Introduction
Nasopharyngeal carcinoma (NPC) has the highest
incidence in Southern China and is one of the most common types of
cancer in Southeast Asia (1,2). The
possible etiological factors identified for NPC are genetic
susceptibility, Epstein-Barr virus (EBV) infection and
environmental risk factors. Although plasma EBV DNA quantification
has been recommended as an independent biomarker for the prediction
of survival (3,4), currently the prediction of prognosis
for patients with NPC mainly depends on clinical staging. However,
NPC patients with the same clinical stage often present different
clinical outcomes, suggesting that the clinical staging is
insufficient for accurate prediction of the prognosis of NPC.
NPC is more radiosensitive than other malignant
tumors arising in the head and neck, making radiotherapy an
important treatment modality for non-disseminated NPC. Although NPC
is usually curable by radiotherapy, there are incidents of local
failure and metastases (5,6). According to the clinical and
biological behavior of NPC patients following radiotherapy, we
subdivided NPC into four types: Type I (no primary and regional
recurrence and no distant metastasis), type II (primary or regional
recurrence and no distant metastasis), type III (no primary and
regional recurrence, and distant metastasis) and type IV (primary
or regional recurrence, and distant metastasis) (7). The identification of prognostic
factors that are more accurately correlated with treatment outcome
would help in determining which NPC patients may benefit from a
higher radiation dose or should undergo combined treatment with
more aggressive chemotherapy. A number of candidate biological
markers, such as p53, EGFR and Bcl-2 genes, have been reported.
However, none of these markers have been established as a marker in
decision-making for the treatment of NPC. Therefore, establishment
of a biological marker that may determine prognosis and response to
a particular treatment is essential in improving prognosis and
developing individualized strategies, thus enhancing treatment
outcome.
Aurora-A kinase (Aur-A), a member of the
serine/threonine kinase family, is required for centrosome
maturation and separation, mitotic commitment, chromosome alignment
and possibly cytokinesis (8,9). Aur-A
is located on chromosome 20q13.2, which is frequently amplified in
epithelial cancers (10,11), suggesting the possible link of its
dysregulation to carcinogenesis. Aur-A has been indicated as an
oncogene since the ectopic overexpression of Aur-A in NIH3T3 and
Rat-1 has been shown to induce cell transformation that generates
tumors when implanted in nude mice (11–13).
In previous clinical studies, the Aur-A gene was found to be
amplified or upregulated in various types of cancer characterized
by chromosome instability (CIN), which is in agreement with the
previous experimental results suggesting that Aur-A abnormalities
are involved in carcinogenesis (14–18).
While a positive correlation of Aur-A abnormalities
with aggressive tumor behavior and poor prognosis has been reported
in recent studies with clinical tissues of certain cancers
(14,16,19–21), a
poor correlation has also been reported for other types of cancer,
indicating a possible dependence of the correlation on specific
types of cancer (22–25). Previous studies indicated that Aur-A
downregulation was capable of inducing cell death, reducing
migration and enhancing radiosensitivity in human NPC. The
epithelial-mesenchymal transition (EMT) is an important process
that mediates cancer invasion and metastasis, and links to
radioresistance (26,27). Additionally, Aur-A inhibition
attenuated EMT (28). Since the
correlation between Aur-A status and disease progression has not
been fully investigated in NPC, the present study was conducted to
assess the expression of Aur-A in NPC and its correlation to the
clinicopathological parameters, overall survival and disease-free
survival in 208 NPC patients.
Materials and methods
Tissue samples
This study was conducted on a total of 208
paraffin-embedded NPC samples obtained from patients who were
histologically and clinically diagnosed at the Sun Yat-Sen
University Cancer Center, China, between 1994 and 1999. Patient
consent and approval from the Institute Research Ethics Committee
was obtained prior to the use of these clinical materials for
research purposes. The clinical characteristics of the patients are
shown in Table I. There were 156
males and 52 females, with a median age of 48.1 years (range,
13–72).
 | Table IClinicopathological characteristics
of patient samples and expression of Aurora-A in NPC. |
Table I
Clinicopathological characteristics
of patient samples and expression of Aurora-A in NPC.
|
Characteristics | N (%) |
|---|
| Gender |
| Male | 156 (75.0) |
| Female | 52 (25.0) |
| Age (y) |
| ≤45 | 97 (46.6) |
| >45 | 111 (53.4) |
| Histological
classification (WHO) |
| Type II | 10 (4.8) |
| Type III | 198 (95.2) |
| Clinical stage (92
stage) |
| I | 26 (12.5) |
| II | 64 (30.8) |
| III | 53 (25.5) |
| IV | 65 (31.3) |
| T
classification |
| T1 | 93 (44.7) |
| T2 | 40 (19.2) |
| T3 | 40 (19.2) |
| T4 | 35 (16.8) |
| N
classification |
| N0 | 128 (61.5) |
| N1 | 33 (15.9) |
| N2 | 30 (14.4) |
| N3 | 17 (8.2) |
| Distant
metastasis |
| Yes | 29 (13.9) |
| No | 179 (86.1) |
| Vital status (at
follow-up) |
| Alive | 136 (65.4) |
| Mortality due to
NPC | 72 (34.6) |
| Therapy |
| Radiation therapy
alone | 162 (77.9) |
| Chemotherapy
alone | 6 (2.9) |
| Radiation therapy
+ chemotherapy | 40 (19.2) |
| Skull base
invasion |
| Yes | 42 (20.2) |
| No | 166 (79.8) |
| Relapse |
| Yes | 23 (11.1) |
| No | 185 (88.9) |
| Expression of
Aurora-A |
| Low | 76 (36.5) |
| High | 132 (63.5) |
The routine staging workup included a detailed
clinical examination of the head and neck, fiberoptic
nasopharyngoscopy, computed tomography (CT) imaging of the entire
neck from the base of the skull, chest radiography, abdominal
sonography, a complete blood count and a biochemical profile. The
disease stages of all 208 patients were classified or reclassified
according to the 1992 NPC staging system of China as mentioned in a
previous study (29). Follow-up
information was obtained from all 208 patients. In total, 162
patients were treated with radiotherapy, six patients with
chemotherapy, and 40 patients received both treatments.
