Introduction
Post-transplant lymphoproliferative disorders
(PTLDs) are defined as lymphoid or plasmacytic proliferations that
develop as a consequence of immunosuppression in a recipient of a
solid organ, bone marrow or stem cell allograft (1) and are classified as
immunodeficiency-associated lymphoproliferative disorders (LPDs) in
the WHO classification (1). The
development of PTLD is usually associated with Epstein-Barr virus
(EBV) and the disorder is also termed EBV-associated LPD. The risk
of developing PTLD varies; patients receiving renal allografts have
the lowest frequency of PTLD (<1%), those with hepatic and
cardiac allografts have an intermediate risk (1–2%) and those with
heart-lung, lung or intestinal allografts have the highest
frequency (>5%) (2–4). Peripheral blood, stem cell and bone
marrow allograft recipients have a low risk of PTLD (~1%) and PTLD
is rare following autologous bone marrow transplantation (5–11).
In the present study, we report a case of
EBV-associated LPD that developed following autologous peripheral
blood stem cell transplantation for relapsing Hodgkin’s
lymphoma.
Materials and methods
Case report
A 51-year-old Japanese male without a noteworthy
past or family history presented with a painless tumor in the left
neck at a dermatological clinic. The dermatologist observed
swelling of the lymph nodes in his left neck and the patient was
referred to the Department of Hematology of our hospital (Shiga
University of Medical Sciences, Shiga, Japan).
A physical examination revealed several swollen
lymph nodes in the left neck of the patient, however, no palpable
superficial lymph nodes, with the exception of those in the left
neck, or hepatosplenomegaly were detected. Laboratory tests
revealed slightly elevated lactate dehydrogenase (333 U/l; range,
119–229) and elevated soluble IL-2 receptor (sIL-2R) (2,070 U/ml;
range, 124–466). In addition, the EBV infection status of the
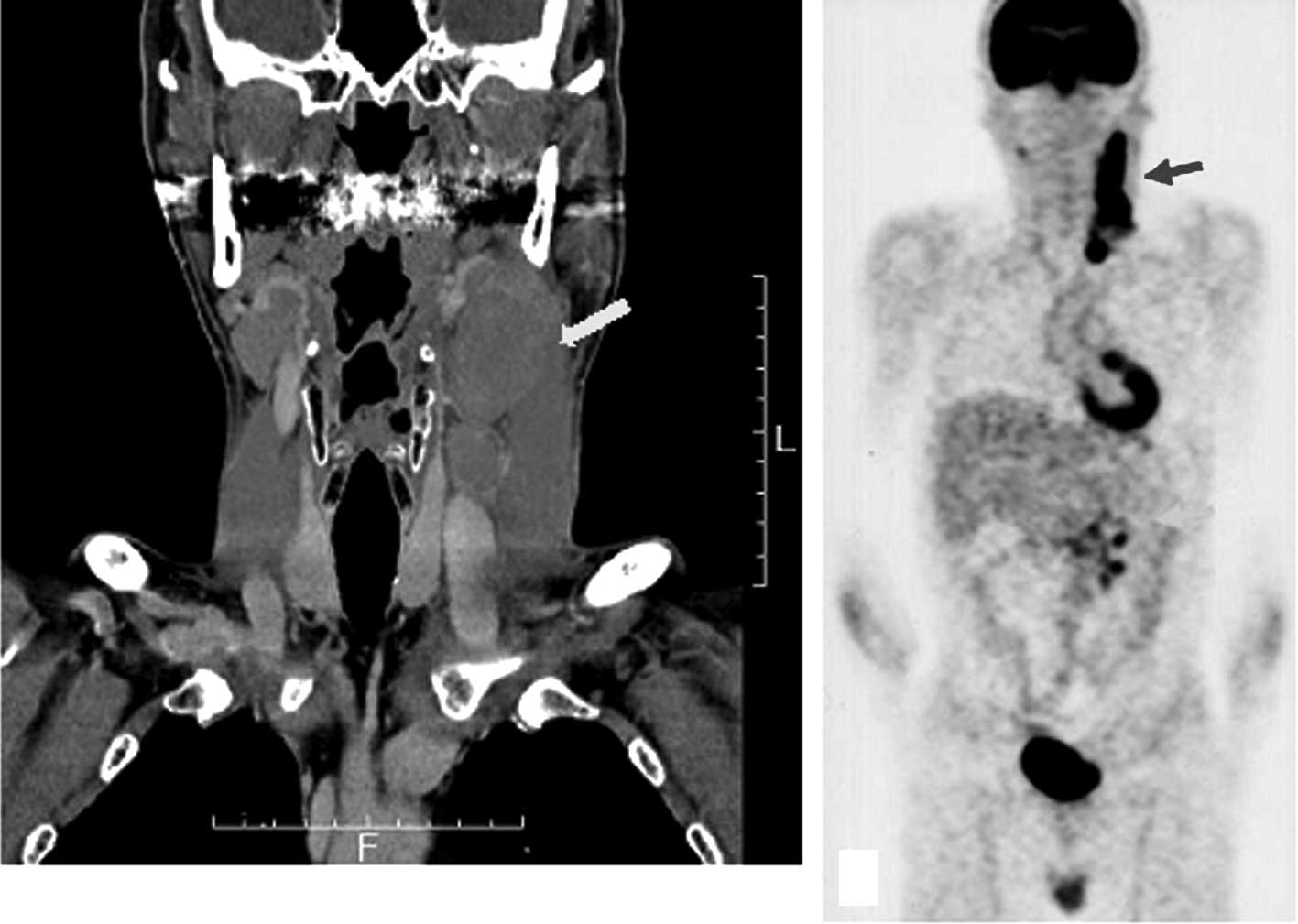
patient was prior infection. Computed tomography scan (CT) revealed
