Introduction
Kidney tumors comprise ~2% of all adult malignancies
and account for approximately 102,000 mortalities worldwide
annually (1). Renal cell carcinoma
(RCC) constitute the majority of all kidney tumors and can be
classified histologically as clear cell (60–80%), papillary
(10–15%), chromophobe (5–10%) and collecting duct carcinoma
(<1%). Approximately 30% of all patients with RCC have
metastatic disease at presentation and ~50% of patients undergoing
curative surgery are expected to experience relapse at distant
sites (2,3).
The treatment of metastatic (m)RCC has markedly
changed over the last 5 years due to the antitumor efficacy of two
groups of targeted agents, i.e., agents that inhibit vascular
endothelial growth factor (VEGF)-signaling pathways and those that
inhibit mammalian target of rapamycin (mTOR) (4). For example, the
VEGF-receptor/c-kit/platelet-derived growth factor
receptor/FMS-like tyrosine kinase 3 tyrosine kinase inhibitor
sunitinib, exhibits a 31% partial response rate in mRCC patients,
disease stabilization in an additional 48% of mRCC patients and an
increase in median progression-free survival from 5 to 11 months
(5). For patients with mRCC that
progressed to VEGF receptor tyrosine kinase inhibitor therapy, the
orally administered mTOR inhibitor everolimus was recently shown to
prolong progression-free survival relative to placebo from 1.9 to
4.9 months (p<0.001), providing an important additional
therapeutic tool for this patient category (6,7).
Well-known side-effects of sunitinib include
hypertension, fatigue, thyroid dysfunction, cardiotoxicity,
gastrointestinal toxicity and skin toxicity (8). In this study, we report the case of a
61-year-old male with papillary mRCC who developed reversible
posterior leukoencephalopathy syndrome (RPLS) during sunitinib
therapy. Our case is the seventh patient reported in the literature
(9) whose treatment with sunitinib
was complicated by RPLS, but the first to additionally suggest that
everolimus may prolong radiological alterations characteristic of
RPLS. Patient consent was obtained for this case report.
Case report
A 61-year-old male with a history of
hypercholesterolemia, hypertension and type 2 diabetes was referred
to our outpatient clinic following diagnosis with mRCC. Due to
hematuria, flank pain, weight loss and a renal mass, the patient
had undergone a right-sided nephrectomy with lymph node dissection
three months earlier. Histopathological examination revealed a
T3bN2Mx papillary cell RCC. Besides a non-productive cough and mild
shortness of breath on exertion, the patient had no specific
complaints. Physical examination revealed no relevant
abnormalities; the patient had a WHO performance score of 1 and a
blood pressure of 150/88 mmHg. A mild anemia [hemoglobin 7.8 mmol/l
(normal 8.5–11 mmol/l)] and a mild renal insufficiency (glomerular
filtration rate 45 ml/min) were noted on laboratory evaluation.
Serum levels of calcium and lactate dehydrogenase were normal.
Computed tomography (CT) of the chest and abdomen demonstrated
metastases in mediastinal and retroperitoneal lymph nodes, as well
as bilateral pulmonary metastases.
