more comprehensive understanding of the
association between the TP53 codon 72 polymorphism and
glioma risk.
In conclusion, our results confirm that TP53
codon 72 polymorphism may be associated with an increased risk of
high-grade glioma development in Europeans.
Acknowledgements
The authors appreciate the assistance from G.R.
Pinto (18), Ahmed Idbaih (41) and Guilherme Francisco (47) for sharing their original data. The
research project was supported by the Key Project Science
Foundation of Heilongjiang Province, China (Grant-ZD200804-01), the
Chinese Postdoctoral Fellowship (Grant-2008043938) and the National
Science Foundation of China (Grant NFSC-30227738), and Ministry of
Education, Science and Technology Development Center College
Research Foundation for the Doctoral Program (20092307110006).
References
|
1
|
Rickert CH and Paulus W: Epidemiology of
central nervous system tumors in childhood and adolescence based on
the new WHO classification. Childs Nerv Syst. 17:503–511. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Phatak P, Selvi SK, Divya T, Hegde AS,
Hegde S and Somasundaram K: Alterations in tumour suppressor gene
p53 in human gliomas from Indian patients. J Biosci. 27:673–678.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olivier M, Eeles R, Hollstein M, Khan MA,
Harris CC and Hainaut P: The IARC TP53 database: new online
mutation analysis and recommendations to users. Hum Mut.
19:607–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felley-Bosco E, Weston A, Cawley HM,
Bennett WP and Harris CC: Functional studies of a germ-line
polymorphism at codon 47 within the p53 gene. Am J Hum Genet.
53:752–759. 1993.PubMed/NCBI
|
|
6
|
Harris N, Brill E, Shohat O, Prokocimer M,
Wolf D, Arai N and Rotteri V: Molecular basis for heterogeneity of
the human p53 protein. Mol Cell Biol. 6:4650–4656. 1986.PubMed/NCBI
|
|
7
|
Lehman TA, Haffty BG, Carbone CJ, Bishop
LR, Gumbs AA, Krishnan S, Shields PG, Modali R and Turner BC:
Elevated frequency and functional activity of a specific germ-line
p53 intron mutation in familial breast cancer. Cancer Res.
60:1062–1069. 2000.PubMed/NCBI
|
|
8
|
Walker KK and Levine AJ: Identification of
a novel p53 functional domain that is necessary for efficient
growth suppression. Proc Natl Acad Sci. 93:15335–15340. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakamuro D, Sabbatini P, White E and
Prendergast GC: The polyproline region of p53 is required to
activate apoptosis but not growth arrest. Oncogene. 15:887–898.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dumont P, Leu JI, Pietra ACD III, George
DL and Murphy M: The codon 72 polymorphic variants of p53 have
markedly different apoptotic potential. Nat Genet. 33:357–365.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langerød A, Bukholm IRK, Bregård A,
Lønning PE, Andersen TI, Rognum TO, Meling GI, Lothe RA and
Børresen-Dale AL: The TP53 codon 72 polymorphism may affect the
function of TP53 mutations in breast carcinomas but not in
colorectal carcinomas. Cancer Epidemiol Biomarkers Prev.
11:1684–1688. 2002.PubMed/NCBI
|
|
12
|
Maarten TB, Struyk L, Tjong-A-Hung SP,
Gruis N, Huurne J, Westendorp RGJ, Vermeer BJ, Bavinck JNB and
Schegget J: Cutaneous squamous cell carcinoma and p53 codon 72
polymorphism: a need for screening? Mol Carcinog. 30:56–61. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Granja F, Moraria J, Moraria EC, Correa
LAC, Assumpcao LVM and Ward LS: Proline homozygosity in codon 72 of
p53 is a factor of susceptibility for thyroid cancer. Cancer Lett.
210:151–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tiwawech D, Srivatankul P, et al: The p53
codon 72 polymorphism in Thai nasopharyngeal carcinoma. Cancer
Lett. 198:69–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Hallani S, Ducray F, Idbaih A, Marie Y,
Boisselier B, Colin C, Laigle-Donadey F, Rodero M, Chinot O,
Thillet J, Hoang-Xuan K, Delattre JY and Sanson M: TP53 codon 72
polymorphism is associated with age at onset of glioblastoma.