several swollen lymph nodes with a maximum diameter of 5×3 cm in
the left neck of the patient and positron emission tomography (PET)
scan revealed an accumulation in the lymph nodes of the left neck
and mesentery (Fig. 1).
Treatment
Clinically, malignant lymphoma was suspected and the
biopsy specimen of the left cervical lymph node revealed nodular
sclerosis classical Hodgkin’s lymphoma (stage IIIa). Following
therapy with ABVd (adriamycin, bleomycin, vinblastine,
dacarbazine), the size of the cervical lymph nodes was reduced and
the concentration of sIL-2R also decreased (710 U/ml).
Nevertheless, following 4 courses of ABVd therapy, PET scan
revealed swelling of the left cervical lymph nodes of the patient
and a repeated biopsy revealed relapsing Hodgkin’s lymphoma.
The patient underwent CHASE (cyclophosphamide,
etoposide, cytarabine, dexamethasone) therapy and autologous
peripheral blood stem cell transplantation was performed. Fever and
skin eruption appeared following transplantation and steroid
therapy was added. The fever went into remission, but the skin
eruption was prolonged. In the 128 days following transplantation,
CT scan revealed swelling of the lymph nodes of the bilateral
axilla as well as the inguinal and para-aortal nodes and a biopsy
of the inguinal lymph node was performed. The serum copy number of
EBV-DNA was 2.7×103 copies/ml at that time. R-CHOP
(rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin,
prednisolone) therapy was performed. Following two courses of
R-CHOP therapy, the swelling of the lymph nodes was reduced and the
serum copy number of EBV-DNA was below the detection
sensitivity.
In the 270 days following transplantation, the fever
reappeared and chest CT scan revealed ground glass opacity in the
bilateral lungs. A diagnosis of cytomegalovirus pneumonia was made,
as the serum C7-HRP was positive. Anti-cytomegalovirus therapy was
performed; however, the patient succumbed to respiratory
dysfunction on the 369th day following transplantation.