Treatment with sunitinib (50 mg daily for 4 weeks
every 6 weeks) was initiated. Although antitumor responses are more
frequently observed in patients with clear cell as compared to
papillary cell RCC (10), the
patient responded clinically with a reduction in his non-productive
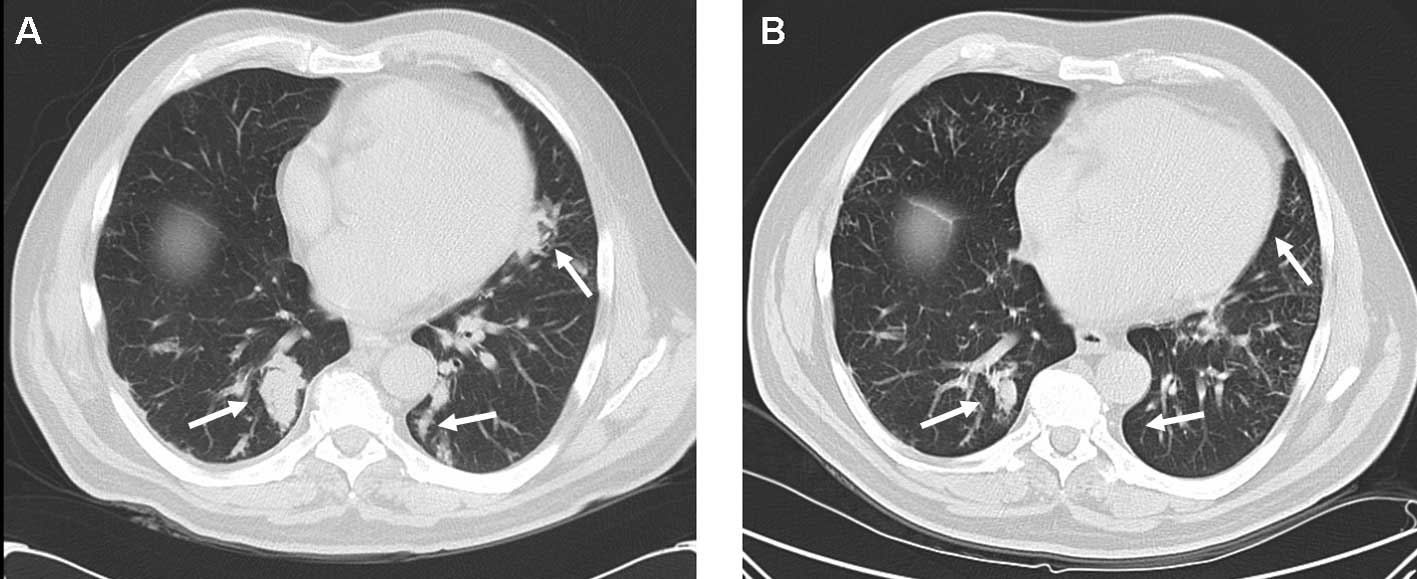
cough and exertional shortness of breath, and radiologically with a
partial response of pulmonary metastases (Fig. 1). Treatment was well tolerated with
no alterations in renal function, blood pressure, thyroid hormone
function and no hematological toxicity.
During the third week of the third cycle of
sunitinib, the patient presented to the emergency department
following an episode of absence and a generalized tonic-clonic
seizure. Physical and neurological examination revealed no other
abnormalities apart from hypertension (202/101 mmHg). The epileptic
seizure responded well to an initial combination of midazolam (7.5
mg i.v.) and phenytoin (1250 mg i.v.). Laboratory tests, including
renal function and serum electrolytes, showed no significant
changes. A CT scan of the brain revealed hypodense areas suggestive
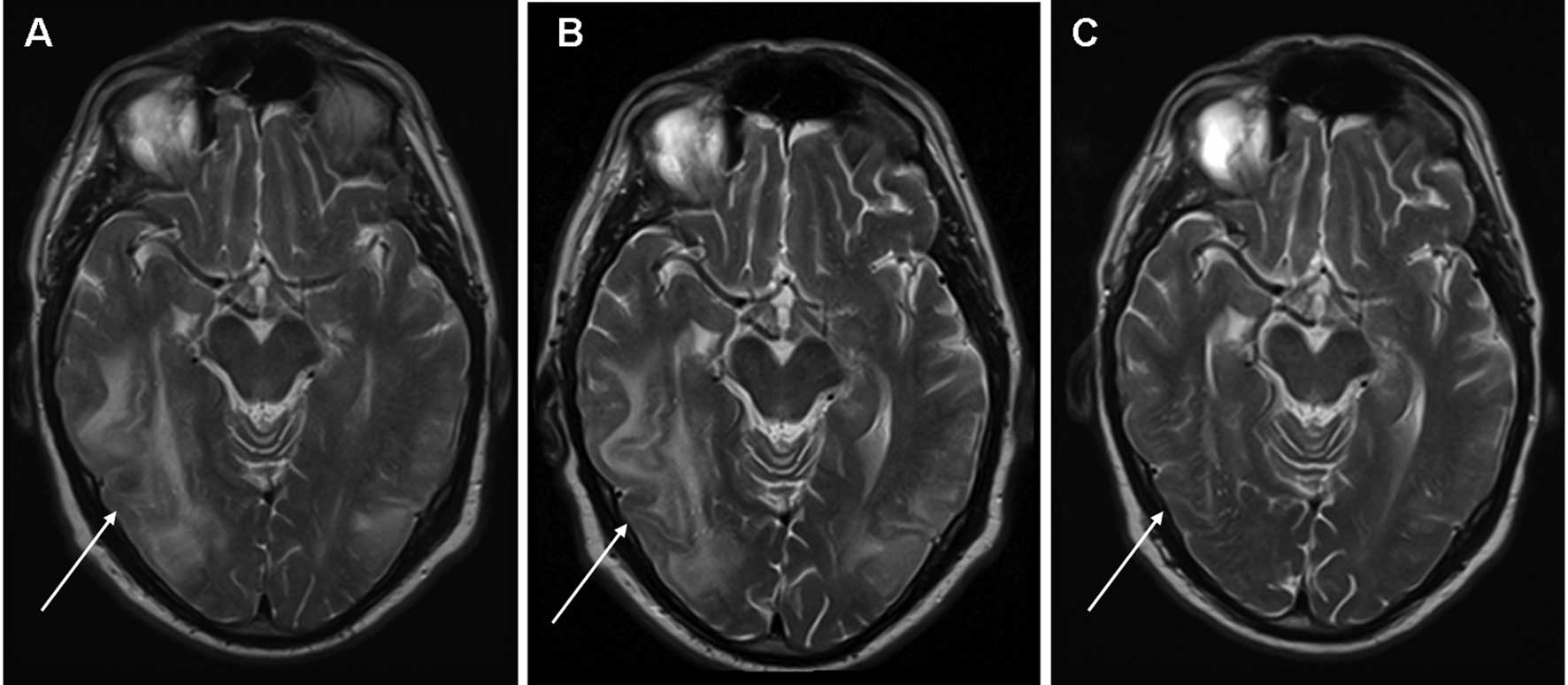
of edema but did not rule out metastases. Subsequent magnetic
resonance imaging (MRI) of the brain was compatible with RPLS with
extensive edema predominantly in the right parieto-temporal, right
parietal and left parieto-occipital areas of the brain with no
evidence of cerebral metastases (Fig.
2A). Treatment with sunitinib was stopped and anti-epileptic
treatment consisting of oral sodium valproate (750 mg bid) was
