Introduction
Renal cell carcinoma (RCC) is one of the most common
types of malignant tumor of the human urinary system. To date, the
benefit of conventional therapies for RCC, including surgical,
radiological and chemotherapeutic approaches, is limited. Treatment
with IFN and IL-2 remains the main immunotherapy method for RCC
after surgery treatment. The efficacy rate is 10–20% when IFN is
used alone to treat metastatic RCC (1). Therefore, a more effective potential
therapy needs to be found. New targeted therapy for RCC may open up
a new avenue for cancer treatment and targeted therapy depends on
the evaluation of target gene status.
HER2, or ErbB-2, is a member of the epidermal growth
factor receptor (EGFR) family with intrinsic protein tyrosine
kinase activity and its increased activity is the assumed mechanism
underlying cell transformation (2).
HER2 combines with the other EGFRs to form heterogeneous dimers and
is involved in signal transduction, cell proliferation,
development, differentiation, migration and tumor formation
(3). Previous studies have reported
that HER2-positive status was an independent predictor of poor
prognosis in multivariate analysis (4). Herceptin, which is targeted against
the HER2 cell-surface receptor, has been successfully used for the
treatment of breast cancer. At present, there are conflicting
reports concerning HER2 expression in RCC due to different
laboratory conditions, case groups or the ethnicity of patients. In
the present study, we evaluated the HER2 status of 42 RCC tumor and
normal tissue specimens using immunohistochemistry (IHC) and 6
specimens using western blotting. Unlike the overexpression
observed in breast cancer, IHC showed that HER2 is commonly
expressed in normal renal, rather than RCC tissues. Since it has
been found that HER2 is expressed by the normal adult kidney, the
presence of this oncoprotein in the normal kidney may affect the
possibility of using HER2-targeted therapy for the treatment of
RCCs overexpressing HER2. The present study represents the
rationale of the analysis.
Materials and methods
Study population and tissue
specimens
A total of 84 paraffin-embedded specimens, including
42 tumor tissues and 42 corresponding adjacent normal tissues,
obtained during a two-year period (between January 2009 and
December 2010) and provided by The Union Hospital of Fujian Medical
University (Fuzhou, China), were analyzed to identify HER2
immunohistochemically stained sections in renal carcinoma cases. Of
these cases, 37 patients had clear cell renal carcinoma, 3 had
papillary renal carcinoma and 2 had carcinoma of the collecting
ducts. Adjacent normal tissues were also identified from the RCC
nephrectomy specimens. A total of 6 patients with RCC, who were
histologically diagnosed following surgery and treated in the
Department of Urology, The Union Hospital of Fujian Medical
University, China, were enrolled in a protein extraction and
western blotting study to verify the HER2 IHC expression. The study
was approved by the Institutional Ethics committee of the Union
Hospital of Fujian Medical University, and written informed consent
was obtained from the participants.
IHC analysis
Immunohistochemical staining was performed using
HER2/ErbB2 (29D8) rabbit mAb (dilution 1:100, Cell Signaling
Technology, Beverly, MA, USA). Briefly, the slides were rehydrated
and antigen retrieval was achieved by microwave for 15 min in
citrate buffer. The slides were incubated in 3% hydrogen peroxide
to quench endogenous horseradish peroxidase (HRP) for 30 min,
followed by incubation with normal goat serum in PBS for 60 min at
room temperature. The slides were then incubated with the primary
antibody at 4°C overnight. Subsequently, the slides were incubated
with biotin-labeled anti-rabbit IgG and preformed avidin-biotin
peroxidase complex. The slides were then counterstained with
hematoxylin, dehydrated and mounted.