Radiotherapy was administered as 2 Gy daily fractions, 5 days per
week, for a total intended dose of 70 Gy. Additional (boost)
radiotherapy of 8–12 Gy was delivered for residual tumor and
destructed skull base following a standard dose of 70 Gy. The neck
received 50–70 Gy depending on the lymph node involvement, 50 Gy
for node-negative necks and 60–70 Gy for node-positive necks. At 3
months following the completion of radiotherapy, the radiotherapy
response was evaluated by clinical examination, CT scan and biopsy
when required.
Immunohistochemical staining (IHC)
IHC staining was performed using Dako Envision
system (Dako, Carpinteria, CA, USA) according to the manufacturer’s
instructions. Briefly, paraffin sections (4 μm) were baked for 1 h
at 65°C. The sections were deparaffinized in xylenes and rehydrated
with graded ethanol to distilled water. The sections were submerged
into EDTA antigenic retrieval buffer (pH 8.0) and microwaved for
antigenic retrieval. After being treated with 0.3%
H2O2 for 15 min to block the endogenous
peroxidase, the sections were treated with normal goat serum for 30
min to reduce the non-specific binding, and then rabbit polyclonal
anti-Aur-A antibody (1:300; Upstate Biotechnology, Charlottesville,
VA, USA) was incubated with the sections overnight at 4°C. After
washing, the sections were incubated with horseradish peroxidase
(HRP) at 4°C for 30 min. Diaminobenzidine (DAB) was used for the
color reaction. For the negative controls, the antibody was
replaced with normal goat serum.
Evaluation of staining
The immunohistochemically stained tissue sections
were scored separately by two pathologists blinded to the clinical
parameters. For Aur-A assessment, the entire tissue section was
scanned to assign the scores. The staining intensity was scored as
0 (negative), 1 (weak), 2 (medium) or 3 (strong). The extent of
staining was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%)
and 4 (76–100%), according to the percentages of the positive
staining areas in relation to the whole carcinoma area, or entire
section for the normal samples. The sum of the intensity and extent
score was used as the final staining scores (0 to 7) for Aur-A.
This relatively simple, reproducible scoring method that yields
highly concordant results between independent evaluators has been
used in previous studies (30,31).
An optimal cut-off value was selected on the basis of a measure of
heterogeneity with the log-rank test with respect to overall
survival. For the purpose of statistical evaluation, tumors having
a final staining score of ≤3 were classified as tumors with a low
expression and those with a score of >3 were classified as
tumors with a high expression of Aur-A antigen.
Statistical analysis
Statistical analysis was carried out using the SPSS
10.0 statistical software package. The Mann-Whitney U test was used
to analyze the Aur-A expression and clinicopathological
characteristics. Survival curves were plotted by the Kaplan-Meier
method and compared by the log-rank test. Univariate and
multivariate regression analyses were performed with the Cox
proportional hazards regression model to analyze the independent
factors related to prognosis. P<0.05 in all cases was considered
to indicate a statistically significant difference.
Results
Clinical outcome
On completion of radiotherapy, 179 (86.1%) of the
208 patients had an initial complete clinical response. All 208
patients had follow-up records and the median follow-up time was
61.7 months (range, 0.93–143). The overall and disease-free
survival rates for all 208 patients were 65.1 and 58.7% at the end
of the follow-up date, respectively, and 42 (20.2%) patients had a
skull base invasion. During follow-up, a total of 29 (13.9%)
patients experienced distant metastasis and 23 (11.1%) patients had
a relapse.
