Introduction
Despite advances in primary surgical therapy and
first line chemotherapy, the majority of patients suffering from
advanced surgical cancer ultimately relapse. Salvage therapy for
platinum refractory disease or heavily pre-treated patients has
proven unsuccessful with regard to survival. Therefore, therapy in
these patients is primarily aimed at improving quality of life.
Symptomatic malignant ascites remains a severe therapeutic
challenge in patients suffering from recurrent ovarian cancer or
other cancer types such as colorectal, stomach, pancreatic or lung
cancer. Malignant ascites and pleural effusion are significant
causes of morbidity and mortality, and frequently impair the
quality of life in patients who are already distressed by the
underlying disease. It is known that the median survival of
patients suffering from malignant ascites is significantly reduced
to 20 weeks following diagnosis if all cancer types are included
(1,2). However, effective treatment of
malignant ascites in palliative patients is a therapeutic challenge
and often frustrating. The established method of treating malignant
ascites is paracentesis and the intraperitoneal (i.p.) instillation
of chemotherapeutic agents such as cisplatin, 5-fluorouracil or
doxorubicin (3,4). However, the efficacy of i.p.
chemotherapy in a palliative setting has only been investigated in
small cohorts of highly selected patients. Although studies have
shown partial responses, the results have been inconsistent and
some studies have reported significant side-effects (5).
A better understanding of the underlying molecular
mechanisms that regulate the formation of malignant effusions have
led to the concept that the angiogenic biomolecule vascular
epithelial growth factor (VEGF) is important in the pathobiology of
a variety of tumours and the pathogenesis of malignant and
non-malignant effusions (6). It
promotes tumour angiogenesis, formation of ascites and pleural
effusions, and metastasis in ovarian cancer (7). Inhibitors of VEGF, such as the
antibody bevacizumab, suppress tumour growth in ovarian cancer
models, possibly by inhibiting angiogenesis (7). It is known that in patients with
malignant effusions, high levels of VEGF are found in serum/plasma
as well as in the malignant effusion (8). Previous studies showed a positive
therapeutic effect of bevacizumab in the systemic treatment of
patients with ovarian carcinoma and formation of ascites (9). Numnum et al demonstrated that
patients undergoing treatment with bevacizumab showed a symptomatic
relief of ascites and required no therapeutic paracentesis after
treatment with the antibody (9).
The most common serious bevacizumab-related toxicities are
hypertension, fatigue, proteinuria, bleeding and pain (10). Some cases of gastrointestinal
perforation or fistula, brain ischemia, pulmonary hypertension,
gastrointestinal bleeding and wound healing complications have been
reported (11).
Intraperitoneal administration of bevacizumab has
recently been demonstrated to be efficacious in locally pretreated
cancer patients suffering from malignant ascites (12). Since malignant pleural effusion
contains high numbers of active tumour cells, the local
intrapleural application of bevacizumab is a rational alternative
to intravenous (i.v.) administration. However, to date only a few
patients with malignant ascites have been treated with
intraperitoneally delivered bevacizumab, and therefore this route
of administration needs to be further explored. In this study, we
have compared i.v. and i.p. administration of bevacizumab in an
ovarian cancer mouse model with intraperitoneal metastasis. We have
examined whether a single i.p. administration of bevacizumab is
capable of reducing ascites-related body surface and prolonging
survival.
Materials and methods
Cells and cell lines
SKOV-3.ip1 ovarian adenocarcinoma cell lines were
kind gifts from Drs Judy Wolf and Janet Price (University of Texas
MD Anderson Cancer Center, Houston, TX, USA), respectively. Cell
lines were maintained in recommended conditions. Cells were grown
at 37˚C in a humidified atmosphere of 5% CO2.
To generate resistant ovarian cancer cells,
SKOV-3.ip1 cells were cultured in growth medium (GM) containing
cisplatin. Initially, 10 ng/ml cisplatin was added to the GM and
the dose was increased by 10 ng/ml per week to a final
concentration of 60 ng/ml.