initiated and the patient made a further uneventful recovery. The
increased blood pressure that was noted upon presentation rapidly
returned to normal and the patient’s regular antihypertensive
treatment consisting of the combination of a thiazide diuretic, an
ACE-inhibitor and a calcium antagonist was continued.
Four weeks later, a CT scan showed progressive
disease of pulmonary metastases and retroperitoneal lymphadenopathy
accompanied by a subjective increase in exertional shortness of
breath, non-productive cough and short episodes of upper abdominal
pain. Second-line treatment with oral everolimus (10 mg once daily)
was initiated. Although the treatment was well tolerated, no
symptomatic improvement in pulmonary complaints occurred and
treatment with everolimus was terminated after 4 weeks due to
progressive disease. Notably, a follow-up MRI scan of the brain
performed in the second week of everolimus therapy (6 weeks after
sunitinib treatment was terminated) still showed radiological
evidence of RPLS (Fig. 2B). A
repeat MRI of the brain performed 2 weeks following cessation of
everolimus showed complete resolution of RPLS (Fig. 2C). Palliative treatment was provided
by the patient’s general practitioner. The patient succumbed to the
disease 6 months later.
Discussion
RPLS is a noteworthy clinical and radiological
entity that was first described by Hinchey et al (11). Clinical symptoms frequently observed
in patients with RPLS include epileptic seizures, altered mental
status, vomiting, visual disturbances and headache.