Elevated Aur-A expression in NPC
tissues
In this study, we examined Aur-A expression in 208
paraffin-embedded NPC samples. Of the 208 NPC samples, 24 samples
contained normal, adjacent and uninvolved nasopharyngeal columnar
epithelia, and 12 samples contained uninvolved nasopharyngeal
squamous epithelia. The majority of studies reported cytoplasmic
staining of Aur-A (32,33), whereas other studies showed nuclear
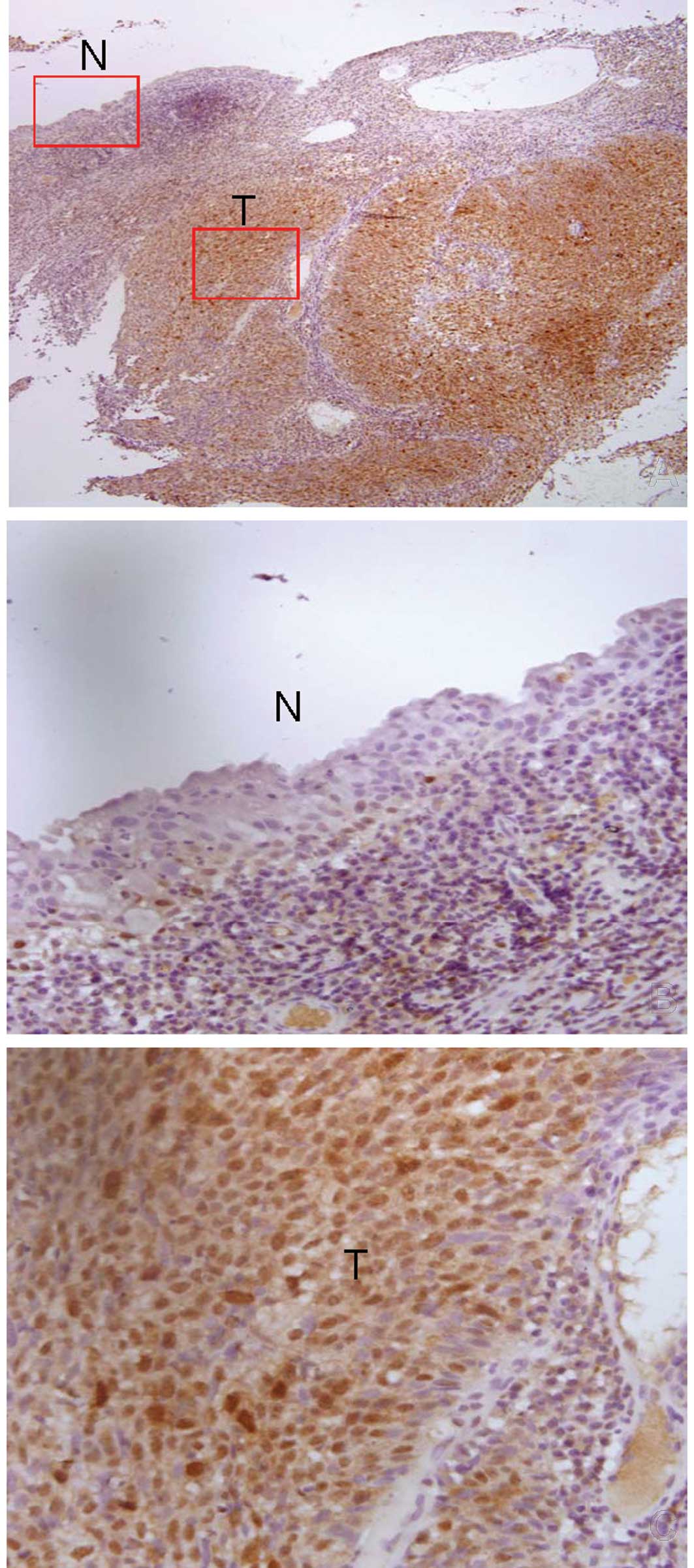
staining (34,35). Our study revealed that Aur-A
localized in the nucleus and cytoplasm in NPC tissues and localized
only in the nucleus in normal nasopharyngeal columnar and squamous
epithelia (Fig. 1). Aur-A was
highly expressed in 132 (63.5%) of the 208 NPC tissues. The Aur-A
expression level was much higher in the tumor cells compared with
the adjacent normal epithelial cells, indicating an increase of
Aur-A in the tumor cells (Fig.
1).
Aur-A expression is associated with NPC T
staging
Table II shows the
association between Aur-A expression and the clinicopathological
parameters. No significant correlation was found between Aur-A
expression and age, gender, histological classification, N
classification, distant metastasis or tumor relapse. However, Aur-A
expression was significantly correlated with T classification
(P=0.012) and skull base invasion (P=0.003). Moreover, Aur-A
expression in late-stage NPCs was significantly higher than that in
early-stage NPCs (P=0.003).
 | Table IICorrelation between the
clinicopathologic characteristics and expression of the Aurora-A
protein. |
Table II
Correlation between the
clinicopathologic characteristics and expression of the Aurora-A
protein.
