Introduction
Curcumin is a non-nutritive yellow pigment found in
the spice turmeric, which is derived from the rhizome of the plant
Curcuma longa Linn. Curcumin lacks toxicity in humans
(1), and extensive research over
several decades has revealed that curcumin possesses anticancer,
anti-inflammatory, antioxidant, antiviral and anti-bacterial
activities (2,3). Curcumin suppressed cell proliferation
or induced apoptosis in cultured prostate cancer cells and other
types of cancer cells (4–10). Curcumin also inhibited prostate
carcinogenesis (11). Studies from
our laboratory and those of other authors have demonstrated
enhanced anticancer activities of curcumin when combined with other
anticancer agents (12–14). Findings of earlier studies showed
that curcumin exerts a wide range of anticancer effects by
modulating a diversity of signaling pathways, including nuclear
factor-κB (NF-κB) and other pathways (15–20).
Curcumin has entered clinical trials for certain types of human
cancer (21–23). However, the clinical efficacy of
curcumin is limited, which is likely to be due to its low
bioavailability (21–23). It was suggested that the β-diketone
moiety of curcumin causes instability and poor metabolic properties
(24–26). Enhanced stability was found in
curcumin analogues by deleting the β-diketone moiety of the
molecule (27). Recently, it was
demonstrated that the cyclohexanone analogues of curcumin have
enhanced stability in biological medium compared to curcumin
(28). The cyclohexanone-containing
curcumin analogue
2,6-bisp[(3-methoxy-4-hydroxyphenyl)methylene)]cyclohexanone was
found to be more potent than curcumin for inhibiting NF-κB in human
breast cancer cells in vitro (29).
In an earlier study, we synthesized a series of
cyclohexanone curcumin analogues and determined their inhibitory
effect on the activity of aldose reductase (30). In the present study, we investigated
the effects of these curcumin analogues on the growth and apoptosis
of human prostate cancer PC-3 cells. We also determined the
inhibitory effect of these analogues on the activation of NF-κB in
PC-3 cells using the luciferase reporter assay. Results of our
study demonstrated that compounds A2-A6 have
stronger effects for inhibiting growth and stimulating apoptosis in
PC-3 cells compared to curcumin. We also found that these curcumin
analogues have stronger effects than curcumin for inhibiting NF-κB
activity in PC-3 cells.
Materials and methods
Chemistry
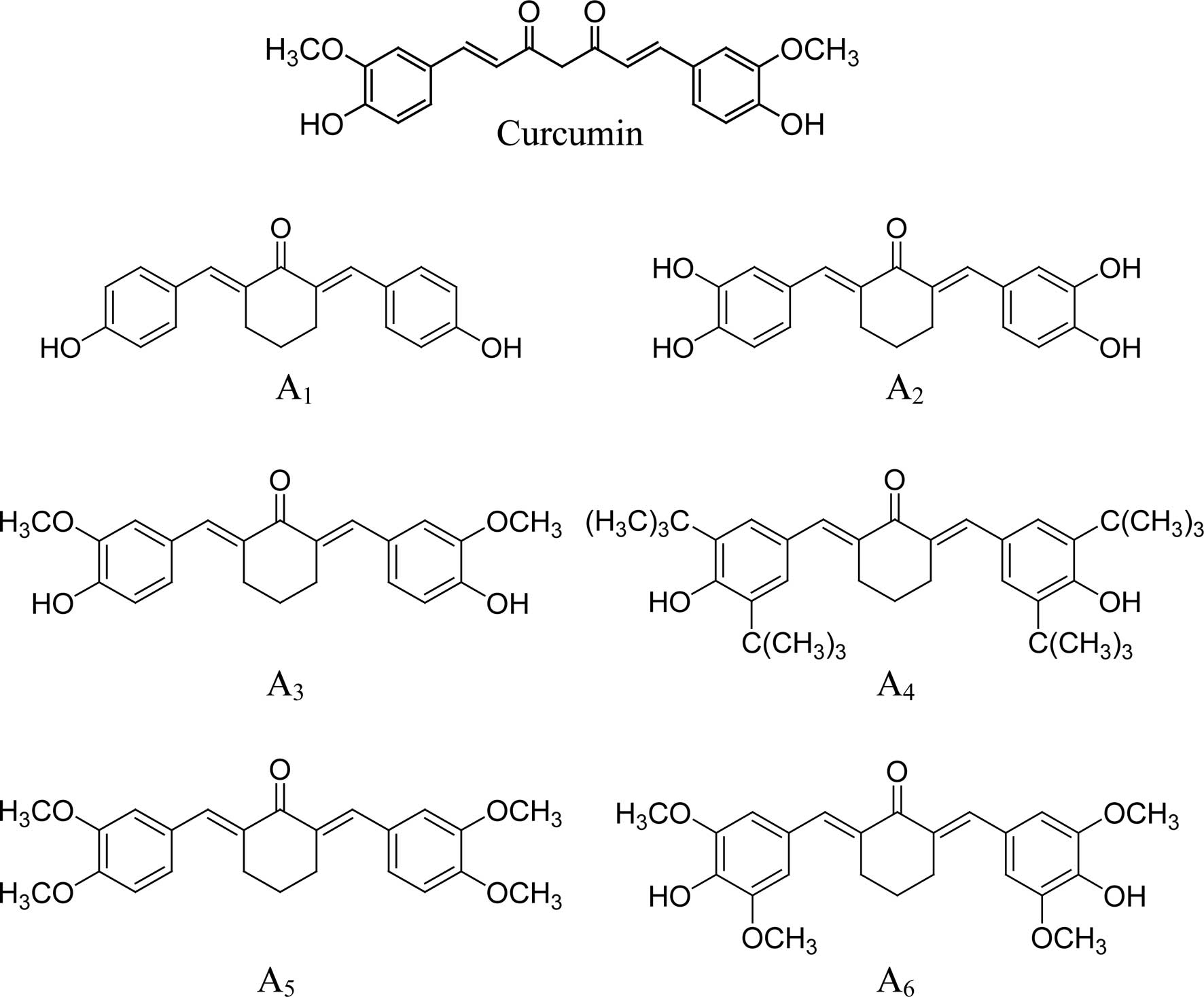
A series of cyclohexanone curcumin analogues were
synthesized by coupling the appropriate substituted benzaldehyde
with cyclohexanone as previously described (30). Characterization of the compounds,
2,6-bis(4-hydroxybenzylidene)-cyclohexanone (A1),
2,6-bis(3,4-dihydroxybenzylidene)-cyclohexanone (A2),
2,6-bis(4-hydroxy-3-methoxybenzylidene)-cyclohexanone
(A3),
2,6-bis(3,5-di-tert-butyl-4-hydroxylbenzylidene)-cyclohexanone
(A4), 2,6-bis(3,4-dimethoxybenzylidene)-cyclohexanone
(A5) and
2,6-bis(4-hydroxy-3,5-dimethoxybenzylidene)-cyclohexanone
(A6), was previously described in detail (30).