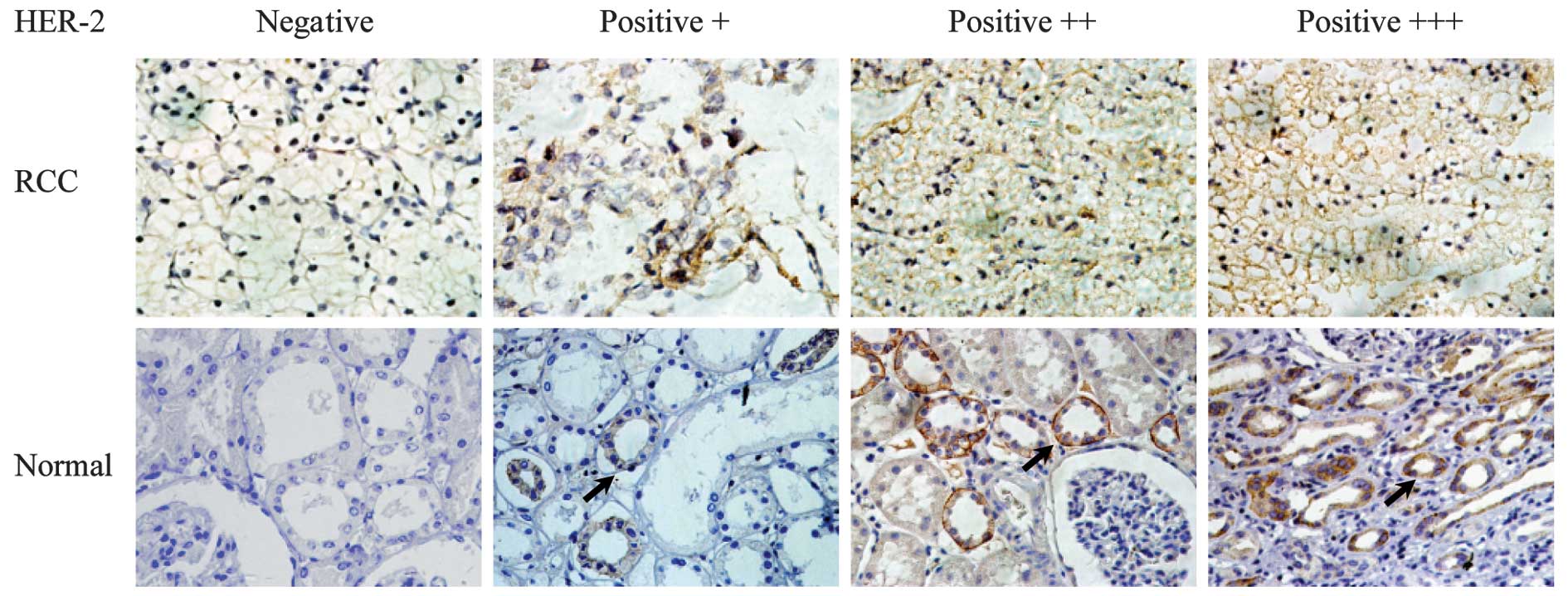
For analysis of HER2 staining, the tumor images were
collected at a magnification of ×400 and the proportion of
positively stained nuclei was determined for a minimum of 5 fields
of view. The integrated optical density (IOD) was then measured
using Image-Pro plus 5.0 software. The expression of HER2 of the 42
specimens was classified by two pathologists in our institute using
the semiquantitative scoring (SQS) recommended in UK guidelines in
separate settings. In this setting, these guidelines define a
positive HER2 status as: IHC score of 3+, uniform, intense
membranous staining in >30% of the tumor; 2+, weak to moderate
complete membranous staining that is non-uniform or weak in
intensity in at least 10% of the cells; 1+, weak and incomplete
membranous staining in <10% of the tumor cells; a negative HER2
status is defined as an IHC score of 0, no staining. Hematoxylin
and eosin staining of the slides of tumors was performed to show
the area of the tumor or adjacent normal tissues and then scanned
using a microscope (Nikon TE2000-U, Tokyo, Japan).
Western blotting
Fresh specimens, including tumor tissues and
corresponding adjacent normal tissues, were obtained during
surgery. A total of 6 RCC tissues, including 1 papillary renal
carcinoma, 5 clear cell renal carcinomas and their normal adjacent
tissues, were used. Cell membrane proteins were extracted using a
Mem-PER Eukaryotic Membrane Protein Extraction kit (Thermo Fisher
Scientific Inc., Logan, UT, USA). For the western blotting
analysis, crude membrane pellets were solubilized in
electrophoresis sample buffer and boiled for 10 min, then separated
on an 8% gel. Following electrophoresis, the proteins were loaded
onto a polyvinylidene fluoride (PVDF) microporous membrane
(Millipore, Billerica, MA, USA). After blocking of non-specific
binding with 5% bovine serum albumin (BSA) for 2 h at room
temperature, the proteins were subsequently identified using a
primary antibody specific to HER2 (dilution 1:1,000) in PBST under
gentle agitation at 4°C overnight. Western blots were developed
with the secondary antibody anti-rabbit IgG (dilution 1:5,000;
Bioss, Beijing, China) and enhanced chemiluminescence (ECL;
Amersham Pharmacia, Freiburg, Germany) detection system. β-actin
was used as a loading control.