Anticancer drugs
Paclitaxel and novantrone were obtained from the
pharmacy of the University of Düsseldorf Medical Centre and handled
according to the manufacturer’s instructions. Paclitaxel (6 mg/ml)
was diluted at a ratio of 1:15 with saline. Novantrone (2 mg/ml)
was diluted at a ratio of 1:50 with saline. Bevacizumab was
purchased from Roche (Basel, Switzerland). Each mouse assigned to a
bevacizumab treatment received a single application. Bevacizumab
was diluted in 200 μl of phosphate-buffered saline (PBS)
immediately prior to administration.
Therapeutic ovarian cancer model
Female CB17 SCID mice were obtained at 3-4 weeks of
age and quarantined at least 1 week prior to the study. Mice were
kept under pathogen-free conditions according to the American
Association for Accreditation of Laboratory Animal Care guidelines.
Animal protocols were reviewed and approved by the state
authorities according to the German animal protection law. Mice
were injected i.p. with 1×107 drug resistant SKOV-3.ip1
cells on day 0. Mice were assigned into six treatment groups, each
consisting of 11 mice: Group 1 served as the control (injection of
PBS); Group 2 and 3 received bevacizumab i.p. or i.v.,
respectively; Group 4 was treated with paclitaxel; the therapy of
Group 5 and 6 consisted of paclitaxel plus bevacizumab i.p. or
i.v., respectively. Each mouse assigned to a chemotherapy treatment
received 2 applications of paclitaxel (10 mg per kg body weight) on
days 11 and 13. Bevacizumab was administered at a total dose of 10
mg per kg body weight on day 12. Drug injections were performed
either intravenously through the lateral tail vein (i.v. group) or
via the peritoneum (i.p. group).
Abdominal circumference and body weight were
measured weekly for calculation of the body surface until 35 days
following initial treatment. Variations in body surface are
dependent on ascites volume plus tumour burden and therefore the
body surface is considered a valuable parameter for disease
progression.
A total of 21 days following the initial treatment,
a representative mouse from each treatment group was sacrificed and
the expression of VEGF was examined in the macroscopic disease and
in the ascites. In the remaining mice, survival was followed daily
until mortality.
RNA preparation and quantitative
RT-PCR
The total cellular RNA of tumour cells was extracted
from 2×105 cells using the RNeasy mini prep kit (Qiagen,
Santa Clarita, CA, USA) and additionally treated with DNase I (Life
Technologies Inc; Rockville, MD, USA) for 30 min. PCR products from
the VEGF gene were used for the creation of the standard curve. A
GeneAmp RNA PCR core kit (Applied Biosystems, Foster City, CA, USA)
was used for cDNA synthesis and PCR amplification of cDNA products.
TaqMan primers and probes were designed by the Primer Express 1.0
software and synthesized by Applied Biosystems. Oligonucleotide
sequences for the amplification of the VEGF gene were: forward
primer CAT GCA GAT TAT GCG GAT CAA; reverse primer TTT GTT GTG CTG
TAG GAA GCT CA; and probe 6FAM-CCT CAC CAA GGC CAG CAC ATA GGA
GA-TAMRA. The human housekeeping gene glyceraldehydes-3-phosphate
dehydrogenase (GAPDH) served as an internal control.
Each single real-time PCR contained 1X
TaqMan® EZ RT-PCT kit (Applied Biosystems), 100 nM
forward primer, 100 nM reverse primer, 100 nM probe and 0.025%
bovine serum albumin in a final volume of 9 μl per reaction. The
PCR contained 1 μl of template or water as the control.