Tissue samples and staining
Formalin-fixed, paraffin- embedded tissue blocks of
the cervical and inguinal lymph nodes were cut into 3 μm sections,
deparaffinized and rehydrated. Each section was stained with
hematoxylin and eosin and then used for immunostaining and in
situ hybridization. Immunohistochemical and in situ
hybridization analyses were performed using an autostainer (XT
system Benchmark, Ventana Medical System, Tucson, AZ, USA)
according to the manufacturer’s instructions. The following primary
antibodies were used: a mouse monoclonal antibody against CD3 (PS1,
Novocastra Laboratories, Ltd., Newcastle upon Tyne, UK), a mouse
monoclonal antibody against CD8 (1A5, Novocastra), a mouse
monoclonal antibody against CD15 (C3D1, DAKO Cytomation, Glostrup,
Denmark), a mouse monoclonal antibody against CD20 (L26,
Novocastra) and a mouse monoclonal antibody against CD30 (1G12,
Novocastra). For in situ hybridization, an INFORM EBER
(EBV-encoded early RNA) probe (Ventana Medical System) was
used.
Results
Biopsy specimen of the cervical lymph
nodes
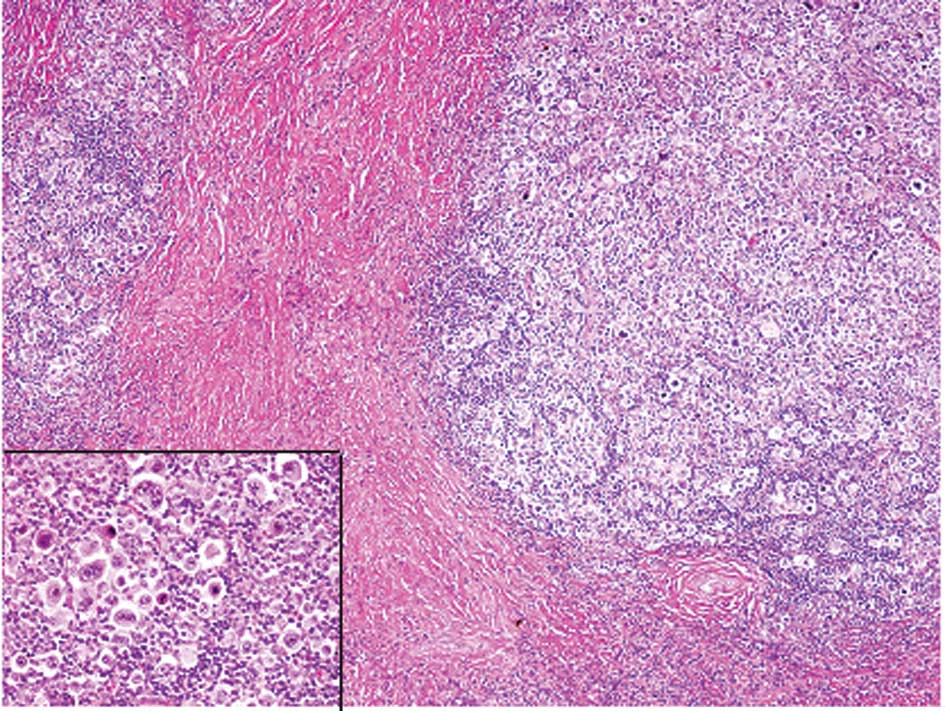
The histopathological analysis of the first biopsy
specimen revealed that normal lymph node architecture was not
preserved and that sclerotic fibrous tissue divided the lymph nodes
into nodules (Fig. 2). In the
nodules, there were atypical large lymphocytes (namely Hodgkin’s
cells) with single large nuclei, prominent nucleoli and large
amounts of slightly eosinophilic cytoplasm; lacuna cells, which had
clear cytoplasm with spider-web-like extensions to the cell
membrane, folded nuclear membranes and less conspicuous nucleoli,
were scattered and mixed with numerous small-sized lymphocytes,
histiocytes and eosinophils (Fig.