| Aurora-A (%) | |
|---|
|
| |
|---|
|
Characteristics | Low expression | High
expression | P-value |
|---|
| Gender |
| Male | 57 (36.5) | 99 (63.5) | 1.000 |
| Female | 19 (36.5) | 33 (63.5) | |
| Age (y) |
| ≤45 | 33 (34.0) | 64 (66.0) | 0.802 |
| >45 | 43 (38.7) | 68 (61.3) | |
| Histological
classification |
| Type II | 6 (60.0) | 4 (40.0) | 0.115 |
| Type III | 70 (35.4) | 128 (64.6) | |
| Clinical stage |
| I–II | 43 (47.8) | 47 (52.2) | 0.003 |
| III–IV | 33 (28.0) | 85 (72.0) | |
| T
classification |
| T1–T2 | 57 (42.9) | 76 (57.1) | 0.012 |
| T3–T4 | 19 (25.3) | 56 (74.7) | |
| N
classification |
| N0 | 49 (38.3) | 79 (61.7) | 0.510 |
| N1–N3 | 27 (33.8) | 53 (66.2) | |
| Distant
metastasis |
| Yes | 7 (24.1) | 22 (75.9) | 0.136 |
| No | 69 (38.5) | 110 (61.5) | |
| Skull base
invasion |
| Yes | 7 (16.7) | 35 (83.3) | 0.003 |
| No | 69 (41.6) | 97 (58.4) | |
| Relapse |
| Yes | 5 (21.7) | 18 (78.3) | 0.119 |
| No | 71 (38.4) | 114 (61.6) | |
Inverse correlation of Aur-A
overexpression with patient survival
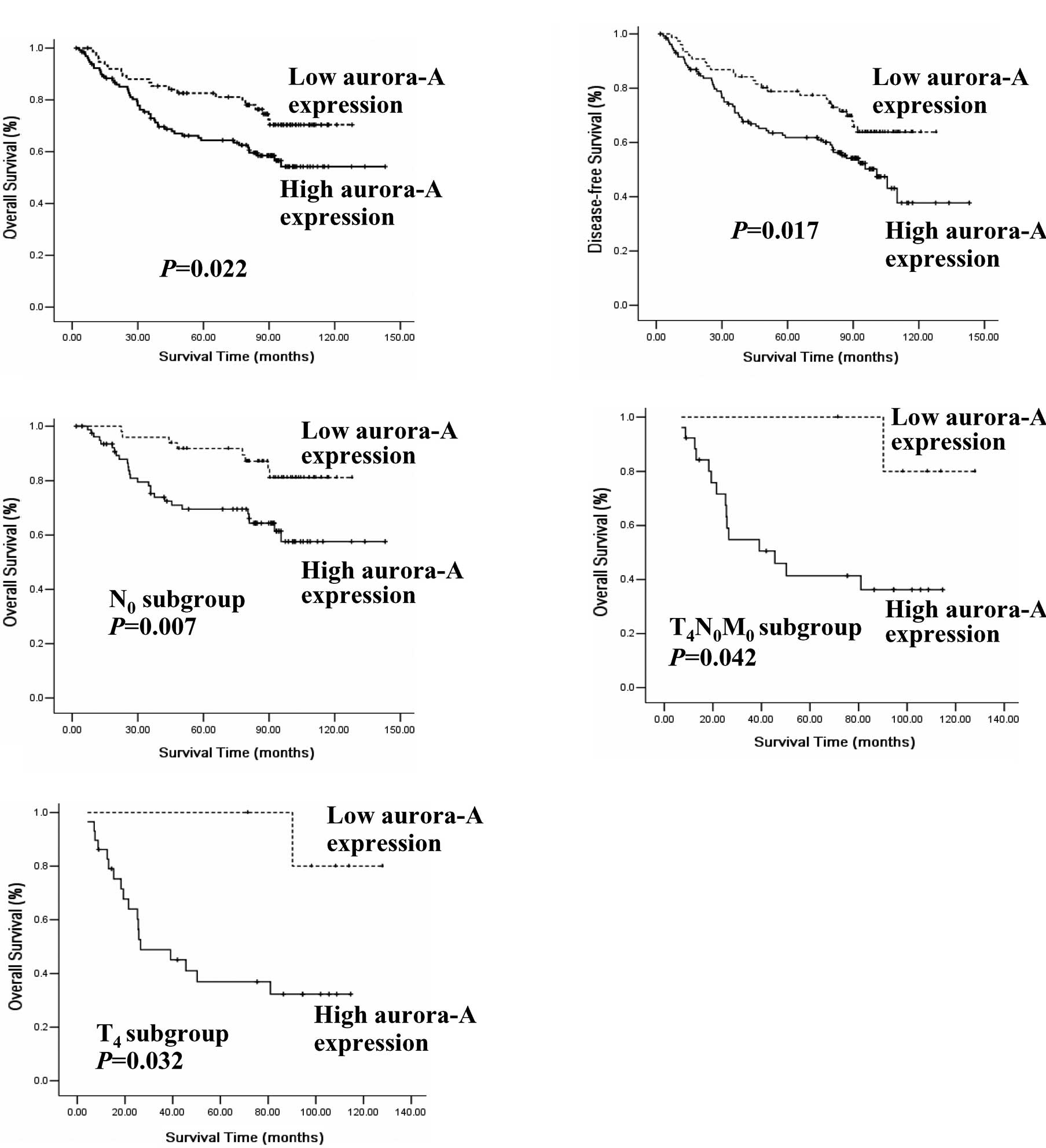
In the entire cohort, the overall survival rate of
the patients with a high Aur-A expression was significantly lower
when compared with that of the low Aur-A expression group (P=0.022,
log-rank test; Fig. 2A). The
disease-free survival rate of the patients with a high Aur-A
expression was also significantly lower (P=0.017, log-rank test;
Fig. 2B). The Aur-A expression
level, N classification, T classification, distance metastasis,
clinical stage (92 stage) and skull base invasion were also found
to be significantly correlated with survival in the Kaplan-Meier
analysis and log-rank test (for N classification, P=0.002; for T
classification, distance metastasis, clinical stage and skull base
invasion, P<0.001). Results of the univariate analysis showed
that histological classification, T classification, clinical stage
and Aur-A expression were statistically significant prognostic
factors. Results of the multivariate analysis of variables in NPC
patients including age, histological classification, T
classification, clinical stage and Aur-A expression, demonstrated
that Aur-A expression was an independent prognostic factor
(Table III).
 | Table IIIUnivariate and multivariate analyses
of various prognostic variables in patients with NPC by Cox
regression analysis. |
Table III
Univariate and multivariate analyses
of various prognostic variables in patients with NPC by Cox
regression analysis.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| P-value | HR (95 CI) | P-value | HR (95 CI) |
|---|
| Age |
| >45 vs.
