Introduction
Cervical cancer is one of the common malignant
tumors in women, with the traditional treatment of early cervical
cancer being radical surgery or radiation therapy. However, there
is some controversy regarding the treatment for bulky cervical
cancer (lesion diameter >4 cm) or locally advanced cervical
cancer. This is due to the limitation that the bulky mass exceeds
the scope of surgical resection, and with the gradual growth of
tumor diameter, the radiation dose required far exceeds the
tolerance of the surrounding normal tissues. Therefore, surgery and
radiation therapy have not proven successful with regards to the
treatment of bulky cervical cancer (1). Advances in the research and
development of the disease have resulted in observations that
cervical cancer is a tumor that is sensitive to chemotherapy
(2). New treatment strategies, such
as neoadjuvant chemotherapy (NAC), have been established, for
example, chemotherapy administered prior to the treatment of cancer
can be differentiated from the second-line treatment following
surgery (3).
Since Friedlander reported the application of NAC on
cervical cancer in 1983, research findings from a large number of
phase II and III clinical trials have been reported. However, as
the studies consisted of different clinical phase cases and various
chemotherapy regimens, the response rate of NAC has been
questioned. In addition, there is no standardized route of
administration or course of treatment for NAC (4,5).
Paclitaxel is now considered a new type of broad-spectrum and
highly efficient anticancer drug, and the efficacy of this agent on
a variety of solid tumors has been noted. For example, one study
has confirmed that paclitaxel kills the human cervical cancer cell
SiHa in vitro (6). In the
current study, paclitaxel combined with carboplatin and cisplatin
therapy was administered to patients between January 2009 and June
2010, who had bulky cervical cancer (FIGO stage Ib2 to IIb2) and
who had received NAC prior to laparoscopic radical hysterectomy and
pelvic lymphadenectomy, in order to explore the response rate and
feasibility of this method on the treatment of bulky cervical
cancer.
Materials and methods
Case selection
A total of 19 patients (average age 42.5, range
29–62) with bulky cervical cancer who were treated with NAC
(paclitaxel combined with carboplatin systemic chemotherapy and
cervical local injection of cisplatin) between January 2009 and
June 2010 in Wuhan Union Hospital (China) were enrolled in the
current study. After 3 courses of treatment, patients were treated
with laparoscopic radical hysterectomy and pelvic lymphadenectomy.
Initial treatment and diagnoses of the patients were confirmed by
cervical biopsy prior to surgery. The clinical stage was identified
in accordance with the standards issued by the International
Federation of Gynecology and Obstetrics (FIGO) in 2000, and
cervical local lesion size was measured by magnetic resonance
imaging (MRI).
Treatment methods
Patients studied were initially treated with NAC
drugs as follows: cisplatin (20 mg, cervical local injection) was
applied for chemotherapy d1 and d4, paclitaxel (trade name:
liposome, 30 mg/bottle, H20030357, batch no.: 070606, Nanjing Sike
Pharmaceutical Co., Ltd.; Nanjing, China) (150 mg/m2
intravenous infusion) for d2 and carboplatin (i.v., AUC=5) for d3.
Each course of medical treatment was administered every 28 days.
The side effects were closely observed during chemotherapy and were
evaluated according to the standard of chemotherapy toxic response
issued by the World Health Organization (WHO). Gynecological
examination was carried out prior to each chemotherapy course in
order to initially assess the lesion development and whether there
was progression of the clinical stage. If the lesion progressed or
parametrial invasion was aggravated, the NAC was replaced by
radiotherapy. Re-examination of MRI was performed prior to therapy
in order to evaluate the efficacy of chemotherapy.
Following administration of chemotherapy,
laparoscopic radical hysterectomy and pelvic lymphadenectomy were
performed. The specific surgical procedures are available in our
previous reports (7). For the
patients with lymph node metastasis, parametrial involvement or
positive vaginal margin after surgery, adjuvant radiotherapy was
carried out.