Neurology. 72:332–336. 2009.PubMed/NCBI
|
|
16
|
Idbaih A, Boisseliera B, Marie Y, Sanson
M, El Hallani S, Crinière E, Fourtassi M, Paris S, Carpentier C,
Rousseau A, et al: Influence of MDM2 SNP309 alone or in combination
with the TP53 R72P polymorphism in oligodendroglial tumors. Brain
Res. 1198:16–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malmer BS, Feychting M, Lönn S, Lindström
S, Grönberg H, Ahlbom A, Schwartzbaum J, Auvinen A,
Collatz-Christensen H, Johansen C, Kiuru A, Mudie N, Salminen T,
Schoemaker MJ, Swerdlow AJ and Henriksson R: Genetic variation in
p53 and ATM haplotypes and risk of glioma and meningioma. J
Neurooncol. 82:229–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinto GR, Yoshioka FKN, Silva RLL, Clara
CA, Santos MJ, Almeida JRW, Burbano RR, Rey JA and Casartelli C:
Prognostic value of TP53 Pro47Ser and Arg72Pro single nucleotide
polymorphisms and the susceptibility to gliomas in individuals from
Southeast Brazil. Genet Mol Res. 7:207–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rajaraman P, Wang SS, Rothman N, Brown MM,
Black PM, Fine HA, Loeffler JS, Selker RG, Shapiro WR, Chanock SJ
and Inskip PD: Polymorphisms in apoptosis and cell cycle control
genes and risk of brain tumors in adults. Cancer Epidemiol
Biomarkers Prev. 16:1655–1661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parhar P, Ezer R, Shao Y, Allen J, Miller
D and Newcomb E: Possible association of p53 codon 72 polymorphism
with susceptibility to adult and pediatric high-grade astrocytomas.
Mol Brain Res. 137:98–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lima-Ramos Vt, Pacheco-Figueiredo L, Costa
S, Pardal F, Silva A, Amorim J, Lopes JM and Reis RM: TP53 codon 72
polymorphism in susceptibility, overall survival, and adjuvant
therapy response of gliomas. Cancer Genet Cytogenet. 180:14–19.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in metaanalysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lau J, Ioannidis JP and Schmid CH:
Quantitative synthesis in systematic reviews. Ann Intern Med.
127:820–826. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tobias A: Assessing the influence of a
single study in the meta-analysis estimate. Stata Tech Bull.
8:15–17. 1999.
|
|
26
|
Begg C and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Idbaiha A, Boisselier B, Sanson M,
Criniere E, Livad S, Marie Y, Carpentier C, Paris S, Laigle-Donadey
F, Mokhtari K, Kujas M, Hoang-Xuan K, Delattre O and Delattre J-Y:
Tumor genomic profiling and TP53 germline mutation analysis of
first-degree relative familial gliomas. Cancer Genet Cytogenet.
176:121–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malmer B, Gronberg H, Andersson U, Jonsson
BA and Henriksson R: Microsatellite instability, PTEN and p53
germline mutations in glioma families. Acta Oncologica. 40:633–637.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malmer B, Feychting M, Lonn S, Ahlbom A
and Henriksson R: p53 Genotypes and risk of glioma and meningioma.