2, inset). Reed-Sternberg cells, which had two large nuclei and
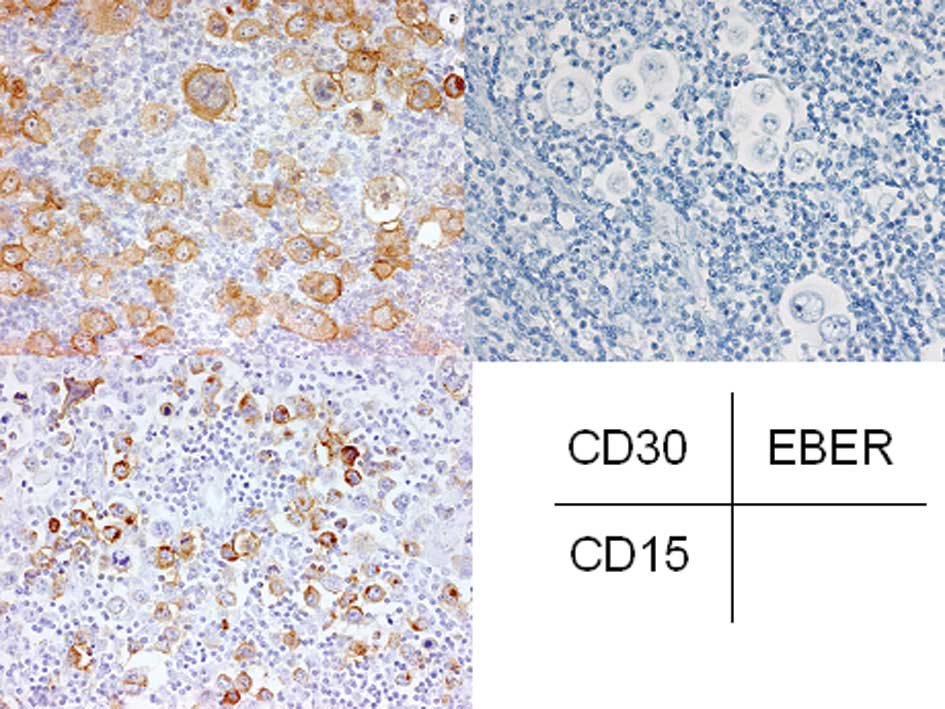
a prominent nucleolus, were also observed (Fig. 2, inset). Immunohistochemical
analyses demonstrated that these atypical large lymphocytes were
positive for CD15 and CD30 (Fig.
3), but negative for CD3 and CD20. EBER was not detected in
these atypical lymphocytes by in situ hybridization
(Fig. 3). These findings led to the
ultimate diagnosis of nodular sclerosis classical Hodgkin’s
lymphoma.
The histopathological and immunohistochemical
findings of the repeated biopsy specimen of the cervical lymph node
were the same as those of the first biopsy specimen as described
above.
Biopsy specimen of the inguinal lymph
node
The histopathological analyses revealed that normal
lymph node architecture was not preserved and that atypical large
lymphocytes with prominent nucleoli were scattered among numerous
small to medium-sized lymphocytes (Fig.
4). Neither typical lacuna cells nor Reed-Sternberg cells were
observed.