PCR was carried out using a LightCyclerTMSystem
(Roche Molecular Biochemicals, Indianapolis, IN, USA) according to
the manufacturer’s instructions. Thermal cycling conditions were: 2
min at 50˚C, 30 min at 60˚C, 5 min at 95˚C and 40 cycles of 20 sec
at 94˚C and 1 min at 60˚C. Data were analyzed with LightCycler
software. Known amounts of human total RNA (200, 20, 2 and 0.2
ng/μl) were amplified to generate a standard curve for the
determination of the concentration of the unknown samples.
Statistical analysis
Data were presented as the mean values ± standard
deviation. The body surface among groups was assessed with a
two-tailed Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference. Survival data were plotted as
a Kaplan-Meier curve. Statistical analyses were performed using
Prism 4 software (GraphPad Software, San Diego, CA, USA).
Results
The orthotopic murine model of peritoneal
disseminated ovarian cancer
One representative mouse per treatment group was
investigated to confirm the tumour model. The dissemination of
ovarian cancer was confirmed surgically. Samples of each tumour
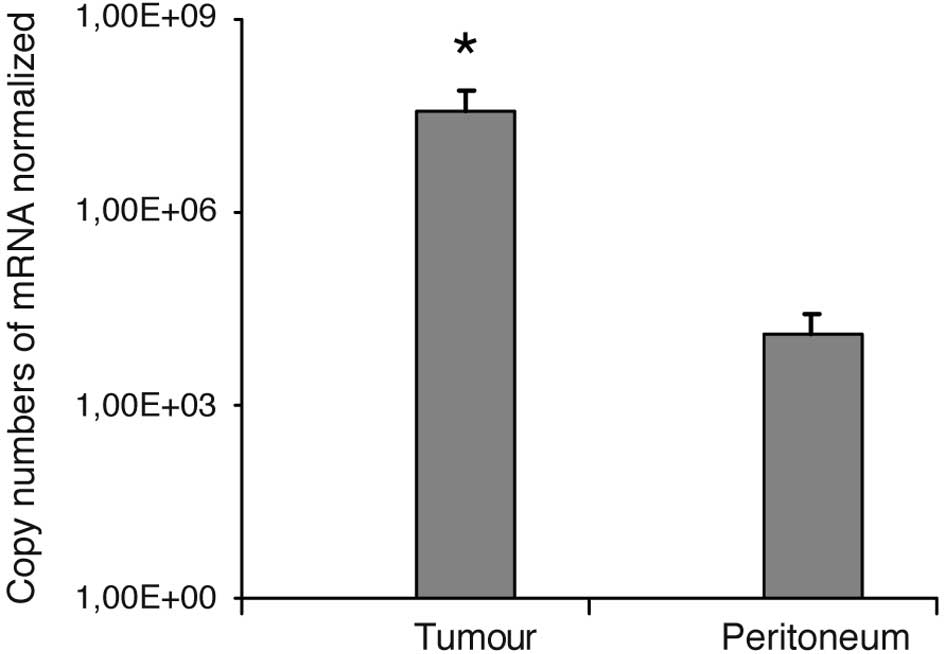
burden were successfully checked for VEGF overexpression. Fig. 1 shows the data of all six sacrificed
mice. VEGF was expressed 2841-fold (p<0.001) higher in the
tumour tissues than in the peritoneum, which was defined as the
healthy control tissue.