Cell culture and reagents
PC-3 cells were obtained from the American Type
Culture Collection (ATCC; Rockville, MD, USA). Curcumin was
obtained from Sigma-Aldrich (St. Louis, MO, USA). The RPMI-1640
tissue culture medium, penicillin-streptomycin, L-glutamine and
fetal bovine serum (FBS) were obtained from Gibco (Grand Island,
NY, USA). The PC-3 cells were maintained in RPMI-1640 culture
medium containing 10% FBS supplemented with penicillin (100
U/ml)-streptomycin (100 μg/ml) and L-glutamine (300 μg/ml).
Cultured cells were grown in a humidified atmosphere of 5%
CO2 at 37°C, and were passaged twice a week. Curcumin
and its analogues were dissolved in DMSO and the final
concentration of DMSO in all experiments was 0.1%.
MTT assay
PC-3 cells were seeded at a density of
0.2×105 cells/ml in medium in 96-well plates (0.2
ml/well) and incubated for 24 h. The cells were then treated with
various concentrations (0.5–10 μM) of the different curcumin
analogues for 72 h. Following treatment, 200 μl
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide (0.5
mg/ml in PBS) was added to each well of the plate and incubated for
2 h. The plate was then centrifuged at 1,000 rpm for 5 min at 4°C.
Following removal of the medium, 0.1 ml DMSO was added to each
well. The absorbance was recorded on a microplate reader at 540 nm.
The effect of different curcumin analogues on cell growth was
assessed as the percentage cell growth compared to DMSO-treated
cells.
Determination of the number of viable
cells
The number of viable cells following each treatment
was determined using the trypan blue exclusion assay (31). In brief, 80 μl of cell suspension
was mixed with 20 μl of 0.4% trypan blue solution and incubated for
2 min. The cells were then examined under a light microscope (Nikon
Optiphot, Japan). Blue cells were counted as dead cells and cells
that did not absorb dye were counted as live cells.
Assessment of apoptotic cells by
morphology and activation of caspase-3
Apoptotic cells were determined by morphological
assessment in cells stained with propidium iodide (32,33).
Cytospin slides were prepared following each experiment and cells
were fixed with acetone/methanol (1:1) at room temperature for 10
min, followed by 10 min of propidium iodide staining (1 μg/ml in
PBS), and were then analyzed using a fluorescence microscope (Nikon
Eclipse TE200, Japan). Apoptotic cells were identified by classical
morphological features, including nuclear condensation, cell
shrinkage and formation of apoptotic bodies (32,33).
Caspase-3 activation was measured using an EnzoLyte
AMC Caspase-3 Assay Fluorimetric kit (AnaSpec, Fremont, CA, USA)
according to the manufacturer's instructions (34). A total of 1×105 cells
were plated in triplicate in a flat-bottomed 96-well plate. Cells
were treated with different curcumin analogues for 72 h. Following
treatment, caspase-3 substrate was added to each well. Plates were
incubated at room temperature for 30 min. Fluorescence intensity
was measured in a Tecan Inifinite M200 plate reader (Tecan US Inc.,
Durham, NC, USA).