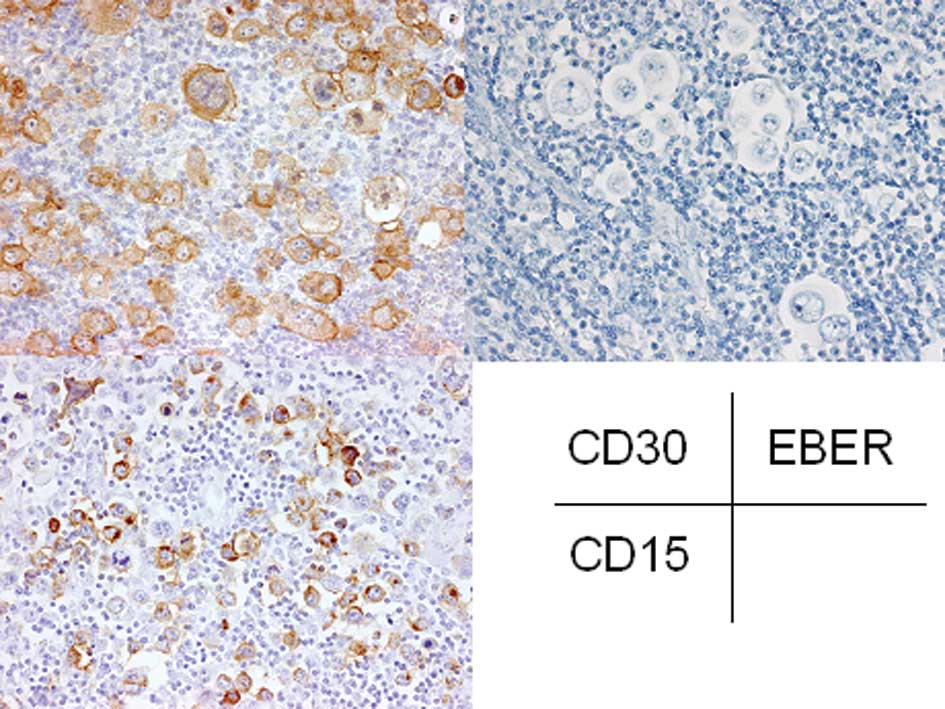
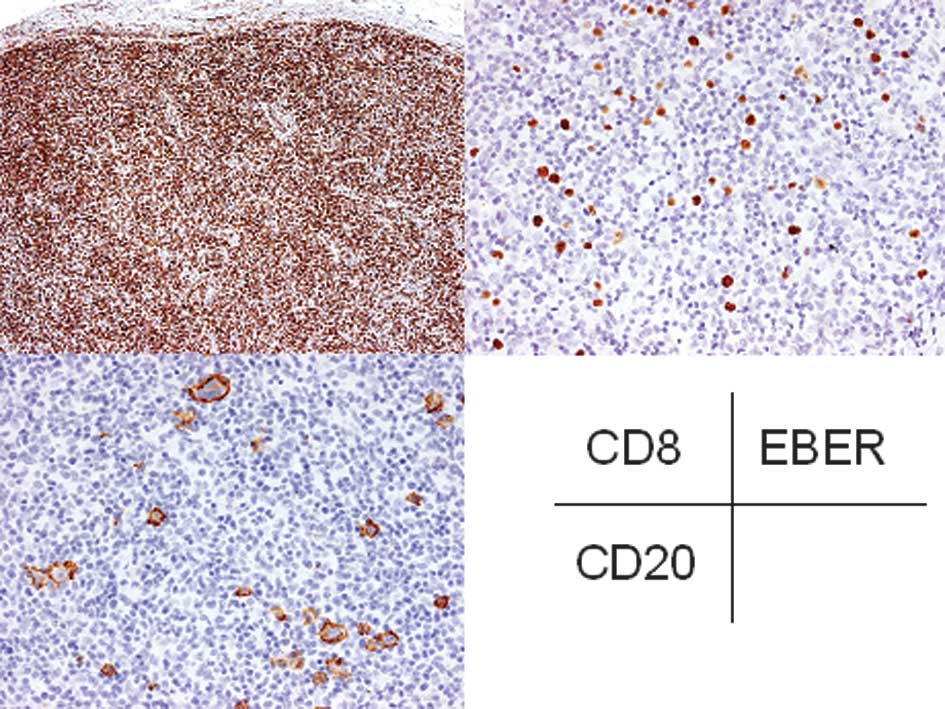
Immunohistochemically, CD20 was expressed in the
atypical large lymphocytes and CD30 was also expressed in most of
these cells (Fig. 5). However, CD15
was not expressed in these cells. The small to medium-sized
lymphocytes were positive for CD3 and CD8 (Fig. 5). EBER was detected in the atypical
large lymphocytes by in situ hybridization. Therefore, a
diagnosis of EBV-associated LPD was made.