Cancer Epidemiol Biomarkers Prev. 14:2220–2223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Portwine C, Chilton-MacNeill S, Brown C,
Sexsmith E, McLaughlin J and Malkin D: Absence of germline and
somatic p53 alterations in children with sporadic brain tumors. J
Neurooncol. 52:227–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zawlik I, Kita D, Vaccarella S,
Mittelbronn M, Franceschi S and Ohgaki H: Common polymorphisms in
the MDM2 and TP53 genes and the relationship between TP53 mutations
and patient outcomes in glioblastomas. Brain Pathol. 19:188–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aka K, Bruner JM, Bondy ML, Ligon K, Nishi
K, Giglio AD, Moser RR, Levin VA and Saya H: Detection of p53
alterations in human astrocytomas using frozen tissue sections for
the polymerase chain reaction. J Neuro Oncol. 16:125–133. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang ZN, Guo CL, Ahronowitz I,
Stemmer-Rachamimov RO, MacCollin M and Nunes FP: A role for the p53
pathway in the pathology of meningiomas with NF2 loss. J
Neurooncol. 91:265–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paunu N, Syrjakoski K, Sankila R, Simola
KOJ, Helen P, Niemela M, Matikainen M, Isola J and Haapasalo H:
Analysis of p53 tumor suppressor gene in families with multiple
glioma patients. J Neurooncol. 55:159–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohgaki H, Dessen P, Jourde B, Horstmann S,
Nishikawa T, Patre PLD, Burkhard C, Schuler D, Probst-Hensch NM,
Maiorka PC, et al: Genetic pathways to glioblastoma: a
population-based study. Cancer Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uno M, Oba-Shinjo SM, Wakamatsu A, Huang
N, Avancini Ferreira Alves V, Rosemberg S, Pires de Aguiar PH,
Leite C, Miura F, Marino Junior R, Scaff M and Nagahashi-Marie SK:
Association of TP53 mutation, p53 overexpression, and p53 codon 72
polymorphism with susceptibility to apoptosis in adult patients
with diffuse astrocytomas. Int J Biol Markers. 21:50–57.
2006.PubMed/NCBI
|
|
38
|
Almeida LO, Custódio AC, Pinto GR, Santos
MJ, Almeida JRW, Clara CA, Rey JA and Casartelli C: Polymorphisms
and DNA methylation of gene TP53 associated with extra-axial brain
tumors. Genet Mol Res. 8:8–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jha P, Jha P, Pathak P, Chosdol K, Suri V,
Sharma MC, Kumar G, Singh M, Mahapatra AK and Chitra S: TP53
polymorphisms in gliomas from Indian patients: Study of codon 72
genotype, rs1642785, rs1800370 and 16 base pair insertion in
intron-3. Exp Mol Pathol. 90:167–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Biros E, Kalina I, Kohut A, Bogyiova E,
Alagovic J and Ulla I: Allelic and haplotype frequencies of the p53
polymorphisms in brain tumor patients. Physiol Res. 51:59–64.
2002.PubMed/NCBI
|
|
41
|
Idbaih A, Boisselier B, Mariea Y, El
Hallani S, Sanson M, Criniere E, Rodero M, Carpentier C, Paris S,
Laigle-Donadey F, Ducray F, Hoang-Xuan K and Delattre JY: TP53
codon 72 polymorphism, p53 expression, and 1p/19q status in
oligodendroglial tumors. Cancer Genet Cytogenet. 177:103–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang LE: Polymorphisms of DNA repair genes
and risk of glioma. Cancer Res. 64:5560–5563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Network TCGAR. Comprehensive genomic
characterization defines human glioblastoma genes and core
pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sjalander A, Birgander R, Saha N, Beckman
L and Beckman G: p53 polymorphisms and haplotypes show distinct
diff erences between major ethnic groups. Hum Hered. 46:41–48.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kashima T, Makino K, Soemantri A and
Ishida T: TP53 codon 72 polymorphism in 12 populations of insular
southeast Asia and Oceania. J Hum Genet. 52:694–697. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thomas M, Kalita A, Labrecque S, Pim D,
Banks L and Matlashwski G: Two polymorphic variants of wild-type
p53 differ biochemically and biologically. Mol Cell Biol.
19:1092–1100. 1999.PubMed/NCBI
|
|
47
|
Francisco G, Menezes PR, Eluf-Neto J and
Chammas R: Arg72Pro TP53 polymorphism and cancer susceptibility: A
comprehensive meta-analysis of 302 case-control studies. Int J
Cancer. 129:920–30. 2011. View Article : Google Scholar : PubMed/NCBI
|