NF-κB-dependent reporter gene expression
assay
NF-κB transcriptional activity was measured using
the NF-κB-luciferase reporter gene expression assay (35). An NF-κB luciferase construct was
stably transfected into PC-3 cells and a single stable clone, PC-3
C4 (35), was used. PC-3 C4 cells
were treated with different curcumin analogues for 24 h, and the
NF-κB-luciferase activities were measured using luciferase assay
kits from Promega (Madison, WI, USA). Following treatment, the
cells were washed with ice-cold phosphate-buffered saline (PBS),
and harvested in 1× reporter lysis buffer. Following
centrifugation, 10 μl aliquots of the supernatants were measured
for luciferase activity using a Luminometer from Turner Designs
Inc., (Sunnyvale, CA, USA). The luciferase activity was normalized
against known protein concentrations, and expressed as the
percentage of luciferase activity in the control cells, which were
treated with DMSO solvent. The protein level was determined using a
Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) according to
the manufacturer's instructions.
Statistical analysis
The analysis of variance (ANOVA) with the
Tukey-Kramer multiple comparison test was used for the comparison
of growth inhibition as determined by the trypan blue assay and
determination of the NF-κB-luciferase activities in cultured PC-3
cells that were treated with different curcumin analogues.
Results
Inhibitory effect of curcumin and its
analogues on the growth of PC-3 cells
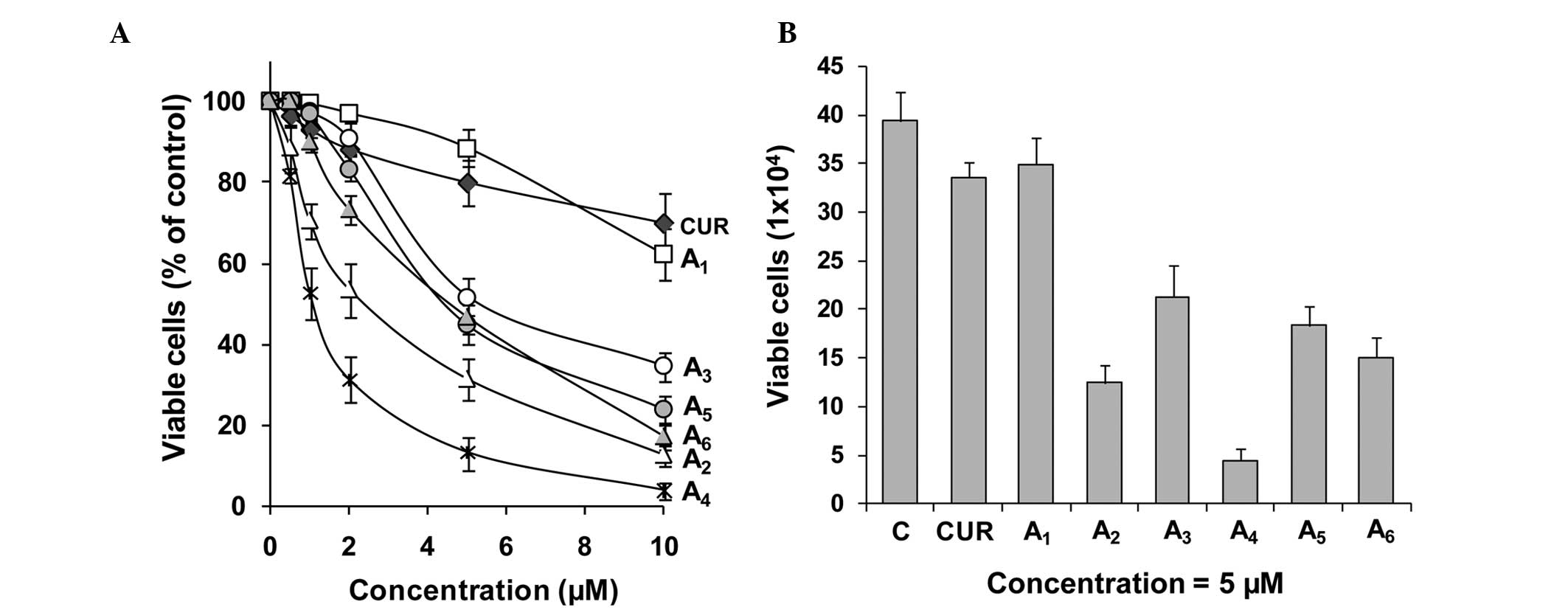
The inhibitory effects of curcumin and its analogues
A1-A6 on the growth of cultured PC-3 cells
were determined using the MTT assay. For each experiment, curcumin
was evaluated as the positive control. The inhibitory effects of
curcumin did not significantly vary between different experiments.