Discussion
PTLD is classified into four categories according to
the WHO classification (1): early
lesions, polymorphic PTLD, monomorphic PTLD and classical Hodgkin’s
lymphoma-type PTLD. The histopathological and immunohistochemical
features of EBV-associated LPD of the present case corresponded to
polymorphic PTLD (1), as EBER- and
CD20-positive atypical large lymphocytes were scattered in numerous
CD8-positive T lymphocytes accompanying lymph node architectural
effacement. A differential diagnosis of relapsing Hodgkin’s
lymphoma was considered. However, CD15 was not expressed in
atypical lymphocytes and typical lacuna cells and Reed-Sternberg
cells were not observed in the present case. Therefore, the
possibility of relapsing Hodgkin’s lymphoma was excluded.
Most individuals become infected with EBV and the
virus persists within the body throughout life in resting memory B
cells. T lymphocytes control proliferating EBV-infected B cells. In
a setting of decreased or impaired T-cell immune surveillance for
EBV-infected B cells, as observed during immunosuppression in
connection with the transplantation of solid organs or bone marrow,
the unchecked replication of B lymphocytes may lead to polyclonal
B-cell hyperplasia and/or the monoclonal proliferation of B cells
(12). In the present case, the
decreased or impaired immune surveillance for EBV-infected B cells
due to chemotherapy and/or blood stem cell transplantation for
relapsing Hodgkin’s lymphoma may be correlated with the development
of EBV-associated LPD.
The development of PTLD is a rare complication in
autologous bone marrow/peripheral blood stem cell transplantation
(5–11). Nash et al reported that 2 of
56 patients (3.6%) with severe autoimmune diseases developed
EBV-associated LPD following autologous stem cell transplantation
and that the CD3+ cell count was 0/μl in these two cases
(5). In addition, Powell et
al reported that 5 of 156 patients (3.5%) with neuroblastoma
developed EBV-associated LPD following autologous peripheral blood
stem cell transplantation (6).
However, only two case reports of EBV-associated LPD which
developed following autologous bone marrow transplantation for
Hodgkin’s lymphoma have been published previously (8,9); one
case was polymorphous B-cell PTLD (8), as was the present case, and the other
was T-cell PTLD (9).
Early detection of the development of EBV-associated
LPD is a significant clinical problem. The monitoring of EBV-DNA
load in the peripheral blood is a useful method to detect the
development of EBV-associated LPD, especially in high-risk patients
(13,14). Moreover, EBV-associated LPD may
occur in the gastrointestinal tract, lung and liver, as well as in
lymph nodes (1). Therefore,
detection of EBER-positive atypical lymphocytes in the biopsy
specimen may lead to the diagnosis of EBV-associated LPD.
In conclusion, we report a case of EBV-associated
LPD that developed following autologous peripheral blood stem cell
transplantation for relapsing Hodgkin’s lymphoma. The occurrence of
EBV-associated LPD may be on the rise due to the increased number
of patients undergoing immunosuppression therapy (15). Therefore, the measurement of serum
EBV-DNA copy number and detection of EBV-infected atypical
lymphocytes by in situ hybridization are significant in
making an early accurate diagnosis and initiating the correct
treatment of EBV-associated LPD in patients undergoing
immunosuppression.
References
|
1
|
Swerdlow SH, Webber SA, Chadburn A and
Ferry JA: Post-transplant lymphoproliferative disorders. WHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
Swerdlow SH, Campo E, Harris NL, et al: IARC; Lyon: pp. 343–349.