Evaluation of chemotherapy
The efficacy of chemotherapy was evaluated according
to the WHO evaluation criteria: 1, complete remission (CR): tumor
disappeared completely with no lymph node metastasis; 2, partial
remission (PR): at least 50% tumor shrinkage; 3, stable disease
(SD): tumors shrank by <50% or enlarged by <25%; 4,
progression disease (PD): tumors enlarged by >25% or there was a
new lesion. Effective chemotherapy refers to patients with CR or
PR, ineffective chemotherapy refers to patients with SD or PD.
Follow-up
Out-patient follow-up was conducted for all 19
patients, once every three months after the first year of therapy.
Follow-up examination consisted of gynecological examination,
vaginal stump brushing cytology examination, pelvic B-us, B-us
examination of kidneys, ureters and bladder, and chest
examination.
Results
The average age of the 19 patients was 42.5 (29–62
years old), and all the individuals had completed at least one
course of chemotherapy and undergone the corresponding evaluation
(Table I). The average diameter of
the bulky tumors was 4.93±0.81 cm (4.0–7.0 cm).
 | Table IClinical data of the cases (n=19). |
Table I
Clinical data of the cases (n=19).
| Clinical
features |
| Age (years,
range) | 42.5±8.2 (29–62) |
| Clinical stage
(FIGO) |
| Ib2 | 9 |
| IIa2 | 9 |
| IIb2 | 1 |
| Pathology |
| Squamous
carcinoma | 14 |
| Adenocarcinoma | 3 |
| Adenosquamous
carcinoma | 2 |
Reduction of tumor size and response to
NAC
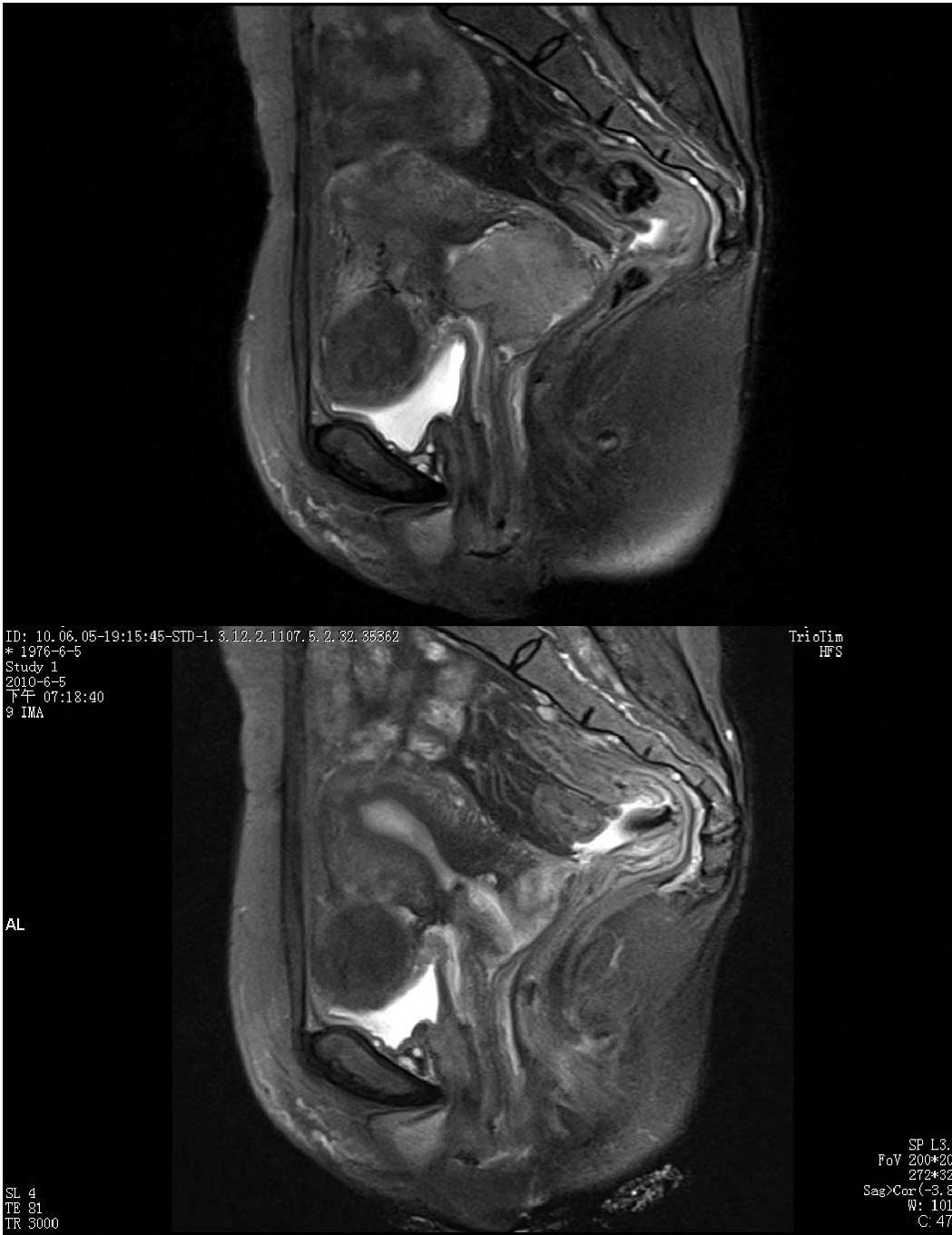
All 19 patients had undergone MRI examination and
measurement of the cervical lesion size prior to NAC and following
the chemotherapy (Table II and
Fig. 1). The diameter of the tumor
after chemotherapy was markely reduced to 2.57±1.90 cm (P<0.01,
vs. the diameter prior to chemotherapy). As confirmed by
pathological diagnosis following chemotherapy, there were 4 CR
cases (21.1%), 11 PR cases (57.9%) and 4 SD cases (21.1%), while no
PD was observed. The chemotherapy response rate (i.e., CR + PR) was