≤45 | 0.085 | 1.018
(0.998–1.038) | 0.161 | 1.244
(0.703–2.201) |
| Histological
classification |
| Type II vs.
III | 0.031 | 2.516
(1.089–5.815) | 0.004 | 3.694
(1.532–8.906) |
| T
classification |
| T1–2 vs. T3–4 | 0.000 | 2.310
(1.852–5.400) | 0.453 | 1.244
(0.703–2.201) |
| Clinical stage |
| I-II vs.
III-IV | 0.000 | 3.162
(1.453–3.672) | 0.009 | 2.448
(1.254–4.777) |
| Expression of
Aurora-A |
| Low vs. high | 0.024 | 1.815
(1.083–3.043) | 0.046 | 1.746
(1.011–3.014) |
Lack of correlation of Aur-A with
metastasis
When NPC patients were classified into N3
(supraclavicular lymph node, diameter >7 cm or fixed or skin
infiltration) or M1 (presence of distant metastasis),
the tumors were considered to have a high metastatic potential. In
N3 or M1 patients, Aur-A expression had no
correlation with clinical outcome (n=40; log-rank, P=0.85) and
further analysis demonstrated that Aur-A expression had no
correlation with lymphatic metastasis and distant metastasis.
Close correlation of Aur-A expression
with T4 subgroup NPC patients
The patients were divided into different subgroups
according to N classification. Notably, in the N0 (no
enlarged lymph node) subgroup, we found that the patients with a
high Aur-A expression had a significantly shorter survival time
than those with a low Aur-A expression (n=128; log-rank, P=0.007;
Fig. 2C). However, in the
N1–N3 subgroups, there were no statistically
significant differences between patients with low or high levels of
Aur-A expression (n=80; log-rank, P=0.846). The N0
subgroup was divided according to T classification and distant
metastasis, and we found that the overall survival rate of patients
with a high Aur-A expression was lower than that of those with a
low Aur-A expression in the T4N0M0
subgroup (n=30; P=0.042; Fig. 2D).
A trend towards shorter overall survival time of patients with a
high Aur-A expression was revealed in the
T1–2N0M0 subgroup, but there was
no statistically significant difference (n=61; log-rank,
P=0.176).
Aur-A was capable of predicting the clinical outcome
of patients in the T4N0M0
subgroup. Thus, we aimed to verify the correlation between Aur-A
expression and the clinical outcome of T4 patients. We
divided all cases into T1–2, T3 and
T4 subgroups. As expected, in the T4
subgroups, a high Aur-A expression had a poor clinical outcome
(n=35; log-rank, P=0.032; Fig. 2E),
whereas Aur-A expression had no correlation to clinical outcome in
either T1–2 or T3 subgroups (P=0.415 and
P=0.498). Thus, regardless of N or M status, a high Aur-A
expression indicated poor prognosis in T4 patients.
Correlation of Aur-A expression with
radiosensitivity
Radiotherapy is the major treatment modality for
NPC. According to our radiotherapy-related definitions for NPC (NPC
patients received five-year follow-up after radical radiotherapy
alone), no primary and regional recurrence was defined as
radiosensitive, and primary or regional recurrence was defined as
radioresistant (7). The
disease-free survival (no recurrence and no metastasis) rate of the
patients with a high Aur-A expression was significantly lower than
a low Aur-A expression (P=0.017, Fig.
2B). Additionally, investigators found that Aur-A
downregulation enhanced radiosensitivity in human NPC in
vitro (36). Taken together, it
indicated that a high Aur-A expression is capable of predicting
radioresistance.
NPC patients with local relapse accompanied by skull
base invasion have been reported to be less sensitive to radiation
therapy and have a poorer clinical outcome than other patients
(37). In this study, there were 7
recurrent NPC patients with skull base invasion. All 7 of these
patients exhibited a high expression of Aur-A and 5 of the 7
patients (71.4%) succumbed to NPC, indicating a possible
correlation of a high Aur-A expression with radiosensitivity.
Previous studies have shown that primary tumor
volume had a close correlation with survival rates and the
treatment outcomes of patients with NPC (3,18,38).
Larger tumor volume may require more aggressive treatment,
suggesting that T4 patients were not sensitive to
radiation therapy. In the present study, we found that Aur-A
expression served as an indicator for clinical outcome in patients
of the T4 subgroup. Therefore, radiotherapy combined
with an Aur-A inhibitor may be of benefit for T4
patients.
Discussion
In this study, we have shown that Aur-A is
overexpressed in NPCs compared with their normal adjacent
epithelia. Our results showed that an increased expression of Aur-A
was significantly associated with tumor aggressive variables,
including T classification, skull base invasion and clinical stage.
These findings are consistent with a previous report that Aur-A is
overexpressed in laryngeal squamous cell carcinoma (LSCC) and
associated with advanced tumor stage (16). Additionally, a significant
correlation was found between a high Aur-A expression with
shortened overall and disease-free survival time in NPC patients,
indicating that Aur-A may be an independent prognostic factor.
Consistent with our findings, certain reports have also suggested
that Aur-A is a potential prognostic biomarker in breast cancer
(39), bladder cancer (40), hepatocellular carcinoma (19), medulloblastoma (41), esophageal squamous cell carcinoma
(35) and head and neck squamous
cell carcinoma (42). However,
there is controversy regarding the use of Aur-A expression as a
progressive marker in glioma (23),
pancreatic carcinoma (22), ovarian
cancer (24) or as a prognostic
marker in breast cancer (25). We
speculated that the differences in the reported clinical studies
may be attributed to the variability in population size, tumor
characteristics, antibodies and IHC staining protocol. Our findings
were obtained by analysis of a relatively large number of cases in
a single institution, and thus, should be of considerable
interest.