2008
|
|
2
|
Opelz G and Döhler B: Lymphomas after
solid organ transplantation: a collaborative transplant study
report. Am J Transplant. 4:222–230. 2003. View Article : Google Scholar
|
|
3
|
Bakker NA, van Imhoff GW, Verschuuren EA,
et al: Early onset post-transplant lymphoproliferative disease is
associated with allograft localization. Clin Transplant.
19:327–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caillard S, Lelong C, Pessione F and
Moulin B: Post-transplant lymphoproliferative disorders occurring
after renal transplantation in adults: report of 230 cases from the
French Registry. Am J Transplant. 6:2735–2742. 2006. View Article : Google Scholar
|
|
5
|
Nash RA, Dansey R, Storek J, et al:
Epstein-Barr virus-associated posttransplantation
lymphoproliferative disorder after high-dose immunosuppressive
therapy and autologous CD34-seletcted hematopoietic stem cell
transplantation for severe autoimmune diseases. Biol Blood Marrow
Transplant. 9:583–591. 2003. View Article : Google Scholar
|
|
6
|
Powell JL, Bunin NJ, Callahan C, Aplenc R,
Griffin G and Grupp SA: An unexpectedly high incidence of
Epstein-Barr virus lymphoproliferative disease after
CD34+ selected autologous peripheral blood stem cell
transplant in neuroblastoma. Bone Marrow Transplant. 33:651–657.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peniket AJ, Perry AR, Williams CD, et al:
A case of EBV-associated lymphoproliferative disease following
high-dose therapy and CD34-purified autologous peripheral blood
progenitor cell transplantation. Bone Marrow Transplant.
22:307–309. 1998. View Article : Google Scholar
|
|
8
|
Hauke RJ, Greiner TC, Smir BN, et al:
Epstein-Barr virus-associated lymphoproliferative disorder after
autologous bone marrow transplantation: report of two cases. Bone
Marrow Transplant. 21:1271–1274. 1998. View Article : Google Scholar
|
|
9
|
Yufu Y, Kimura M, Kawano R, et al:
Epstein-Barr virus-associated T cell lymphoproliferative disorder
following autologous blood stem cell transplantation for relapsed
Hodgkin’s disease. Bone Marrow Transplant. 26:1339–1341. 2000.
|
|
10
|
Lones MA, Kirov I, Said JW, Shintaku IP
and Neudorf S: Post-transplant lymphoproliferative disorder after
autologous peripheral stem cell transplantation in a pediatric
patient. Bone Marrow Transplant. 26:1021–1024. 2000. View Article : Google Scholar
|
|
11
|
Heath JA, Broxson EH Jr, Dole MG, et al:
Epstein-Barr virus-associated lymphoma in a child undergoing an
autologous stem cell rescue. J Pediatr Hematol Oncol. 24:160–163.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
List AF, Greco FA and Vogler LB:
Lymphoproliferative diseases in immunocompromised hosts: the role
of Epstein-Barr virus. J Clin Oncol. 5:1673–1689. 1987.PubMed/NCBI
|
|
13
|
Shaffer DR, Rooney CM and Gottschalk S:
Immunotherapeutic options for Epstein-Barr virus-associated
lymphoproliferative disease following transplantation.
Immunotherapy. 2:663–671. 2010. View Article : Google Scholar
|
|
14
|
Meijer E and Cornelissen JJ: Epstein-Barr
virus-associated lymphoproliferative disease after allogenic
haematopoietic stem cell transplantation: molecular monitoring and
early treatment of high-risk patients. Curr Opin Hematol.
15:576–585. 2008. View Article : Google Scholar
|
|
15
|
Ohta M, Taga T, Nomura A, et al:
Epstein-Barr virus-related lymphoproliferative disorder,
cytomegalovirus reactivation, and varicella zoster virus
encephalitis during treatment of medulloblastoma. J Med Virol.
83:1582–1584. 2011. View Article : Google Scholar
|