78.9% (15/19) (Table III). Of the
19 cases, 1 (Ib2) case reached the maximum lesion diameter of 7.0
cm, with no obvious shrinkage of the lesion after a single course
of chemotherapy. Therefore, the patient rejected chemotherapy using
radiation therapy. For the remaining 18 patients, no significant
remission was observed for 1 IIb2 and 1 Ib2 cases following
chemotherapy, therefore radiation therapy was proposed. The
remaining case with SD strongly requested surgery; however, this 1
IIa2 case was found to have been infiltrated by the tumor at the
bladder serosa during the surgery, and the procedure was
terminated. Thus, 15 patients completed the surgery with the
surgical resection rate at 83.3%. Of these 15 patients, only two
cases, 1 Ib2 SD case, who requested surgery, and 1 IIa2 PR case,
with lymph node metastasis after surgery were observed. The lymph
node metastasis rate was 13.3%, and no parametrial involvement and
positive vaginal surgical margin cases were observed.
 | Table IITumor size before and after NAC. |
Table II
Tumor size before and after NAC.
| Stage | Before NAC tumor size
(height × length × width) cm | After NAC tumor size
(height × length × width) cm | Note |
|---|
| Ib2 | 4.5×4.0 | 2.0×2.0 | |
| 4.2×3.5×2.7 | No obvious mass | |
| 4.2×4.5 | 1.0×1.5 | |
| 5.4×4.3×3.0 | 5.0×4.5×3.0 | |
| 5.5×3.9 | No obvious mass | |
| 4.1×3.6×4.0 | 1.0×1.5 | |
| 7.0×4.5 | 6.5×5.0 | Rejected after one
course of chemotherapy |
| 4.0×3.0×5.3 | 2.0×1.5 | |
| 6.0×5.0 | 4.0×5.5 | |
| IIa2 | 4.1×3.9×4.2 | 3.0×2.5 | |
| 6.0×4.9×5.0 | 2.5×3.0×2.2 | |
| 4.5×2.0×5.0 | 3.0×2.0×2.5 | |
| 5.0×3.0 | 2.9×1.3 | |
| 4.0×4.1×3.5 | 2.2×3.5×2.2 | |
| 3.0×3.5×4.0 | No obvious mass | |
| 5.1×3.9×4.0 | 2.5×3.0×2.0 | |
| 3.8×3.4×5.2 | No obvious mass | |
| 3.5×4.0×4.1 | 1.5×2.0×1.5 | |
| IIb2 | 4.5×3.5 | 4.5×4.0 | |
 | Table IIIAnalysis of neoadjuvant chemotherapy
response rate. |
Table III
Analysis of neoadjuvant chemotherapy
response rate.