Data from the curcumin incubations were averaged (Fig. 1). Curcumin and its analogues
A1-A6 inhibited the growth of PC-3 cells in a
concentration-dependent manner (Fig.
1). A4 was the strongest curcumin analogue for
inhibiting the growth of PC-3 cells, as determined by the MTT
assay, followed by A2, A6, A5,
A3 and A1 (Fig.
1A). In additional experiments, the effects of different
curcumin analogues on cell growth were determined by the trypan
blue exclusion assay. Compounds A2-A6 were
more potent for decreasing the number of viable PC-3 cells as
compared to curcumin (Fig. 1B).
Statistical analysis using ANOVA with the Tukey-Kramer test
demonstrated that the differences in the number of viable cells
between the curcumin-treated group and any curcumin
analogue-treated group (except the A1-treated group)
were statistically significant (P<0.001). The number of viable
cells was significantly lower in the A4-treated group
than in the curcumin-treated or any other curcumin analogue-treated
group (P<0.05 compared to the A2-treated group;
P<0.001 compared to other curcumin analogue-treated groups).
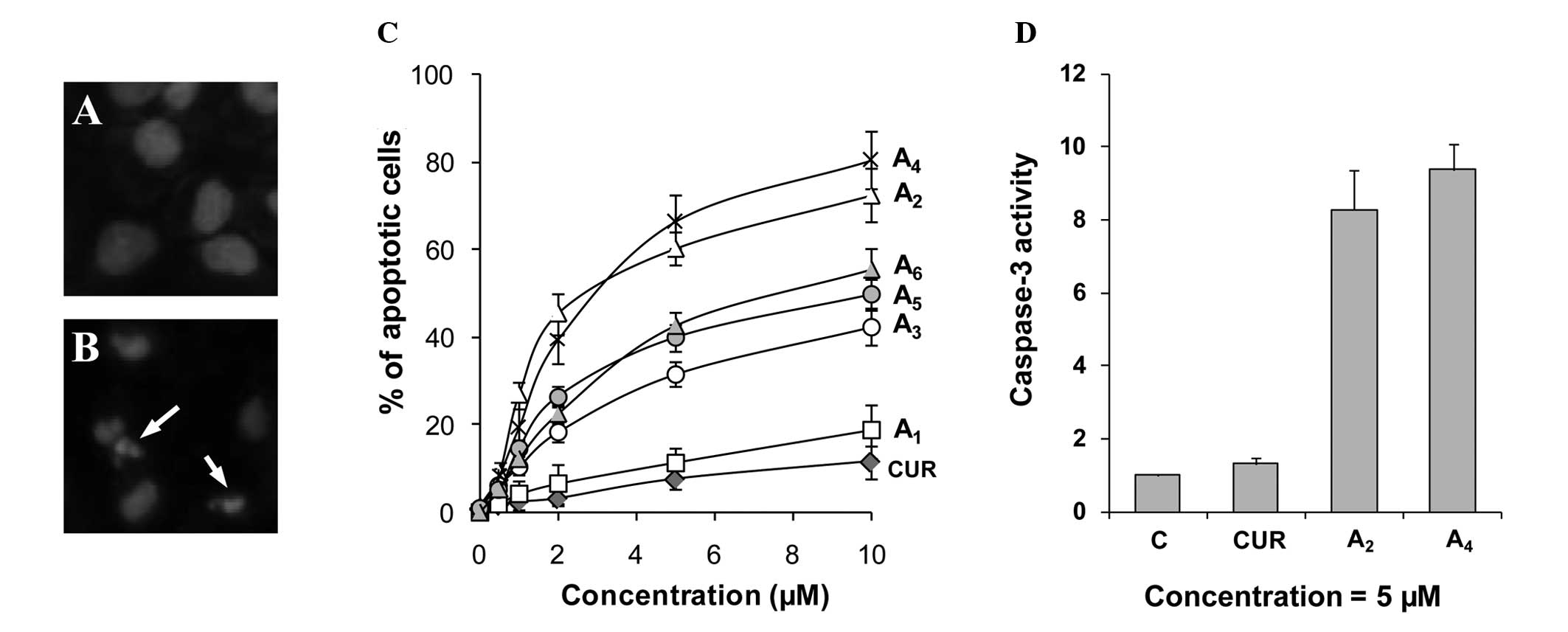
Stimulatory effect of curcumin analogues
on apoptosis in PC-3 cells
Effects of the curcumin analogues
A1-A6 on apoptosis in PC-3 cells were
determined by morphological assessment of apoptotic cells.
Apoptotic cells were identified by classical morphological
features, including nuclear condensation, cell shrinkage and
formation of apoptotic bodies. Morphologically distinct apoptotic
cells from representative samples are shown in Fig. 2B. Treatment of PC-3 cells with
curcumin resulted in a small increase in apoptotic cells (Fig. 2C). Treatment with compounds
A1-A6 stimulated apoptosis in PC-3 cells in a
concentration-dependent manner (Fig.