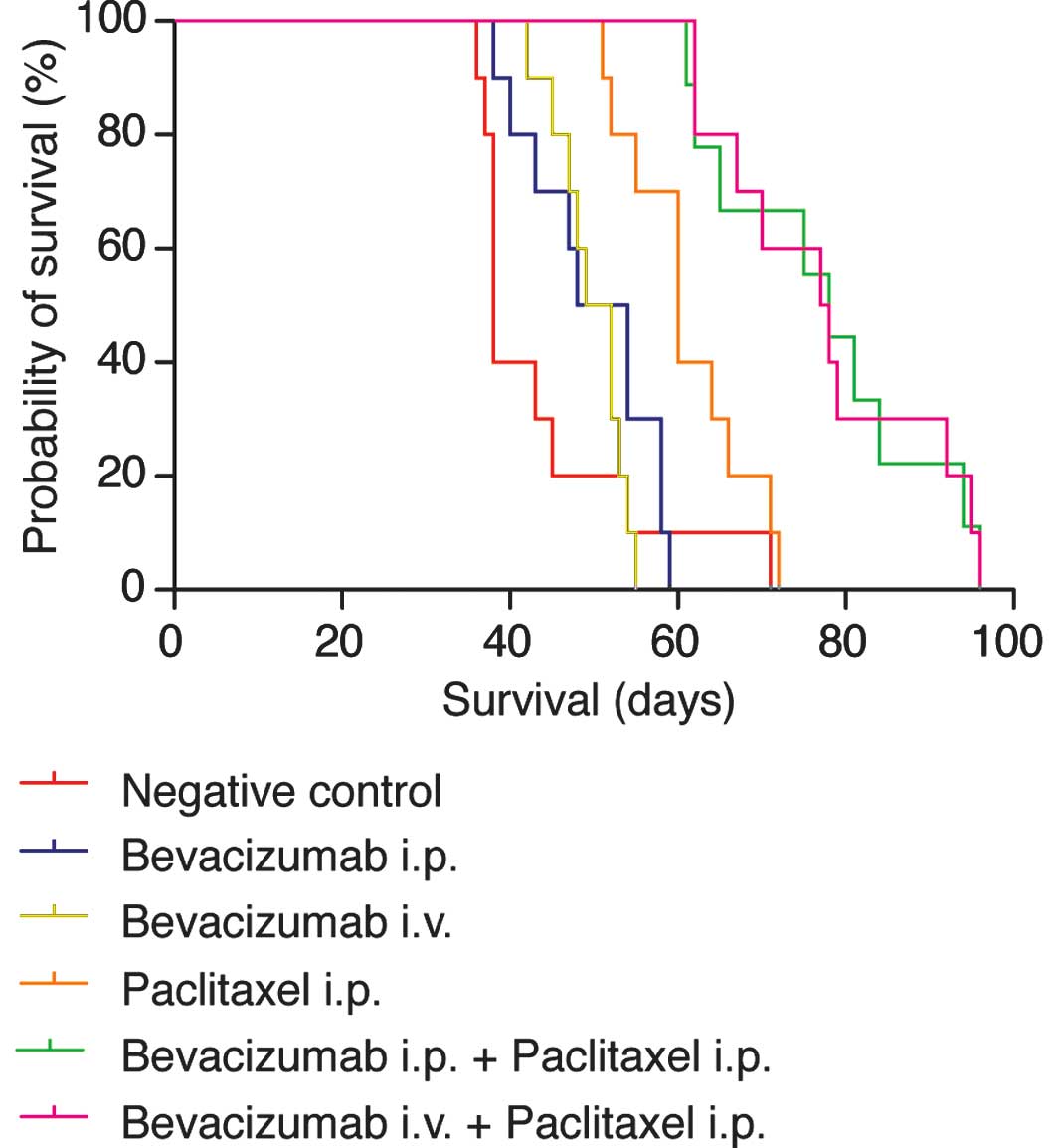
Overall survival
Advanced carcinomatosis was allowed to develop for
12 days and mice were treated with either a single dose of PBS
(negative control), bevacizumab i.v., bevacizumab i.p., paclitaxel
i.p. or a combination of bevacizumab and paclitaxel. Fig. 2 shows the results in a plot
according to Kaplan-Meier calculations. In pairwise comparisons,
survival of mice in all treatment groups was significantly enhanced
when compared to the non-treatment control group (p<0.001 for
bevacizumab + paclitaxel and p<0.05 for single agent bevacizumab
i.p., bevacizumab i.v. and paclitaxel i.p.). Survival of mice
treated with a combination regimen of bevacizumab and paclitaxel
was significantly prolonged compared to mice treated with a single
agent (p<0.001). However, differences between i.p. and i.v.
administration of bevacizumab were not statistically significant in
the single agent regimen or combination regimen with
paclitaxel.