| Chemotherapy
response | Clinical stage
(case) | Total | Ratio (cases/total
cases) % |
|---|
|
| | |
|---|
| Ib2 | IIa2 | IIb2 | | |
|---|
| CR | 2 | 2 | 0 | 4 | 21.1 |
| PR | 4 | 7 | 0 | 11 | 57.9 |
| SD | 3 | 0 | 1 | 4 | 21.1 |
| PD | 0 | 0 | 0 | 0 | 0 |
| Total | 9 | 9 | 1 | 19 | |
Side effects of chemotherapy
The side effects caused by paclitaxel combined with
carboplatin systemic chemotherapy and local injection of cisplatin
were evaluated in accordance with the standards of toxic response
issued by WHO. Of the 19 patients studied, 1 discontinued treatment
following a single course of chemotherapy; therefore the
chemotherapy completion rate was 18/19 (94.7%). The most common
toxicity was hematological toxicity, 11 cases (61.1%) presented
with decreased white blood cell and granulocyte cell counts, of
which 2 cases (11.1%) had a 3–4 level white blood cell and
granulocyte decrease. The incidence of thrombocytopenia was 3/18
(16.7%), and no 3–4 level of thrombocytopenia was observed. Minor
gastrointestinal reactions were evident; 3 cases (16.7%) presented
with mild nausea and vomiting. No peripheral nervous system side
effects occurred. Of the 18 cases, 1 (5.6%) presented with liver
damage, chemotherapy was continued after liver protection and
enzyme reduction treatment, and there was no renal dysfunction. No
drug allergy was observed in any of the patients.
Follow-up after surgery
With the exception of 1 case who discontinued NAC,
all patients were followed up until the last day, July 2010. No
cases of recurrence or metastasis after surgery have been observed
as yet.
Discussion
The efficacy of NAC on the treatment of bulky or
locally advanced cervical cancer remains to be determined. NAC is
potentially capable of reducing localized lesions, improving
parametrial involvement and increasing the surgical resection rate
(8). NAC prior to radiotherapy or
surgery-induced blood rheology variation in the local tumor is
beneficial for the control or elimination of pelvic micrometastases
as it decreases the lymph node metastasis rate, thereby reducing
the radiotherapy rate, decreasing the risk of recurrence and
improving quality of life following surgery. Recent meta-analyses
have confirmed this hypothesis (3).
However, on the basis of a randomized, controlled study of stage
Ib2-IIb cervical cancer, Katsumata et al found that there
was no significant difference between a NAC-combined surgery group
and the surgery alone group in terms of resection and survival rate
(9). This discrepancy is attributed
to the differences of chemotherapy regimens, courses and case
selections. Since platinum drugs, particularly cisplatin, are
sensitizers of radiation therapy, early research on NAC has mainly
focused on cisplatin-based chemotherapy, and numerous investigators
have confirmed that the response rate of cisplatin-combined duplex
treatment is superior to that of single treatment with cisplatin
(10). The application of
paclitaxel provides a new option for NAC chemotherapy. One
randomized controlled study in Italy has shown that the
chemotherapy remission rate in ifosfamide and cisplatin combined
with the paclitaxel (TIP) group was significantly increased
compared with that in the paclitaxel-negative group (IP) (48 vs.
23%), and that the survival time for patients who achieved CR and
PR was longer than that of patients with a poor response to
chemotherapy (11). Moore et
al confirmed that a chemotherapy response rate of 36% can be
achieved in paclitaxel combined with cisplatin NAC (12). Thus, paclitaxel-based NAC specimens
have attracted extensive attention.