2C). Compounds A2 and A4 demonstrated
stronger stimulatory effects on apoptosis in PC-3 cells compared to
the other compounds. The effect of the two strongest compounds
A2 and A4 on activation of caspase-3 in
comparison to curcumin was determined. Treatment of PC-3 cells with
curcumin caused only a small increase in caspase-3 activity, while
treatment with A2 and A4 caused an 8.2- and
9.3-fold increase in caspase-3 activity, respectively (Fig. 2D). Our results identified
A2 and A4 as the two curcumin analogues that
had the greatest effect for stimulating apoptosis in PC-3
cells.
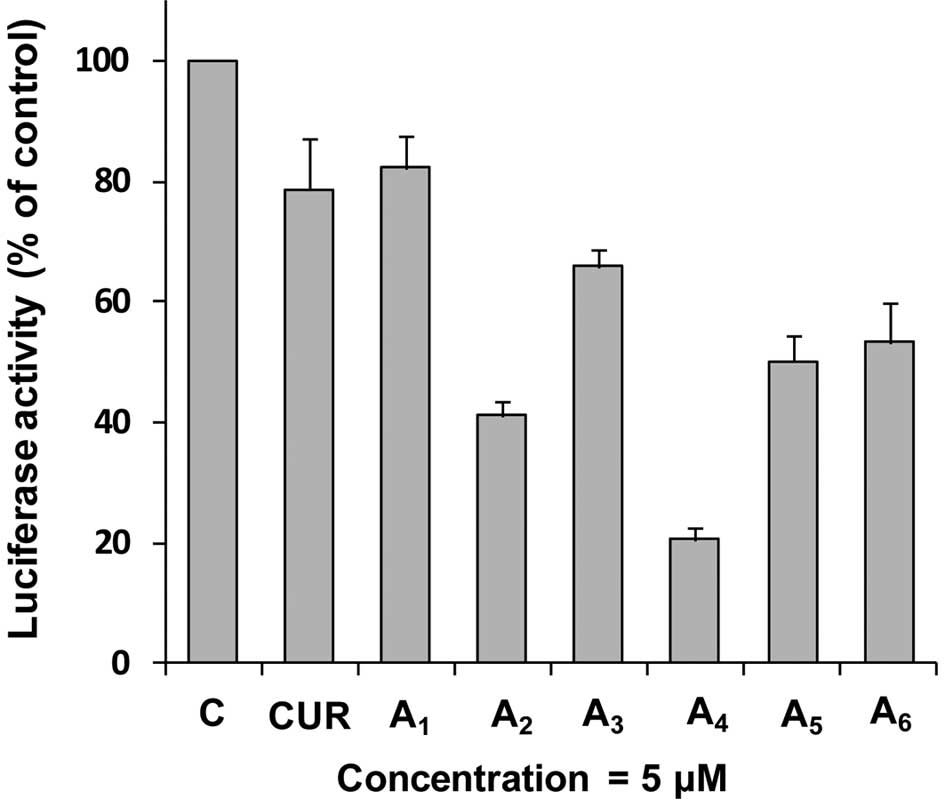
Effect of curcumin analogues on NF-κB
activity
To investigate the effect of
A1-A6 on activation of NF-κB activity, we
used an NF-κB-luciferase reporter gene expression assay in PC-3 C4
cells. PC-3 C4 is a cell line derived from the stable transfection
of PC-3 cells with an NF-κB luciferase construct (35). In these experiments, PC-3 C4 cells
were treated with different concentrations of curcumin and its
analogues A1-A6 for 24 h. Treatment of PC-3
C4 cells with curcumin or A1 (both 5 μM) caused only
modest decreases in the activity of NF-κB (Fig. 3). Treatment with
A2-A6 (all at 5 μM) caused a further decrease
in NF-κB transcriptional activity. Statistical analysis using ANOVA
with the Tukey-Kramer test demonstrated that NF-κB activity was
significantly lower in the A4-treated group than in any
other treated group (P<0.01 compared to the
A2-treated group; P<0.001 compared to other curcumin
analogue-treated groups). There were good correlations between
inhibition of NF-κB activity and cell growth inhibition (r=0.97),
and between inhibition of NF-κB activity and apoptosis stimulation
(r=0.96) in the PC-3 cells treated with all compounds at 5 μM.
Analysis of structure-activity
correlation
Six curcumin analogues (A1-A6)
that contain a five-carbon linker with a mono-carbonyl group
(cyclohexanone linker) were evaluated for anticancer activities in
human prostate cancer PC-3 cells. All of the curcumin analogues,
with the exception of A1, had stronger inhibitory
effects on cell growth and stronger stimulatory effects on the
apoptosis of PC-3 cells compared to curcumin. Although the
structures of A3 and curcumin are the same, with the
exception of their middle linker (Fig.