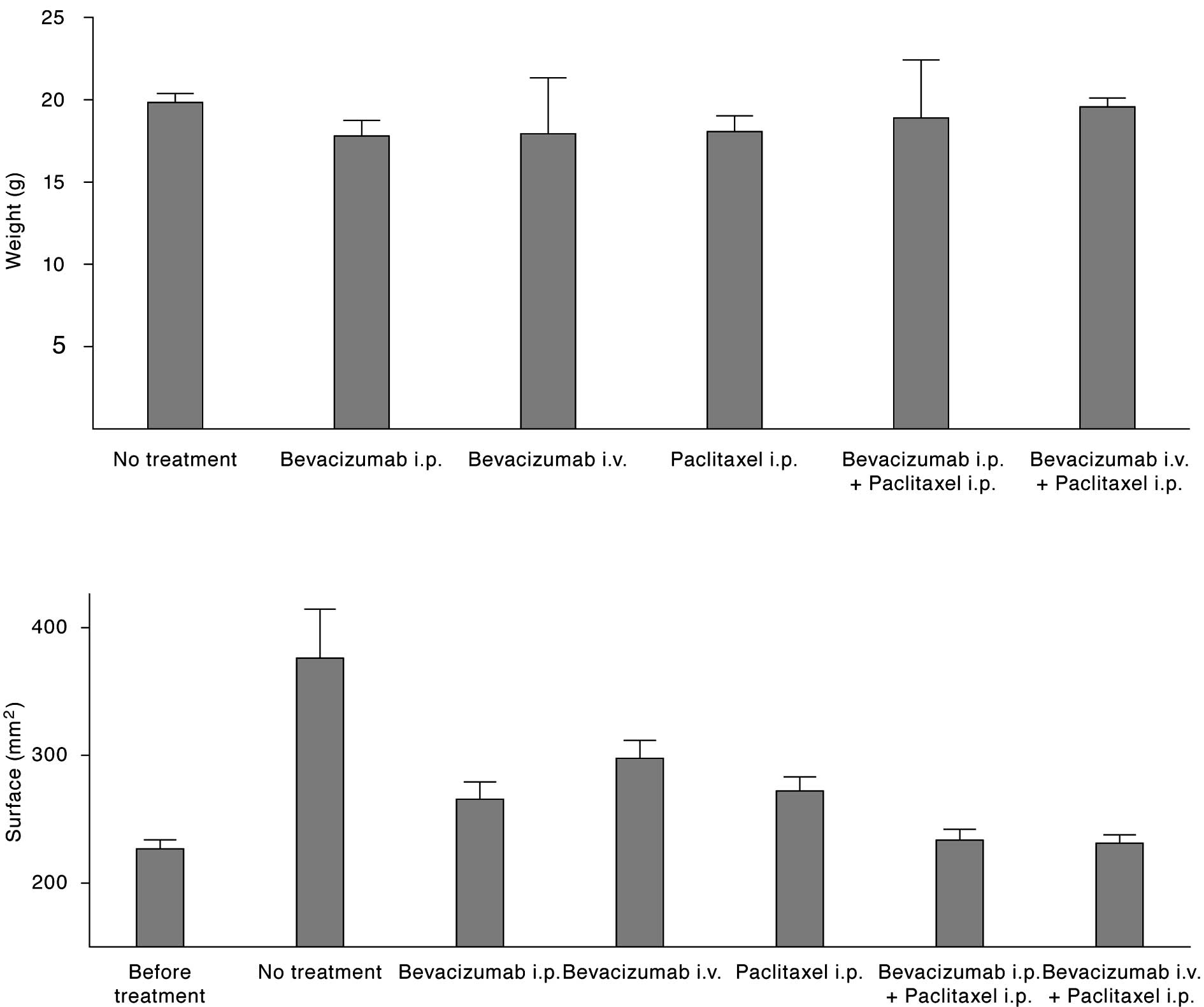
Therapeutic success
Success of the treatment was assessed via reduction
of the body surface, which is proportional to the intracorporal
ascites volume.