Statistical analysis
The Mann-Whitney U test was used to analyze the
statistical contrast between HER2 expression in RCC and normal
tissues. The correlation coefficients (r and P-values) between the
HER2 status of normal tissue and the TNM stage were obtained using
the Spearman test. P<0.05 was considered to indicate a
statistically significant result. Statistical analyses were
performed using SPSS 11.5 software (SPSS, Inc., Chicago, IL,
USA).
Results
The clinical and demographic details of the 42
patients are shown in Table I,
based on TNM classification. HER2 expression was assessed generally
by IHC. Our aim was to determine the frequency of HER2 expression
in RCC and corresponding normal tissue and the correlation between
HER2 expression and tumor grading. A distinctive HER2 expression in
renal normal tissue was confirmed at the protein level by IHC. The
sections of tumor and adjacent normal tissues are shown in Fig. 1. Of a total of 42 tumor and adjacent
normal tissues, HER2 expression was observed in 7 of the 42 tumors
(16.67%) using IHC. For the majority of the tumors, HER2 expression
was negative. However, 33 adjacent normal tissues (78.57%)
expressed the HER2 protein (Table
II), which was confined to the renal tubule and renal
collecting duct as shown in Fig. 1.
This is an unfavorable outcome contrary to our intent.
 | Table IClinical data of 42 patients with
RCC. |
Table I
Clinical data of 42 patients with
RCC.
| | | | | | HER2 expression |
|---|
| | | | | |
|
|---|
| Case | Gender | Age (years) | Clinical stage | Tumor size (cm) | TNM stage | RCC tissues | Normal tissues |
|---|
| 1 | F | 63 | 1 | 3 | T1N0M0 | 0 | 3+ |
| 2 | M | 43 | 1 | 3.5 | T1N0M0 | 0 | 3+ |
| 3 | M | 61 | 1 | 2.9 | T1N0M0 | 0 | 3+ |
| 4 | F | 57 | 1 | 4.3 | T1N0M0 | 0 | 3+ |
| 5 | M | 38 | 1 | 6.0 | T1N0M0 | 2+ | 0 |
| 6 | M | 63 | 1 | 4.3 | T1N0M0 | 0 | 1+ |
| 7 | M | 59 | 2 | 8.5 | T2N0M0 | 3+ | 1+ |
| 8 | F | 25 | 1 | 4.3 | T1N0M0 | 0 | 3+ |
| 9 | M | 17 | 4 | 10 | T4N1M0 | 0 | 0 |
| 10 | M | 55 | 1 | 3.6 | T1N0M0 | 2+ | 0 |
| 11 | F | 60 | 1 | 3.5 | T1N0M0 | 0 | 3+ |