4), the anticancer activity of A3 was stronger than
that of curcumin (Fig. 1)
suggesting that a cyclohexanone linker increases anticancer
activity. A comparison of the six curcumin analogues (all with the
same mono-carbonyl linker) revealed that anticancer activity was
significantly influenced by substituents on the benzene rings. The
presence of a methoxy group on both sides of the p-phenol
group markedly increased activity compared to a compound with a
methoxy group on only one side (A3 vs. A6).
Tert-butyl substituents on both sides of the p-phenol group
(A4) had the strongest anticancer effect among all of
the studied compounds. Comparison of A1 and
A2 suggested that o-dihydroxyl substituents on both
benzene rings (A2) had stronger activity than an
analogue with a single hydroxyl group on each side
(A1).
Discussion
In the present study, we demonstrated that a series
of cyclohexanone curcumin analogues (A2-A6)
had stronger anticancer activities than curcumin in cultured human
prostate cancer PC-3 cells. Among the curcumin analogues,
A4 demonstrated a stronger inhibitory effect on the
growth of PC-3 cells than any of the other curcumin analogues.
Compounds A2 and A4 were stronger than the
other compounds for stimulating apoptosis. In addition, we found
that all curcumin analogues tested (except for A1) were
more potent inhibitors of NF-κB in PC-3 cells than curcumin.
A4 was the most potent compound among the six curcumin
analogues tested for inhibiting the activation of NF-κB.
Extensive studies have shown that curcumin exerts a
wide range of antitumor effects through modulation of significant
signaling pathways, including transcription factor NF-κB and other
pathways (15–20,36).
Of those involved in antitumor effects, NF-κB is generally regarded
as an important target of curcumin (16,37).
NF-κB has been linked to cell proliferation, invasion,
angiogenesis, metastasis, suppression of apoptosis and
chemoresistance in multiple tumors (38,39).
In addition, evidence suggests that NF-κB is significant in the
growth and radio/chemoresistance of prostate cancer (40–44).
Curcumin is able to suppress NF-κB activation by an Akt-dependent
or Akt-independent inhibition of IKK (15,16,45).
Certain curcumin analogues, including
3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24) have been found
to have a potent inhibitory effect on NF-κB (46). In the present study, we identified
that 5 out of 6 cyclohexanone curcumin analogues tested had a more
potent inhibitory effect than curcumin on activation of NF-κB in
PC-3 cells. The effects of these curcumin analogues on growth
inhibition and apoptosis stimulation were associated with their
inhibitory effect on activation of NF-κB. This result indicates
that inhibition of NF-κB activation may be involved in growth
inhibition and apoptosis induction in PC-3 cells treated with these
curcumin analogues.
Based on the analysis of the correlation between the
structures of curcumin analogues and their effects on the growth
and apoptosis of human prostate cancer PC-3 cells, analogues with a
cyclohexanone linker between the two benzene rings enhance
anticancer effects. Substituents on the benzene rings of the
analogues also affect their activities. The analogue with a
tert-butyl substituent on both sides of the p-phenol group
(A4) demonstrated stronger anticancer activity than the
other analogues, suggesting that the introduction of more
hydrophobic groups on both sides of the p-phenol group may
be an important strategy for the development of more potent
compounds with anti-prostate cancer activity.
Acknowledgements
This study was supported by the 2011 Guangdong
Province Leadership Grant, and by departmental funds from the
Department of Chemical Biology at the Ernest Mario School of
Pharmacy at Rutgers University. The authors thank Ms. Annette
Dionisio for her excellent help in the preparation of this
manuscript.
References
|
1
|
Lao CD: Dose escalation of a curcuminoid
formulation. BMC Complement Altern Med. 6:102006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008.
|
|
3
|
Aggarwal BB and Sung B: Pharmacological
basis for the role of curcumin in chronic diseases: an age-old
spice with modern targets. Trends Pharmacol Sci. 30:85–94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moragoda L, Jaszewski R and Majumdar AP:
Curcumin induced modulation of cell cycle and apoptosis in gastric
and colon cancer cells. Anticancer Res. 21:873–878. 2001.PubMed/NCBI
|
|
5
|
Anto RJ, Mukhopadhyay A, Denning K and
Aggarwal BB: Curcumin (diferuloylmethane) induces apoptosis through
activation of caspase-8, BID cleavage and cytochrome c release: its
suppression by ectopic expression of Bcl-2 and Bcl-xl.
Carcinogenesis. 23:143–150. 2002. View Article : Google Scholar
|
|
6
|
Squires MS, Hudson EA, Howells L, Sale S,
Houghton CE, Jones JL, Fox LH, Dickens M, Prigent SA and Manson MM:
Relevance of mitogen activated protein kinase (MAPK) and
phosphotidylinositol–3-kinase/protein kinase B (PI3K/PKB) pathways
to induction of apoptosis by curcumin in breast cells. Biochem
Pharmacol. 65:361–376. 2003.PubMed/NCBI
|
|
7
|
Hanif R, Qiao L, Shiff SJ and Rigas B:
Curcumin, a natural plant phenolic food additive, inhibits cell
proliferation and induces cell cycle changes in colon
adenocarcinoma cell lines by a prostaglandin-independent pathway. J
Lab Clin Med. 130:576–584. 1997. View Article : Google Scholar
|
|
8
|
Mukhopadhyay A, Bueso-Ramos C, Chatterjee
D, Pantazis P and Aggarwal BB: Curcumin downregulates cell survival
mechanisms in human prostate cancer cell lines. Oncogene.