The precise mechanism by which the upregulation of
Aur-A affects the process of carcinogenesis and tumor progression
has yet to be clearly elucidated. A number of studies have reported
that deregulation of the Aur-A function impairs genomic integrity
and thus contributes to tumorigenesis by its association or
interaction with its effectors or activators such as p53, TPX2,
BRCA1, TACC and RASSF1 (43,44).
Aur-A may phosphorylate p53 at Ser215 or Ser315 and result in
inactivation of the p53 transcriptional activity or facilitation of
MDM2-mediated degradation (45,46).
Aur-A also induces telomerase activity by upregulation of c-Myc
(47). Phosphorylated Aur-A may be
correlated with tumor cell proliferation, inhibition of apoptosis
and resistance to chemotherapeutic drugs (48). Disturbance of these factors by
abnormal Aur-A expression or activity may affect tumorigenesis and
tumor progression. Notably, results of previous studies revealed
that a high Aur-A expression is associated with radioresistance and
EMT in human NPC (28,36).
A number of useful biological markers have already
been identified to predict prognosis and aid in clinical
decision-making in NPC in the following regards: high Met protein
and syndecan-1 expression levels correlate with poorer survival in
late-stage NPC (49,50); cyclin D1 predicts NPC recurrence and
tumor response to radiation therapy (51); quantification of plasma EBV DNA
correlates with the response to treatment and the likelihood of
relapse and survival (52); and
other biomarkers such as Bmi-1, CENP-H and cathepsin D could
predict the poor clinical outcome of NPC patients (53–55).
Although these biological markers are likely to improve the
validity of molecular classification in the future, none are
capable of predicting the T4 subgroup and no small
molecule inhibitor has been developed thus far.
The present data have demonstrated a statistically
significant correlation between Aur-A and T classification of NPC.
Particularly, in the T4 subgroup, and even in the
T4N0M0 subgroup, patients with a
high Aur-A expression had a poorer prognosis than those with a low
Aur-A expression. Thus, we speculated that T4 subgroup
patients would benefit from the chemical inhibition of Aurora
kinase to improve treatment outcome. Recently, Aurora kinase
inhibitors have been developed and VX-680 has shown promising
results in in vivo studies (44). The potential of sensitizing NPC
cells to radiotherapy by inhibiting Aur-A activity provided the
feasibility of combinational treatment for the T4 NPC
patient subgroup.
The correlation analysis in the present study does
not appear to indicate a significant correlation of Aur-A with
lymphatic or distant metastasis, in contrast to a previous report
on head and neck squamous cell carcinoma (HNSCC) showing that the
upregulation of Aur-A was significantly associated with tumor
distant metastasis (42).
Furthermore, the present study has shown that in N3 or
M1 subgroup patients, Aur-A expression does not appear
to have a correlation with clinical outcome, indicating that Aur-A
activity is not linked to metastasis and that Aur-A may not be
involved in the metastatic process, at least in NPC. The difference
of Aur-A expression in NPC and HNSCC may be attributed to the
unique features of NPC. NPC is distinctive in head and neck
carcinoma for its marked geographical variation shown in incidence,
its close association with EBV, its highly metastatic nature and
its unique clinical development features (56,57).
Taken together, results of the present study have
demonstrated that the level of Aur-A expression was highly elevated
in NPC, inversely correlated with survival and directly correlated
with the malignant status of NPC. The present findings suggests
that Aur-A is a biological marker that may be used to identify a
subgroup of patients with poor prognosis for new treatment
approaches. An Aurora kinase inhibitor that sensitizes NPC cells to
radiotherapy would offer an opportunity for future target-guided
combinational therapy.
Acknowledgements
We thank Professor Quentin Liu for his valuable
comments and extensive editing of the manuscript. This study was
supported by grants from the National High Technology Research and
Development Program of China (No. 2006AA02Z4B4) and the National
Natural Science Foundation of China (No. 30770641 and
31170805).
References
|
1
|
Licitra L, Bernier J, Cvitkovic E, et al:
Cancer of the nasopharynx. Crit Rev Oncol Hematol. 45:199–213.
2003. View Article : Google Scholar
|
|
2
|
Titcomb CP Jr: High incidence of
nasopharyngeal carcinoma in Asia. J Insur Med. 33:235–238.
2001.PubMed/NCBI
|
|
3
|
Chen MK, Chen TH, Liu JP, Chang CC and
Chie WC: Better prediction of prognosis for patients with
nasopharyngeal carcinoma using primary tumor volume. Cancer.
100:2160–2166. 2004.PubMed/NCBI
|
|
4
|
Leung SF, Zee B, Ma BB, et al: Plasma
Epstein-Barr viral deoxyribonucleic acid quantitation complements
tumor-node-metastasis staging prognostication in nasopharyngeal
carcinoma. J Clin Oncol. 24:5414–5418. 2006. View Article : Google Scholar
|
|
5
|
Sham JS and Choy D: Prognostic factors of
nasopharyngeal carcinoma: a review of 759 patients. Br J Radiol.
63:51–58. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270.