An advantage of NAC involves its ability to reduce
tumor size, improving the resection rate and controlling the lymph
node metastasis rate. To accurately evaluate changes in the tumor
volume, the patients included in this study were examined by MRI
prior to and following chemotherapy in order to ensure the accuracy
of the assessment. We confirmed that the lesion size in all cases
after NAC was reduced to varying degrees; the tumor diameter prior
to chemotherapy was reduced from 4.93±0.81 cm to 2.57±1.90 cm after
chemotherapy (P<0.01). Even the 7-cm cervical lesion of the Ib2
case was reduced to 6.5 cm after only a single course of
chemotherapy. However, the patient eventually discontinued therapy
due to financial reasons. Findings of various reports have shown
that the response rate of paclitaxel-based NAC on the treatment of
bulky and locally advanced cervical cancer was 36–95%, and the CR
rate was 9–39% (12–16). However, no cervical local injection
method was observed in any of the programs. In the present study,
paclitaxel-based systemic and local chemotherapy were studied. The
effective rate reached 78.9% and the CR rate reached 21.1%, which
was second only to the 39% CR rate reported by Park et al
(13). However, the course applied
by these authors was 10 days/course, which was shorter and had
greater side effects (13).
Cervical local injection was applied in this study, which reduced
the toxicity of drugs and, at the same time, increased the rate of
the direct effect on the lesions. Of the 18 cases, 4 were
pathologically confirmed as the lesion having completely
disappeared, demonstrating that the method was safe and
therapeutically effective. In addition, 15 cases completed the
surgery with a surgical resection rate of 83.3%, which is in line
with the removal rate of other countries of 73–100% (17). Two cases presented with lymph node
metastasis following surgery with a metastasis rate of 13.3%, which
is slightly lower than the transfer rate of 22–25% noted in other
studies (18). Therefore, NAC
(i.e., paclitaxel combined with carboplatin and cisplatin systemic
and local chemotherapy) on the treatment of bulky cervical cancer
is effective. However, a limited number of patients was included in
the present study. Therefore, a larger cohort of patients is
required in future studies to confirm our results.
The toxicity of NAC is one of the main elements of
focus. The most common side effects in paclitaxel combined with
platinum drugs are blood system and renal toxicity. However, the
toxic response to level 3–4 is relatively small, and it is capable
of being mitigated through symptomatic treatment. In this study,
61.1% of the patients presented with bone marrow suppression,
mainly white blood cell and granulocyte decrease, but only 2 cases
were level 3–4, which were ameliorated after granulocyte
colony-stimulating factor treatment, and no serious infection was
observed. On the basis of reducing allergic reaction and
maintaining the response rate, paclitaxel liposome was applied. No
cases of allergic reaction were observed in the study. With the
exception of systemic treatment, there was local cervical injection
of cisplatin. As for the response rate, this method of treatment is
capable of significantly reducing the lesion size since cisplatin
is unable to directly infiltrate into the blood; the side effects
caused by cisplatin, such as bone marrow suppression and renal
toxicity, were lighter than with systemic chemotherapy. Therefore,
even if combined with local treatment, no serious liver or kidney
damage was observed, with the exception of 1 case with mild liver
dysfunction. Therefore, the chemotherapy regimen applied in this
study is safe and feasible.
In conclusion, NAC with paclitaxel, combined with
carboplatin and cisplatin, for the treatment of bulky cervical
cancer is capable of reducing tumor volume and improving the
operative rate effectively with low toxicity. It is therefore
capable of improving the survival rate and reducing the recurrence
of cancer. However, NAC should be investigated in a larger cohort
of patients in randomized prospective studies in order to confirm
results obtained in the present study.
References
|
1
|
Eddy GL, Bundy BN, Creasman WT, et al:
Treatment of (‘bulky’) stage IB cervical cancer with or without
neoadjuvant vincristine and cisplatin prior to radical hysterectomy
and pelvic/para-aortic lymphadenectomy: a phase III trial of the
gynecologic oncology group. Gynecol Oncol. 106:362–369. 2007.
|
|
2
|
Benedetti Panici P, Greggi S, Scambia G,
et al: Long-term survival following neoadjuvant chemotherapy and
radical surgery in locally advanced cervical cancer. Eur J Cancer.
34:341–346. 1998.PubMed/NCBI
|
|
3
|
Choi CH, Kim T, Lee J, et al: Phase II
study of neoadjuvant chemotherapy with mitomycin-c, vincristine and
cisplatin (MVC) in patients with stages IB2-IIB cervical cancer.