20:7597–7609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piantino CB, Salvadori FA, Ayres PP, Kato
RB, Srougi V, Leite KR and Srougi M: An evaluation of the
anti-neoplastic activity of curcumin in prostate cancer cell lines.
Int Braz J Urol. 35:354–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hilchie AL, Furlong SJ, Sutton K,
Richardson A, Robichaud MR, Giacomantonio CA, Ridgway ND and Hoskin
DW: Curcumin-induced apoptosis in PC3 prostate carcinoma cells is
caspase-independent and involves cellular ceramide accumulation and
damage to mitochondria. Nutr Cancer. 62:379–389. 2010. View Article : Google Scholar
|
|
11
|
Teiten MH, Gaascht F, Eifes S, Dicato M
and Diederich M: Chemopreventive potential of curcumin in prostate
cancer. Genes Nutr. 5:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cabrespine-Faugeras A, Bayet-Robert M, Bay
JO, Chollet P and Barthomeuf C: Possible benefits of curcumin
regimen in combination with taxane chemotherapy for
hormone-refractory prostate cancer treatment. Nutr Cancer.
62:148–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ide H, Tokiwa S, Sakamaki K, Nishio K,
Isotani S, Muto S, Hama T, Masuda H and Horie S: Combined
inhibitory effects of soy isoflavones and curcumin on the
production of prostate-specific antigen. Prostate. 70:1127–1133.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khor TO, Keum YS, Lin W, Kim JH, Hu R,
Shen G, Xu C, Gopalakrishnan A, Reddy B, Zheng X, Conney AH and
Kong AN: Combined inhibitory effects of curcumin and phenethyl
isothiocyanate on the growth of human PC-3 prostate xenografts in
immunodeficient mice. Cancer Res. 66:613–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin YG, Kunnumakkara AB, Nair A, Merritt
WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM,
Lutgendorf SK, Aggarwal BB and Sood AK: Curcumin inhibits tumor
growth and angiogenesis in ovarian carcinoma by targeting the
nuclear factor-kappaB pathway. Clin Cancer Res. 13:3423–3430. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar
|
|
18
|
Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou
Q, Zhu LX and Zhao YF: Curcumin dually inhibits both mammalian
target of rapamycin and nuclear factor-κB pathways through a
crossed phosphatidylinositol 3-kinase/Akt/IκB kinase complex
signaling axis in adenoid cystic carcinoma. Mol Pharmacol.
79:106–118. 2011.PubMed/NCBI
|
|
19
|
Jutooru I, Chadalapaka G, Lei P and Safe
S: Inhibition of NFkappaB and pancreatic cancer cell and tumor
growth by curcumin is dependent on specificity protein
down-regulation. J Biol Chem. 285:25332–25344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo H, Yu JH, Chen K and Ye ZQ: Curcumin
induced the expression of inhibitor kappaBalpha protein in human
prostate cancer cells. Zhonghua Wai Ke Za Zhi. 44:1256–1259.
2006.PubMed/NCBI
|
|
21
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: an ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164.
2008.
|
|
22
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, et al: Phase
I clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
24
|
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS,
Hsieh CY and Lin JK: Stability of curcumin in buffer solutions and
characterization of its degradation products. J Pharm Biomed Anal.
15:1867–1876. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomren MA, Másson M, Loftsson T and
Tønnesen HH: Studies on curcumin and curcuminoids XXXI. Symmetric
and asymmetric curcuminoids: stability, activity and complexation
with cyclodextrin. Int J Pharm. 338:27–34. 2007.PubMed/NCBI
|
|
26
|
Grogan G: Emergent mechanistic diversity
of enzyme-catalysed beta-diketone cleavage. Biochem J. 388:721–730.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang G, Shao L, Wang Y, Zhao C, Chu Y,
Xiao J, Zhao Y, Li X and Yang S: Exploration and synthesis of
curcumin analogues with improved structural stability both in vitro
and in vivo as cytotoxic agents. Bioorg Med Chem. 17:2623–2631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Somers-Edgar TJ, Taurin S, Larsen L,
Chandramouli A, Nelson MA and Rosengren RJ: Mechanisms for the
activity of heterocyclic cyclohexanone curcumin derivatives in
estrogen receptor negative human breast cancer cell lines. Invest
New Drugs. 29:87–97. 2011. View Article : Google Scholar
|
|
29
|
Yadav B, Taurin S, Rosengren RJ,
Schumacher M, Diederich M, Somers-Edgar TJ and Larsen L: Synthesis
and cytotoxic potential of heterocyclic cyclohexanone analogues of
curcumin. Bioorg Med Chem. 18:6701–6707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du ZY, Bao YD, Liu Z, Qiao W, Ma L, Huang
ZS, Gu LQ and Chan AS: Curcumin analogs as potent aldose reductase
inhibitors. Arch Pharm (Weinheim). 339:123–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng X, Cui XX, Avila GE, Huang MT, Liu
Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS
and Conney AH: Atorvastatin and celecoxib inhibit prostate PC-3
tumors in immunodeficient mice. Clin Cancer Res. 13:5480–5487.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ploszaj T, Motyl T, Orzechowski A,
Zimowska W, Wareski P, Skierski J and Zwierzchowski L:
Antiapoptotic action of prolactin is associated with up-regulation
of Bcl-2 and down-regulation of Bax in HC11 mouse mammary
epithelial cells. Apoptosis. 3:295–304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng X, Chang RL, Cui XX, Avila GE, Lee
S, Lu YP, Lou YR, Shih WJ, Lin Y, Reuhl K, Newmark H, Rabson A and
Conney AH: Inhibitory effect of12-O-tetradecanoylphorbol-13-acetate
alone or in combination with all-trans-retinoic acid on the growth
of LNCaP prostate tumors in immunodeficient mice. Cancer Res.