1992.PubMed/NCBI
|
|
7
|
Li ZQ, Xia YF, Liu Q, et al:
Radiotherapy-related typing in 842 patients in canton with
nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
66:1011–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bolanos-Garcia VM: Aurora kinases. Int J
Biochem Cell Biol. 37:1572–1577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andrews PD, Knatko E, Moore WJ and Swedlow
JR: Mitotic mechanics: the auroras come into view. Curr Opin Cell
Biol. 15:672–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sen S, Zhou H and White RA: A putative
serine/threonine kinase encoding gene BTAK on chromosome 20q13 is
amplified and overexpressed in human breast cancer cell lines.
Oncogene. 14:2195–2200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bischoff JR, Anderson L, Zhu Y, et al: A
homologue of Drosophila aurora kinase is oncogenic and
amplified in human colorectal cancers. EMBO J. 17:3052–3065.
1998.PubMed/NCBI
|
|
12
|
Zhou H, Kuang J, Zhong L, et al: Tumour
amplified kinase STK15/BTAK induces centrosome amplification,
aneuploidy and transformation. Nat Genet. 20:189–193. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giet R, Petretti C and Prigent C: Aurora
kinases, aneuploidy and cancer, a coincidence or a real link?
Trends Cell Biol. 15:241–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogawa E, Takenaka K, Katakura H, et al:
Perimembrane Aurora-A expression is a significant prognostic factor
in correlation with proliferative activity in non-small-cell lung
cancer (NSCLC). Ann Surg Oncol. 15:547–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lentini L, Amato A, Schillaci T and Di
Leonardo A: Simultaneous Aurora-A/STK15 overexpression and
centrosome amplification induce chromosomal instability in tumour
cells with a MIN phenotype. BMC Cancer. 7:2122007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guan Z, Wang XR, Zhu XF, et al: Aurora-A,
a negative prognostic marker, increases migration and decreases
radiosensitivity in cancer cells. Cancer Res. 67:10436–10444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landen CN Jr, Lin YG, Immaneni A, et al:
Overexpression of the centrosomal protein Aurora-A kinase is
associated with poor prognosis in epithelial ovarian cancer
patients. Clin Cancer Res. 13:4098–4104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CC, Ho HC, Lee MS, et al: Primary
tumor volume of nasopharyngeal carcinoma: significance for
survival. Auris Nasus Larynx. 35:376–380. 2008.PubMed/NCBI
|
|
19
|
Jeng YM, Peng SY, Lin CY and Hsu HC:
Overexpression and amplification of Aurora-A in hepatocellular
carcinoma. Clin Cancer Res. 10:2065–2071. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia LP, Zhou FF, Yang MT and Liu Q: Roles
of Aurora-A in tumorigenesis and prognosis of breast cancer. Ai
Zheng. 28:668–672. 2009.PubMed/NCBI
|
|
21
|
Zou LJ, Li GQ, Gong LL, et al: Expression
of aurora-A kinase in human lung cancer cell lines PG, A549, and
NCI-H460. Ai Zheng. 24:792–795. 2005.PubMed/NCBI
|
|
22
|
Li D, Zhu J, Firozi PF, et al:
Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human
pancreatic cancer. Clin Cancer Res. 9:991–997. 2003.PubMed/NCBI
|
|
23
|
Klein A, Reichardt W, Jung V, Zang KD,
Meese E and Urbschat S: Overexpression and amplification of STK15
in human gliomas. Int J Oncol. 25:1789–1794. 2004.PubMed/NCBI
|
|
24
|
Gritsko TM, Coppola D, Paciga JE, et al:
Activation and overexpression of centrosome kinase BTAK/Aurora-A in
human ovarian cancer. Clin Cancer Res. 9:1420–1426. 2003.PubMed/NCBI
|
|
25
|
Royce ME, Xia W, Sahin AA, et al:
STK15/Aurora-A expression in primary breast tumors is correlated
with nuclear grade but not with prognosis. Cancer. 100:12–19. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurrey NK, Jalgaonkar SP, Joglekar AV, et
al: Snail and slug mediate radioresistance and chemoresistance by
antagonizing p53-mediated apoptosis and acquiring a stem-like
phenotype in ovarian cancer cells. Stem Cells. 27:2059–2068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan XB, Long ZJ, Yan M, et al: Inhibition
of Aurora-A suppresses epithelial-mesenchymal transition and
invasion by downregulating MAPK in nasopharyngeal carcinoma cells.
Carcinogenesis. 29:1930–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong MH, Mai HQ, Min HQ, Ma J, Zhang EP
and Cui NJ: A comparison of the Chinese 1992 and fifth-edition
International Union Against Cancer staging systems for staging
nasopharyngeal carcinoma. Cancer. 89:242–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: a
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masunaga R, Kohno H, Dhar DK, et al:
Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.PubMed/NCBI
|
|
32
|
Tong T, Zhong Y, Kong J, et al:
Overexpression of Aurora-A contributes to malignant development of
human esophageal squamous cell carcinoma. Clin Cancer Res.