As shown in Fig. 3,
the mean abdominal surface (AS) 24 days after initiation of
treatment was 376, 266, 298, 272, 231 and 234 mm2 for
mice treated with PBS, bevacizumab i.p., bevacizumab i.v.,
paclitaxel i.p, bevacizumab i.p. + paclitaxel i.p. or bevacizumab
i.v. + paclitaxel i.p., respectively. The mean AS of all mice prior
to treatment was 227±22 mm2. The results of the
statistical analyses among the treatment arms are shown in Table I. Each treatment resulted in a
significant decrease of body surface with the exception of
bevacizumab i.v. In that group, two mice were non-responders to the
therapy and showed a body surface comparable to the animals without
treatment. It is noteworthy that the combination of paclitaxel plus
bevacizumab significantly improved body surface as well as overall
survival in comparison to a treatment with one of the drugs.
 | Table ISummary of the statistical analyses
shown as the p-values following body surface comparisons using the
t-test. |
Table I
Summary of the statistical analyses
shown as the p-values following body surface comparisons using the
t-test.
| P-value |
|---|
| vs. no treatment |
| Bevacizumab
i.p. | 0.00113 |
| Bevacizumab
i.v. | 0.7159 |
| Paclitaxel i.p. | 0.00279 |
| Paclitaxel i.p. +
bevacizumab i.p. | 0.000148 |
| Paclitaxel i.p. +
bevacizumab i.v. | 0.0000065 |
| vs. paclitaxel or
bevacizumab |
| Paclitaxel +
bevacizumab | 0.00000577 |
Discussion
Ovarian carcinoma is the leading cause of mortality
from gynaecological cancer (7). In
general, the tumour progression is already advanced at the time of
diagnosis. Despite advances in primary surgical therapy and first
line chemotherapy, the majority of patients suffering from advanced
surgical cancer ultimately relapse. Salvage therapy for platinum
refractory disease has shown limited efficacy with regard to
survival. Therapy in these patients is therefore primarily aimed at
improving quality of life. Secondary symptoms such as symptomatic
malignant ascites are a therapeutic challenge in the palliative
setting. In addition, it is well known that the prognosis of
patients with malignant pleural effusion is worse compared to those
without secondary symptoms (1). The
effect of paracentesis is usually of short duration and the effect
of i.p. administration of chemotherapeutic agents is limited.
The humanized monoclonal antibody bevacizumab that
targets angiogenesis by binding to VEGF has shown significant
single agent activity in patients suffering from recurrent ovarian
cancer. Studies have shown response rates between 16 and 18% in
platinum-sensitive and platinum-refractory disease (10,13).
Despite the improvement of progression-free survival in these
trials, bevacizumab appears to improve the control of ascites.
These data are in line with the observation of elevated levels of
VEGF in malignant ascites (6,14).
Evidence suggests that angiogenesis is important in
the prognosis and progression of epithelial ovarian cancer. Several
studies have demonstrated a direct association of microvessel
density and VEGF in primary tumours with extent of disease and time
to progression (15). In addition,
VEGF has relatively low levels of expression in normal tissue
(16) and is therefore considered
attractive as a potential target for an anti-VEGF agent such as
bevacizumab. Preclinical models have demonstrated inhibition of
tumour growth in various tumour cell types (17). Several alterations in vascular
function, such as decreases in vessel density, diameter and
permeability, result in a decreased production of malignant
ascites. Consequently, various approaches have been examined to
target VEGF and VEGF receptors (18), with several studies having
demonstrated a reduction of ascites and tumour growth inhibition
following the administration of bevacizumab in various ovarian
cancer animal models (19).