64:1811–1820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Romero-Weaver AL, Wang HW, Steen HC,
Scarzello AJ, Hall VL, Sheikh F, Donnelly RP and Gamero AM:
Resistance to IFN-alpha-induced apoptosis is linked to a loss of
STAT2. Mol Cancer Res. 8:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng X, Chang RL, Cui XX, Avila G, Huang
MT, Liu Y, Kong AN, Rabson AB and Conney AH: Inhibition of NF-κB by
(E)3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile
(BAY11-7082; BAY) is associated with enhanced
12-O-tetradecanoylphorbol-13-acetate-induced growth
suppression and apoptosis in human prostate cancer PC-3 cells. Int
J Oncol. 32:257–264. 2008.
|
|
36
|
Chen A and Zheng S: Curcumin inhibits
connective tissue growth factor gene expression in activated
hepatic stellate cells in vitro by blocking NF-κB and ERK
signalling. Br J Pharmacol. 153:557–567. 2008.PubMed/NCBI
|
|
37
|
Li L, Aggarwal BB, Shishodia S, Abbruzzese
J and Kurzrock R: Nuclear factor-κB and IκB kinase are
constitutively active in human pancreatic cells, and their
down-regulation by curcumin (diferuloylmethane) is associated with
the suppression of proliferation and the induction of apoptosis.
Cancer. 101:2351–2362. 2004.
|
|
38
|
Aggarwal BB: Nuclear factor-κB: the enemy
within. Cancer Cell. 6:203–208. 2004.
|
|
39
|
Arlt A, Gehrz A, Müerköster S, Vorndamm J,
Kruse ML, Fölsch UR and Schäfer H: Role of NF-kappaB and Akt/PI3K
in the resistance of pancreatic carcinoma cell lines against
gemcitabine-induced cell death. Oncogene. 22:3243–3251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Holley AK, Xu Y, St Clair DK and St Clair
WH: RelB regulates manganese superoxide dismutase gene and
resistance to ionizing radiation of prostate cancer cells. Ann NY
Acad Sci. 1201:129–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karin M: NF-κB as a critical link between
inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009.
|
|
42
|
Royuela M, Rodríguez-Berriguete G, Fraile
B and Paniagua R: TNF-α/IL-1/NF-κB transduction pathway in human
cancer prostate. Histol Histopathol. 23:1279–1290. 2008.
|
|
43
|
Paule B, Terry S, Kheuang L, Soyeux P,
Vacherot F and de la Taille A: The NF-κB/IL-6 pathway in metastatic
androgen-independent prostate cancer: new therapeutic approaches?
World J Urol. 25:477–489. 2007.
|
|
44
|
Flynn V Jr, Ramanitharan A, Moparty K,
Davis R, Sikka S, Agrawal KC and Abdel-Mageed AB:
Adenovirus-mediated inhibition of NF-κB confers chemo-sensitization
and apoptosis in prostate cancer cells. Int J Oncol. 23:317–323.
2003.
|
|
45
|
Wang D, Veena MS, Stevenson K, Tang C, Ho
B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES and Wang MB:
Liposome-encapsulated curcumin suppresses growth of head and neck
squamous cell carcinoma in vitro and in xenografts through the
inhibition of nuclear factor κB by an AKT-independent pathway. Clin
Cancer Res. 14:6228–6236. 2008.PubMed/NCBI
|
|
46
|
Kasinski AL, Du Y, Thomas SL, Zhao J, Sun
SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, Liotta D and Fu
H: Inhibition of IkappaB kinase-nuclear factor-kappaB signaling
pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a
novel monoketone analog of curcumin. Mol Pharmacol. 74:654–661.
2008. View Article : Google Scholar : PubMed/NCBI
|