Gynecol Oncol. 104:64–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neoadjuvant Chemotherapy for Locally
Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant
chemotherapy for locally advanced cervical cancer: a systematic
review and meta-analysis of individual patient data from 21
randomised trials. Eur J Cancer. 39:2470–2486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bae JH, Lee S, Lee A, et al: Neoadjuvant
cisplatin and etoposide followed by radical hysterectomy for stage
1B–2B cervical cancer. Gynecol Oncol. 111:444–448. 2008.PubMed/NCBI
|
|
6
|
Zhu Lihong, Wei Lihui, Li Xiaoping, et al:
Killing effect of paclitaxel liposome on cervical carcinoma SiHa
cells in vitro. Bull Chin Cancer. 17:704–707. 2008.
|
|
7
|
Shen Y and Wang Z: Total laparoscopic
radical hysterectomy for treatment of uterine malignant tumors:
analysis of short-term therapeutic efficacy. J Huazhong Univ Sci
Technol [Med Sci]. 30:376–379. 2010.PubMed/NCBI
|
|
8
|
Lai CH, Hsueh S, Chang TC, et al:
Prognostic factors in patients with bulky stage IB or IIA cervical
carcinoma undergoing neoadjuvant chemotherapy and radical
hysterectomy. Gynecol Oncol. 64:456–462. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsumata N, Yoshikawa H, Hirakawa T, et
al: Phase III randomized trial of neoadjuvant chemotherapy (NAC)
followed by radical hysterectomy (RH) versus RH for bulky stage
I/II cervical cancer. ASCO Annu Meet Proc. 24:abs. 50132006.
|
|
10
|
Monk B, Huang HQ, Cella D, et al: Quality
of life outcomes from a randomized phase III trial of cisplatin
with or without topotecan in advanced carcinoma of the cervix: a
Gynecologic Oncology Group Study. J Clin Oncol. 23:4617–4625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buda A, Fossati R, Colombo N, et al:
Randomized trial of neoadjuvant chemotherapy comparing paclitaxel,
ifosfamide, and cisplatin with ifosfamide and cisplatin followed by
radical surgery in patients with locally advanced squamous cell
cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio)
Italian Collaborative Study. J Clin Oncol. 23:4137–4145. 2005.
|
|
12
|
Moore DH, Blessing JA, McQuellon MP, et
al: Phase III study of cisplatin with or without paclitaxel in
stage IVB, recurrent, or persistent squamous cell carcinoma of the
cervix: a gynecologic oncology group study. J Clin Oncol.
22:3113–3119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park DC, Kim JH, Lew YO, Kim DH and
Namkoong SE: Phase II trial of neoadjuvant paclitaxel and cisplatin
in uterine cervical cancer. Gynecol Oncol. 92:59–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dueñas-Gonzalez A, López-Graniel C,
González-Enciso A, et al: A phase II study of multimodality
treatment for locally advanced cervical cancer: neoadjuvant
carboplatin and paclitaxel followed by radical hysterectomy and
adjuvant cisplatin chemoradiation. Ann Oncol. 14:1278–1284.
2003.
|
|
15
|
D’Agostino G, Distefano M, Greggi S, et
al: Neoadjuvant treatment of locally advanced carcinoma of the
uterine cervix with epirubicin, paclitaxel and cisplatin. Cancer
Chemother Pharmacol. 49:256–260. 2004.PubMed/NCBI
|
|
16
|
Zanetta G, Lissoni A, Pellegrino A, et al:
Neoadjuvant chemotherapy with cisplatin, ifosfamide and paclitaxel
for locally advanced squamous-cell cervical cancer. Ann Oncol.
9:977–980. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang YY, Moon H, Cho SH, et al: Ten-year
survival of patients with locally advanced stage IB-IIB cervical
cancer after neoadjuvant chemotherapy and radical hysterectomy.
Gynecol Oncol. 82:88–93. 2001.PubMed/NCBI
|
|
18
|
Eddy GL, Manetta A, Alvarez RD, et al:
Neoadjuvant chemotherapy with Vincristin and cisplatin followed by
radical hysterectomy and pelvic lymphadenectomy for FIGO stage IB
bulky cervical cancer: a gynecologic oncology group pilot study.
Gynecol Oncol. 57:412–416. 1995. View Article : Google Scholar
|