Findings of a recently published study have shown
prolonged survival following maintenance therapy in an
intraperitoneal xenograft mouse model of previously untreated
ovarian cancer (20). This study
showed a significant inhibition of tumour growth following
administration of 5 mg/kg bevacizumab as a single agent. In
addition, bevacizumab significantly enhanced sensitivity to
cisplatin. It may be speculated that enhancement of drug-induced
tumour cell apoptosis by VEGF immunoneutralisation is the molecular
basis which has been shown recently for cisplatin-treated ovarian
cancer (21).
Another study has recently demonstrated rapid
absorption of bevacizumab following i.p. administration, with a
bioavailability of 92.8% (23). The
authors reported a rapid and near complete absorption of
bevacizumab following i.p. dosing, as well as increased animal
survival following combined therapy with bevacizumab and i.p.
bevacizumab and i.p. paclitaxel. As yet, little is known about the
effect of bevacizumab on malignant ascites in pre-treated ovarian
cancer patients. To the best of our knowledge, the present study is
the first that evaluated i.v. as well as i.p. administration of
bevacizumab in a xenograft model of cisplatin pre-treated ovarian
cancer. Of note, we only administered a single dose of bevacizumab,
paclitaxel or the combination therapy. We demonstrated
significantly prolonged survival and reduction of ascites.
Moreover, the addition of bevacizumab to a common cytostatic
therapy such as paclitaxel resulted in further improvement in
ascites reduction and survival. These findings are in agreement
with preclinical and clinical studies which demonstrated that the
efficacy of i.p. chemotherapy is limited by poor penetration of
drugs into peritoneal tumours (23). It has been hypothesized, that
adjunct therapy with anti-angiogenic agents may lead to decreased
drug removal from peritoneal tumours, increased drug concentration
in tumours and increased efficacy of i.p. chemotherapy (21). In a recently published report, four
heavily pre-treated ovarian cancer patients received bevacizumab
intravenously at a dose of 15 mg/kg every 3 weeks (9,17).
Ascites were significantly reduced in all patients and no patient
required a paracentesis following the initiation of therapy. Only a
few patients with malignant ascites have received i.p. bevacizumab
(4). Nine patients with malignant
ascites from colon, breast, uterine and ovarian cancer, for whom
repeated paracentesis prior to treatment was required, were
administered intraperitoneal bevacizumab at 5 mg/kg monthly. Eight
out of nine patients (89%) experienced prolongation of the interval
between paracentesis (23).
However, the i.p. route of administration in a palliative patient
population needs to be examined stringently regarding the safety of
the treatment. Intraperitoneal application may be the route of
choice in this particular setting as it allows for the accumulation
of the study drug within the body compartment where malignant
ascites is promoted by VEGF secretion.
The effects of bevacizumab, either alone or in
combination with i.p. paclitaxel, were not found to be
significantly dependent on the route of administration. We conclude
from this study that treatment of malignant ascites with i.p.
bevacizumab is an effective and powerful alternative to i.v.
treatment.
References
|
1
|
Eitan R, Levine DA, Abu-Rustum N, et al:
The clinical significance of malignant pleural effusions in
patients with optimally debulked ovarian carcinoma. Cancer.
103:1397–1401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherer DM, Eliakim R and Abulafia O: The
role of angiogenesis in the accumulation of peritoneal fluid in
benign conditions and the development of malignant ascites in the
female. Gynecol Obstet Invest. 50:217–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markman M: Intraperitoneal antineoplastic
drug delivery: rationale and results. Lancet Oncol. 4:277–283.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobold S, Hegewisch-Becker S, Oechsle K,
Jordan K, Bokemeyer C and Atanackovic D: Intraperitoneal VEGF
inhibition using bevacizumab: a potential approach for the
symptomatic treatment of malignant ascites? Oncologist.