| 12 | F | 56 | 2 | 10 | T2N0M0 | 2+ | 1+ |
| 13 | F | 58 | 2 | 13.1 | T2N0M0 | 0 | 3+ |
| 14 | M | 61 | 1 | 0.9 | T1N0M0 | 0 | 0 |
| 15 | M | 60 | 1 | 4.1 | T1N0M0 | 3+ | 0 |
| 16 | M | 46 | 1 | 4.3 | T1N0M0 | 0 | 2+ |
| 17 | M | 62 | 3 | 5.9 | T3bN1M0 | 0 | 3+ |
| 18 | M | 31 | 1 | 2.2 | T1N0M0 | 0 | 1+ |
| 19 | M | 69 | 3 | 2.4 | T3N0M0 | 0 | 1+ |
| 20 | F | 60 | 3 | 8.6 | T2N1M0 | 0 | 3+ |
| 21 | F | 58 | 2 | 9.9 | T2N0M0 | 0 | 0 |
| 22 | M | 65 | 1 | 6.5 | T1N0M0 | 0 | 3+ |
| 23 | M | 67 | 3 | 1.7 | T3N0M0 | 0 | 2+ |
| 24 | M | 40 | 1 | 2.2 | T1N0M0 | 0 | 3+ |
| 25 | M | 70 | 3 | 2.5 | T2N1M0 | 0 | 3+ |
| 26 | M | 61 | 3 | 1.2 | T3N0M0 | 0 | 0 |
| 27 | M | 48 | 2 | 13.6 | T2N0M0 | 0 | 2+ |
| 28 | M | 47 | 3 | 4.5 | T3N1M0 | 0 | 0 |
| 29 | M | 44 | 1 | 6.0 | T1N0M0 | 1+ | 3+ |
| 30 | M | 47 | 1 | 1.7 | T1N0M0 | 0 | 3+ |
| 31 | M | 43 | 1 | 4.8 | T1N0M0 | 0 | 3+ |
| 32 | M | 50 | 1 | 3.0 | T1N0M0 | 0 | 3+ |
| 33 | M | 53 | 1 | 4.2 | T1N0M0 | 0 | 2+ |
| 34 | M | 41 | 1 | 4.5 | T1N0M0 | 0 | 2+ |
| 35 | M | 69 | 1 | 1.5 | T1N0M0 | 0 | 3+ |
| 36 | F | 62 | 1 | 5.9 | T1N0M0 | 0 | 3+ |
| 37 | M | 45 | 1 | 6.1 | T1N0M0 | 0 | 2+ |
| 38 | M | 51 | 4 | 14.0 | T2N2M0 | 0 | 0 |
| 39 | F | 62 | 1 | 4.5 | T1N0M0 | 0 | 3+ |
| 40 | F | 45 | 1 | 5.2 | T1N0M0 | 2+ | 3+ |
| 41 | M | 48 | 2 | 3.8 | T2N0M0 | 0 | 2+ |
| 42 | F | 52 | 1 | 5.7 | T1N0M0 | 0 | 2+ |
 | Table IIThe correlation between HER2
expression in RCC and normal tissue. |
Table II
The correlation between HER2
expression in RCC and normal tissue.
| HER2 | | | |
|---|
|
| | | |
|---|
| − | + | ++ | +++ | Total | r | P-value |
|---|
| RCC | 35 | 1 | 4 | 2 | 42 | −0.410 | 0.007 |
| Normal | 9 | 5 | 8 | 20 | 42 | | |
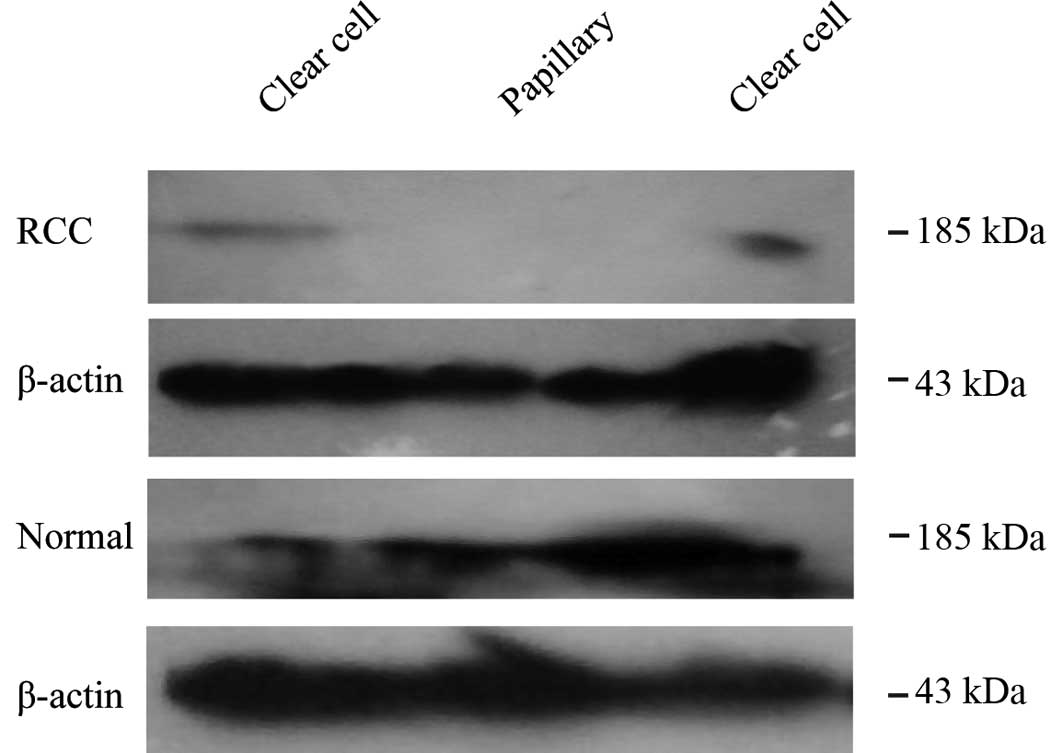
To confirm this result, we investigated the HER2
expression in 6 fresh RCC and adjacent normal tissues by western
blotting. Of the 6 tumor and adjacent normal tissues, only 2 cases
of tumor tissue expressed HER2 weakly. However, the adjacent normal
tissues exhibited positive expression (Fig. 2). This outcome is in accordance with
the results previously described in the paraffin-embedded tissues.