Mechanistically, RPLS corresponds to a cerebral vasogenic edema
associated with a variety of conditions including arterial
hypertension, eclampsia, collagen vascular disorders, Guillain
Barre syndrome, thrombotic thrombocytic purpura, acute porphyria
and postcarotid endarterectomy. However, it is most commonly caused
by immunosuppressive and cytotoxic drugs including cyclosporin A,
tacrolimus, cisplatin, cytarabine, intravenous immunoglobulins,
L-asparaginase, 5-fluorouracil, interferon-α, and more recently by
anti-angiogenic agents including bevacizumab, sorafenib and
sunitinib (9,11-20).
Typical MRI findings in patients with RPLS include edema involving
the white matter, predominantly in the posterior portions of the
cerebral hemispheres, particularly bilaterally in the
parieto-occipital regions, although other areas of the brain may
also be affected (11,17).
Although the exact pathogenesis of RPLS remains
unclear, it is most likely due to a disruption of cerebral vascular
endothelial cells and impaired cerebrovascular autoregulation
leading to edema (11). During
hypertensive periods the upper limit of cerebral autoregulation,
which normally maintains a constant perfusion rate during blood
pressure changes, is exceeded resulting in hyperperfusion and the
formation of vasogenic edema of the brain (21). Furthermore, renal dysfunction
appears to predispose patients to the development of RPLS, likely
due to fluid overload (16).
Cytotoxic drugs may have direct toxic effects on the vascular
endothelium leading to capillary leakage and cerebral edema, even
in normotensive patients using drugs in normal therapeutic ranges
(11,22,23).
Angiogenesis inhibitors, including sunitinib, may trigger RPLS
through the induction of endothelial dysfunction and/or
hypertension (9,12–14).
In our patient, there was pre-existing hypertension and mild renal
dysfunction. Although his blood pressure was well controlled during
the first cycles of sunitinib, an increase was observed when the
patient was admitted with RPLS. This increased blood pressure
rapidly normalized after the initiation of anti-epileptic treatment
and the discontinuation of sunitinib. Notably, radiological
alterations compatible with RPLS persisted during second-line
therapy with everolimus. We believe that everolimus may have
contributed to this lengthy (>42 days) persistence of
radiological abnormalities, as these rapidly resolved when
everolimus was discontinued. Additionally, other authors have
demonstrated that radiological signs of RPLS normally improve with
a median of 20 days in 88% of patients, with complete or
near-complete resolution in 70% of patients (17). RPLS has not been reported in
association with everolimus or other mTOR inhibitors. As mTOR
inhibitors are believed to exert antitumor effects by direct
inhibition of tumor cell growth and proliferation, as well as the
inhibition of angiogenesis (via inhibition of tumor cell VEGF
production and VEGF-induced proliferation of endothelial cells),
the latter mechanism may contribute to or sustain RPLS (24).
Our case is the seventh report in the literature of
RPLS associated with sunitinib, and the first to additionally
suggest that everolimus sustains and therefore potentially
contributes to the occurrence of RPLS. Early recognition of RPLS is
critical as neurological damage may be irreversible if RPLS is not
treated immediately by adequate blood pressure regulation and the
discontinuation of any causative drugs, as permanent neurological
damage may result from cerebral infarction or hemorrhages (25,26).
References
|
1
|
Parkin DM, Bray F, Ferlay J, et al:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Veldt AA, Haanen JB, van den
Eertwegh AJ, et al: Targeted therapy for renal cell cancer: current
perspectives. Discov Med. 10:394–405. 2010.PubMed/NCBI
|
|
3
|
Linehan WM, Zbar B, Bates SE, et al:
Cancer of the kidney and ureter. Cancer: Principles and Practice of
Oncology. 6th edition. DeVita VT Jr, Hellman S and Rosenberg SA:
Lippincott Williams & Wilkins; Philadelphia: pp. 1362–1396.