10:7304–7310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoque A, Carter J, Xia W, et al: Loss of
aurora A/STK15/BTAK overexpression correlates with transition of in
situ to invasive ductal carcinoma of the breast. Cancer Epidemiol
Biomarkers Prev. 12:1518–1522. 2003.PubMed/NCBI
|
|
34
|
Burum-Auensen E, De Angelis PM, Schjolberg
AR, Kravik KL, Aure M and Clausen OP: Subcellular localization of
the spindle proteins Aurora A, Mad2, and BUBR1 assessed by
immunohistochemistry. J Histochem Cytochem. 55:477–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka E, Hashimoto Y, Ito T, et al: The
clinical significance of Aurora-A/STK15/BTAK expression in human
esophageal squamous cell carcinoma. Clin Cancer Res. 11:1827–1834.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan XB, Fan XJ, Chen MY, et al: Inhibition
of Aurora-A results in increased cell death in 3-dimensional
culture microenvironment, reduced migration and is associated with
enhanced radiosensitivity in human nasopharyngeal carcinoma. Cancer
Biol Ther. 8:1500–1506. 2009. View Article : Google Scholar
|
|
37
|
Farias TP, Dias FL, Lima RA, et al:
Prognostic factors and outcome for nasopharyngeal carcinoma. Arch
Otolaryngol Head Neck Surg. 129:794–799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chu ST, Wu PH, Chou P and Lee CC: Primary
tumor volume of nasopharyngeal carcinoma: prognostic significance
for recurrence and survival rate. Eur Arch Otorhinolaryngol.
265(Suppl 1): S115–S120. 2008.PubMed/NCBI
|
|
39
|
Miyoshi Y, Iwao K, Egawa C and Noguchi S:
Association of centrosomal kinase STK15/BTAK mRNA expression with
chromosomal instability in human breast cancers. Int J Cancer.
92:370–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sen S, Zhou H, Zhang RD, et al:
Amplification/overexpression of a mitotic kinase gene in human
bladder cancer. J Natl Cancer Inst. 94:1320–1329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neben K, Korshunov A, Benner A, et al:
Microarray-based screening for molecular markers in medulloblastoma
revealed STK15 as independent predictor for survival. Cancer Res.
64:3103–3111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reiter R, Gais P, Jutting U, et al: Aurora
kinase A messenger RNA overexpression is correlated with tumor
progression and shortened survival in head and neck squamous cell
carcinoma. Clin Cancer Res. 12:5136–5141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marumoto T, Zhang D and Saya H: Aurora-A -
a guardian of poles. Nat Rev Cancer. 5:42–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Agnese V, Bazan V, Fiorentino FP, et al:
The role of Aurora-A inhibitors in cancer therapy. Ann Oncol.
18(Suppl 6): vi47–vi52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Q, Kaneko S, Yang L, et al: Aurora-A
abrogation of p53 DNA binding and transactivation activity by
phosphorylation of serine 215. J Biol Chem. 279:52175–52182. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Katayama H, Sasai K, Kawai H, et al:
Phosphorylation by aurora kinase A induces Mdm2-mediated
destabilization and inhibition of p53. Nat Genet. 36:55–62. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang H, Ou CC, Feldman RI, Nicosia SV,
Kruk PA and Cheng JQ: Aurora-A kinase regulates telomerase activity
through c-Myc in human ovarian and breast epithelial cells. Cancer
Res. 64:463–467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tanaka E, Hashimoto Y, Ito T, et al: The
suppression of aurora-A/STK15/BTAK expression enhances
chemosensitivity to docetaxel in human esophageal squamous cell
carcinoma. Clin Cancer Res. 13:1331–1340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qian CN, Guo X, Cao B, et al: Met protein
expression level correlates with survival in patients with
late-stage nasopharyngeal carcinoma. Cancer Res. 62:589–596.
2002.PubMed/NCBI
|
|
50
|
Chen CL and Ou DL: Expression of
syndecan-1 (CD138) in nasopharyngeal carcinoma is correlated with
advanced stage and poor prognosis. Hum Pathol. 37:1279–1285. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lai JP, Tong CL, Hong C, et al:
Association between high initial tissue levels of cyclin d1 and
recurrence of nasopharyngeal carcinoma. Laryngoscope. 112:402–408.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin JC, Wang WY, Chen KY, et al:
Quantification of plasma Epstein-Barr virus DNA in patients with
advanced nasopharyngeal carcinoma. N Engl J Med. 350:2461–2470.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Song LB, Zeng MS, Liao WT, et al: Bmi-1 is
a novel molecular marker of nasopharyngeal carcinoma progression
and immortalizes primary human nasopharyngeal epithelial cells.
Cancer Res. 66:6225–6232. 2006. View Article : Google Scholar
|
|
54
|
Cheng AL, Huang WG, Chen ZC, et al:
Identificating cathepsin D as a biomarker for differentiation and
prognosis of nasopharyngeal carcinoma by laser capture
microdissection and proteomic analysis. J Proteome Res.
7:2415–2426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liao WT, Song LB, Zhang HZ, et al:
Centromere protein H is a novel prognostic marker for
nasopharyngeal carcinoma progression and overall patient survival.
Clin Cancer Res. 13:508–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chang KP, Hsu CL, Chang YL, et al:
Complementary serum test of antibodies to Epstein-Barr virus
nuclear antigen-1 and early antigen: a possible alternative for
primary screening of nasopharyngeal carcinoma. Oral Oncol.
44:784–792. 2008. View Article : Google Scholar
|
|
57
|
Chin D, Boyle GM, Porceddu S, Theile DR,
Parsons PG and Coman WB: Head and neck cancer: past, present and
future. Expert Rev Anticancer Ther. 6:1111–1118. 2006. View Article : Google Scholar : PubMed/NCBI
|