Therefore, HER2 is mainly expressed in normal kidney tissue
specimens, rather than in RCC tissues. To examine the change in
HER2 expression during RCC pathogenesis, we analyzed the
correlation between normal renal tissue and tumor tissue. The HER2
status of normal tissue was negatively correlated with that of the
RCC tissues (r=−0.410, P=0.007; Table
II). Furthermore, the HER2 status of normal tissue was
negatively correlated with the TNM stage (r=−0.246, P=0.027)
following statistical analysis according to the difference in stage
(Table III).
 | Table IIIHER2 status of normal tissue is
negatively correlated with the TNM stage. |
Table III
HER2 status of normal tissue is
negatively correlated with the TNM stage.
| Normal tissue | | |
|---|
|
| | |
|---|
| TNM | − | + | ++ | +++ | r | P-value |
|---|
| T1 | 4 | 2 | 5 | 16 | −0.246 | 0.027 |
| T2 | 2 | 2 | 2 | 3 | | |
| T3 | 2 | 1 | 1 | 1 | | |
| T4 | 1 | 0 | 0 | 0 | | |
Discussion
The incidence rate of RCC, including clear cell
renal cell carcinoma (70–80%), papillary renal cell carcinoma
(10–15%), chromophobe renal cell carcinoma (5%) and carcinoma of
the collecting ducts (1%), comprises 90–95% of all neoplasms of the
kidney. As a malignant tumor, RCC has a poor prognosis, due in
large part to the fact that 40% of patients develop distant
metastases following removal of the primary tumor and that
available chemotherapeutic agents are ineffective against RCC
(5). Thus, there is a need for the
development of new treatment strategies. In recent years, molecular
targeted therapy has become a new treatment modality for RCC.
Herceptin, which is targeted against the HER2 cell-surface
receptor, has been reported to increase the cure rate of breast
cancer by 20% (6,7). HER2 has been identified as a potential
therapeutic target. However, molecular targeted therapy for RCC
depends on the evaluation of the target gene status.
HER2 amplification and overexpression play
significant roles in signal transduction, cell proliferation,
development, differentiation, migration and tumor formation.
Findings of available reports indicate a discrepancy with regard to
HER2 expression in RCC. It has been reported that the HER2 positive
rate in RCC is 40% (8). However,
other authors have found that the positive rate of HER2 expression
was 11% in RCC tissues and was expressed at heterogeneous levels
independently of tumor grading and staging (9). Another study reported that the HER2
gene was neither overexpressed nor amplified in cases of Wilms
tumor (10). This discrepancy may
partly be explained by differences in staining technique and
patient cohorts (11,12). Results of the present study
emphasize the low rate of expression and complete concordance
between IHC and western blotting analyses in RCC. Therefore,
although a high positive rate was observed in breast cancer,
gastric carcinoma and bladder cancer (13,14),
HER2 expression in RCC may be rare, as in endometrial cancer
(15). It is not appropriate to
employ antibody- or CTL-based immunotherapy, such as
antibody-coated tumor cell vaccine, in cases of RCC (16).