2001
|
|
4
|
Powles T, Chowdury S, Jones R, et al:
Sunitinib and other targeted therapies for renal cell carcinoma. Br
J Cancer. 104:741–745. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Escudier B, Oudard S, et al:
Efficacy of everolimus in advanced renal cell carcinoma: a
double-blind, randomised, placebo-controlled phase III trial.
Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motzer RJ, Escudier B, Oudard S, et al:
Phase 3 trial of everolimus for metastatic renal cell carcinoma.
Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verheul HM and Pinedo HM: Possible
molecular mechanisms involved in the toxicity of angiogenesis
inhibition. Nat Rev Cancer. 7:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Padhy BM, Shanmugam SP, Gupta YK, et al:
Reversible posterior leucoencephalopathy syndrome in an elderly
male on sunitinib therapy. Br J Clin Pharmacol. 71:777–779. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choueiri TK, Plantade A, Elson P, et al:
Efficacy of sunitinib and sorafenib in metastatic papillary and
chromophobe renal cell carcinoma. J Clin Oncol. 26:127–131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinchey J, Chaves C, Appignani B, et al: A
reversible posterior leukoencephalopathy syndrome. N Engl J Med.
334:494–500. 1996. View Article : Google Scholar
|
|
12
|
Martín G, Bellido L and Cruz JJ:
Reversible posterior leukoencephalopathy syndrome induced by
sunitinib. J Clin Oncol. 25:35592007.
|
|
13
|
Medioni J, Cojocarasu O, Banu E, et al:
Reversible encephalopathy syndrome secondary to sunitinib for
metastatic renal cell carcinoma patient. Targ Oncol. 2:193–195.
2007. View Article : Google Scholar
|
|
14
|
Cumurciuc R, Martinez-Almoyna L, Henry C,
et al: Posterior reversible encephalopathy syndrome during
sunitinib therapy. Rev Neurol (Paris). 164:605–607. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen A and Agarwal N: Reversible posterior
leucoencephalo-pathy syndrome associated with sunitinib. Intern Med
J. 39:341–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kapiteijn E, Brand A, Kroep J, et al:
Sunitinib induced hypertension, thrombotic microangiopathy and
reversible posterior leukencephalopathy syndrome. Ann Oncol.
18:1745–1747. 2007. View Article : Google Scholar
|
|
17
|
Fugate JE, Claassen DO, Cloft HJ, et al:
Posterior reversible encephalopathy syndrome: associated clinical
and radiologic findings. Mayo Clin Proc. 85:427–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Govindarajan R, Adusumilli J, Baxter DL,
et al: Reversible posterior leukencephalopathy syndrome induced by
RAF kinase inhibitor BAY 43–9006. J Clin Oncol.
24:e482006.PubMed/NCBI
|
|
19
|
Glusker P, Recht L and Lane B: Reversible
posterior leukoencephalopathy syndrome and bevacizumab. N Engl J
Med. 354:980–981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozcan C, Wong SJ and Hari P: Reversible
posterior leukencephalopathy syndrome and bevacizumab. N Engl J
Med. 354:981–982. 2006.
|
|
21
|
Paulson OB, Strandgaard S and Edvinsson L:
Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 2:161–192.
1990.
|
|
22
|
Covarrubias DJ, Luetmer PH and Campeau NG:
Posterior reversible encephalopathy syndrome: prognostic utility of
quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol.
23:1038–1048. 2002.
|
|
23
|
Ito Y, Arahata Y, Goto Y, et al: Cisplatin
neurotoxicity presenting as reversible posterior
leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 19:415–417.
1998.PubMed/NCBI
|
|
24
|
Agarwala SS and Case S: Everolimus
(RAD001) in the treatment of advanced renal cell carcinoma: a
review. Oncologist. 15:236–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwartz RB: A reversible posterior
leukencephalopathy syndrome. N Engl J Med. 334:17431996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weingarten K, Barbut D, Fillipi C, et al:
Acute hypertensive encephalopathy: findings on spin-echo and
gradient-echo MR-imaging. Am J Roentgenol. 162:665–670. 1994.
View Article : Google Scholar : PubMed/NCBI
|