14:1242–1251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aslam N and Marino CR: Malignant ascites:
new concepts in pathophysiology, diagnosis, and management. Arch
Intern Med. 161:2733–2737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983. View Article : Google Scholar
|
|
7
|
Huynh H, Teo CC and Soo KC: Bevacizumab
and rapamycin inhibit tumor growth in peritoneal model of human
ovarian cancer. Mol Cancer Ther. 6:2959–2966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sack U, Hoffmann M, Zhao XJ, et al:
Vascular endothelial growth factor in pleural effusions of
different origin. Eur Respir J. 25:600–604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Numnum TM, Rocconi RP, Whitworth J and
Barnes MN: The use of bevacizumab to palliate symptomatic ascites
in patients with refractory ovarian carcinoma. Gynecol Oncol.
102:425–428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cannistra SA, Matulonis UA, Penson RT, et
al: Phase II study of bevacizumab in patients with
platinum-resistant ovarian cancer or peritoneal serous cancer. J
Clin Oncol. 25:5180–5186. 2007. View Article : Google Scholar
|
|
11
|
Simpkins F, Belinson JL and Rose PG:
Avoiding bevacizumab related gastrointestinal toxicity for
recurrent ovarian cancer by careful patient screening. Gynecol
Oncol. 107:118–123. 2007. View Article : Google Scholar
|
|
12
|
Hamilton CA, Maxwell GL, Chernofsky MR,
Bernstein SA, Farley JH and Rose GS: Intraperitoneal bevacizumab
for the palliation of malignant ascites in refractory ovarian
cancer. Gynecol Oncol. 111:530–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burger RA, Sill MW, Monk BJ, Greer BE and
Sorosky JI: Phase II trial of bevacizumab in persistent or
recurrent epithelial ovarian cancer or primary peritoneal cancer: a
Gynecologic Oncology Group Study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zebrowski BK, Liu W, Ramirez K, Akagi Y,
Mills GB and Ellis LM: Markedly elevated levels of vascular
endothelial growth factor in malignant ascites. Ann Surg Oncol.
6:373–378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rein DT, Breidenbach M, Nettelbeck DM, et
al: Evaluation of tissue-specific promoters in carcinomas of the
cervix uteri. J Gene Med. 6:1281–1289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerber HP and Ferrara N: Pharmacology and
pharmacodynamics of bevacizumab as monotherapy or in combination
with cytotoxic therapy in preclinical studies. Cancer Res.
65:671–680. 2005.PubMed/NCBI
|
|
18
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu L, Hofmann J, Zaloudek C, Ferrara N,
Hamilton T and Jaffe RB: Vascular endothelial growth factor
immunoneutralization plus Paclitaxel markedly reduces tumor burden
and ascites in athymic mouse model of ovarian cancer. Am J Pathol.
161:1917–1924. 2002. View Article : Google Scholar
|
|
20
|
Mabuchi S, Terai Y, Morishige K, et al:
Maintenance treatment with bevacizumab prolongs survival in an in
vivo ovarian cancer model. Clin Cancer Res. 14:7781–7789. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah DK, Shin BS, Veith J, Toth K,
Bernacki RJ and Balthasar JP: Use of an anti-vascular endothelial
growth factor antibody in a pharmacokinetic strategy to increase
the efficacy of intraperitoneal chemotherapy. J Pharmacol Exp Ther.
329:580–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davies S, Dai D, Pickett G, Thiel KW,
Korovkina VP and Leslie KK: Effects of bevacizumab in mouse model
of endometrial cancer: Defining the molecular basis for resistance.
Oncol Rep. 25:855–862
|
|
23
|
Shah DK, Veith J, Bernacki RJ and
Balthasar JP: Evaluation of combined bevacizumab and
intraperitoneal carboplatin or paclitaxel therapy in a mouse model
of ovarian cancer. Cancer Chemother Pharmacol. 68:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|