Notably, in our study there was a negative
correlation between the HER2 expression in normal tissue and that
of RCC (P=0.007, r=−0.410). Coincidently, the location where HER2
was expressed in normal tissues was the same site from which RCC
later originated. Taken together, this finding indicates that a
decrease in the expression of this gene occurred during RCC
oncogenesis. Furthermore, for the patients in whom RCC had already
occurred, the HER2 status of the normal tissue was negatively
correlated with the TNM stage to a certain extent (P=0.027,
r=−0.246), which also confirms that HER2 is involved in RCC
oncogenesis. This observation suggests that HER2 expression was
reduced during the process of RCC oncogenesis and also played a
significant role in RCC development later, indicating a new
mechanism of HER2 in the progression of RCC. In conclusion, HER2 is
not an important target for the treatment of RCC. In fact, since
tumors which overexpressed HER2 showed negative adjacent normal
tissues, HER2 in this specific patient subgroup could serve as a
candidate treating target.
Acknowledgements
This study was partly supported by grants from the
Fujian Natural Sciences Foundation (2009J05069, 2009J05067) and
Fujian Education Department Foundation (JA09113). We gratefully
acknowledge Professor Yinghong Yang (Department of Pathology, The
Union Hospital of Fujian Medical University) for the generous and
critical advice.
References
|
1
|
Fossa SD: Interferon in metastatic renal
cell carcinoma. Semin Oncol. 27:187–193. 2000.PubMed/NCBI
|
|
2
|
Klapper LN, Glathe S, Vaisman N, et al:
The ErbB-2/HER2 oncoprotein of human carcinomas may function solely
as a shared coreceptor for multiple stroma-derived growth factors.
Proc Natl Acad Sci USA. 96:4995–5000. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reese DM and Slamon DJ: HER-2/neu signal
transduction in human breast and ovarian cancer. Stem Cells.
15:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Revillion F, Bonneterre J and Peyrat JP:
ERBB2 oncogene in human breast cancer and its clinical
significance. Eur J Cancer. 34:791–808. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar
|
|
6
|
Disis ML, Wallace DR, Gooley TA, et al:
Concurrent trastuzumab and HER2/neu-specific vaccination in
patients with metastatic breast cancer. J Clin Oncol. 27:4685–4692.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tokugawa T, Kobayashi A, Matsuyama T, Imai
S and Koyama H: A patient with axillary node metastasis from breast
cancer who responded to trastuzumab/capecitabine combination
therapy. Gan To Kagaku Ryoho. 36:467–469. 2009.(In Japanese).
|
|
8
|
Zhang XH, Takenaka I, Sato C and Sakamoto
H: p53 and HER-2 alterations in renal cell carcinoma. Urology.
50:636–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ragab SM, Samaka RM and Shams TM: HER2/neu
expression: a predictor for differentiation and survival in
children with Wilms tumor. Pathol Oncol Res. 16:61–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vasei M, Modjtahedi H, Ale-Booyeh O, et
al: Amplification and expression of EGFR and ERBB2 in Wilms tumor.
Cancer Genet Cytogenet. 194:88–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Latif Z, Watters AD, Bartlett JM,
Underwood MA and Aitchison M: Gene amplification and overexpression
of HER2 in renal cell carcinoma. BJU Int. 89:5–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seliger B, Rongcun Y, Atkins D, et al:
HER-2/neu is expressed in human renal cell carcinoma at
heterogeneous levels independently of tumor grading and staging and
can be recognized by HLA-A2.1-restricted cytotoxic T lymphocytes.
Int J Cancer. 87:349–359. 2000. View Article : Google Scholar
|
|
13
|
Laé M, Couturier J, Oudard S, Radvanyi F,
Beuzeboc P and Vieillefond A: Assessing HER2 gene amplification as
a potential target for therapy in invasive urothelial bladder
cancer with a standardized methodology: results in 1005 patients.
Ann Oncol. 21:815–819. 2010.PubMed/NCBI
|
|
14
|
Shiroiwa T, Fukuda T and Shimozuma K:
Cost-effectiveness analysis of trastuzumab to treat HER2-positive
advanced gastric cancer based on the randomised ToGA trial. Br J
Cancer. 105:1273–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Srijaipracharoen S, Tangjitgamol S,
Tanvanich S, et al: Expression of ER, PR, and Her-2/neu in
endometrial cancer: a clinicopathological study. Asian Pac J Cancer
Prev. 11:215–220. 2010.PubMed/NCBI
|
|
16
|
Wang H, Wang D, Li M, et al: Enhanced
anti-tumor immunity generated by Rituximab-coated tumor cell
vaccine. Cancer Lett. 268:129–136. 2008. View Article : Google Scholar : PubMed/NCBI
|