Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of solid malignant tumor worldwide, and both the
incidence and mortality rates have increased in recent years
(1,2). The development of HCC usually follows
a multistep sequence, and de novo development of HCC appears
to be rare. The carcinogenic sequence of chronic hepatitis,
cirrhosis, dysplastic nodule (DN) and HCC has been well-established
(3). Nodular lesions that differ
from the surrounding liver parenchyma and that are characterized by
cytological or structural atypia are termed DNs. DN is a well-known
precancerous lesion of HCC. DNs are classified as low-grade (LGDN)
or high-grade (HGDN) depending on the degree of atypia (3,4).
Keratin 19 (K19) and K7, molecular markers of
hepatic progenitor cells and cholangiocytes, are expressed in a
proportion of HCCs (5,6). K19 expression in HCC is associated
with recurrence, metastasis and poor prognosis (5–8).
Recent studies have postulated that K19-positive HCCs originates
from hepatic progenitor cells (HPCs) (5,9,10).
However, it remains unknown whether K19-positive HCCs are generated
through the carcinogenesis of K19-positive HPCs or whether K19
expression in HCC may be the result of dedifferentiation during HCC
progression. If K19-positive HCCs arise from HPCs, HPC would be
expected to already be present in precursor HCC lesions. Clarifying
the histogenesis of K19-positive HCC is significant, as it may
provide a rationale for novel therapeutic approaches to HCC.
Therefore, in the present study, we examined K19 and
K7 expression in 27 DNs and 79 HCC tissue sections from resected
liver samples and 132 HCC tissue microarrays (TMA). We also
analyzed the clinicopathological characteristics of these HCCs.
Patients and methods
Patients
The total 107 samples consisted of preoperatively
untreated tissue sections surgically resected (13 LGDNs, 15 HGDNs
and 79 HCCs) between September 2004 and August 2008 at the Chonbuk
National University Hospital and Samsung Medical Center, Korea.
This study was approved by the Ethics Committees of Chonbuk
National University and Samsung Medical Center. Group 1 HCCs were
comprised 27 small HCCs (≤2 cm) and 52 advanced HCCs (>2 cm). Of
the 27 small HCCs, 5 were vaguely nodular, 20 were distinctly
nodular and 2 were infiltrative types. Representative 4-μm sections
were prepared from 10% formalin-fixed, paraffin-embedded tissue
samples for immunohistochemical staining. In each case,
clinicopathological characteristics, such as patient age at
diagnosis, gender, etiology, serological data, including
α-fetoprotein (AFP) and albumin levels, background liver disease,
tumor size, Edmonson-Steiner grade, microvessel invasion, presence
of intrahepatic metastasis and ascites, as well as follow-up data
were obtained from hospital records. Tumors were staged according
to the 2010 American Joint Committee on Cancer
tumor-node-metastasis classification (11). The follow-up period was determined
from the date of initial surgery until the date of the last
follow-up or death.
TMA construction
We constructed TMA slides (Superbiochips
Laboratories, Seoul, Korea) to compare the concordance rates of K19
and K7 expression in HCC between whole sections and TMA. After
screening, hematoxylin and eosin-stained slides, cores measuring 3
mm in the greatest dimension, were obtained from 132 representative
paraffin-embedded HCC blocks, which were resected at the Chonbuk
National University Hospital between January 1998 and December
2009. A subset of 36 HCCs from the 79 whole-tissue sections was
compared with the corresponding TMA samples.
Immunohistochemistry
Immunohistochemical staining for K19 and K7 (Dako,
Carpinteria, CA, USA) was performed as previously described
(12). Samples demonstrating
membrane and cytoplasmic staining of at least 5% of tumor cells
were defined as positive (5).
Positive immunoreactivity in whole tissue sections was classified
as: diffuse pattern, >50% of tumor cells were positive;
geographic pattern, 5–49% were positive.
Statistical analysis
Comparisons between K19 or K7 expression and
clinicopathological factors were assessed by the Chi-square test.
Survival analyses were performed using the Kaplan-Meier method, and
differences in survival between the various clinical groups were
determined by the log-rank test. A Cox proportional hazards
regression analysis was performed to estimate the impact of
clinicopathological factors on patient survival. P<0.05 was
considered to indicate a statistically significant difference. SPSS
version 15.0 statistical software (SPSS Inc., Chicago, IL, USA) was
used for the statistical analysis.
Results
Clinical characteristics
The 175 patients with HCC were aged between 25 and
79 years old and comprised 146 males and 29 females. A total of 126
patients were positive for hepatitis B virus surface antigen; 22
were alcohol-related, 10 were positive for anti-hepatitis C virus
antibody and 17 patients were of unknown etiology (Table I).
 | Table IAssociation between pathological
features and K19-positive patients with HCC. |
Table I
Association between pathological
features and K19-positive patients with HCC.
| Overall HCC
(n=175) | Non-viral HCC
(n=39) | Viral HCC
(n=136) |
|---|
|
|
|
|
|---|
| Characteristics | Total | K19+ | P-value | Total | K19+ | P-value | Total | K19+ | P-value |
|---|
| Gender |
| Male | 146 | 13 | 0.062 | 33 | 2 | 0.003 | 113 | 11 | 0.634 |
| Female | 29 | 6 | | 6 | 3 | | 23 | 3 | |
| Age (years) |
| <55 | 66 | 6 | 0.632 | 7 | 0 | 0.263 | 57 | 6 | 0.940 |
| ≥55 | 109 | 13 | | 32 | 5 | | 79 | 8 | |
| Liver cirrhosis |
| Absence | 85 | 7 | 0.279 | 22 | 2 | 0.428 | 63 | 5 | 0.401 |
| Presence | 90 | 12 | | 17 | 3 | | 73 | 9 | |
| Ascites |
| Absence | 158 | 18 | 0.488 | 33 | 5 | 0.307 | 125 | 13 | 0.891 |
| Presence | 17 | 1 | | 6 | 0 | | 11 | 1 | |
| Albumin (g/dl) |
| ≥3.5 | 151 | 18 | 0.257 | 34 | 5 | 0.358 | 117 | 13 | 0.437 |
| <3.5 | 24 | 1 | | 5 | 0 | | 19 | 1 | |
| Preoperative AFP
(ng/ml) |
| <100 | 115 | 6 | 0.001 | 32 | 3 | 0.169 | 83 | 3 | 0.001 |
| ≥100 | 60 | 13 | | 7 | 2 | | 53 | 11 | |
| Intrahepatic
metastasis |
| Absence | 119 | 13 | 0.967 | 30 | 4 | 0.170 | 89 | 9 | 0.516 |
| Presence | 56 | 6 | | 9 | 1 | | 47 | 5 | |
| Microvessel
invasion |
| Absence | 75 | 7 | 0.575 | 17 | 2 | 0.862 | 58 | 5 | 0.580 |
| Presence | 100 | 12 | | 22 | 3 | | 78 | 9 | |
| Histological
grade |
| 1 and 2 | 98 | 6 | 0.023 | 23 | 2 | 0.356 | 75 | 4 | 0.035 |
| 3 and 4 | 77 | 13 | | 16 | 3 | | 61 | 10 | |
| pT stage |
| 1 | 69 | 6 | 0.651 | 16 | 2 | 0.974 | 53 | 4 | 0.518 |
| 2 | 72 | 8 | | 14 | 2 | | 58 | 6 | |
| 3 and 4 | 34 | 5 | | 9 | 1 | | 25 | 4 | |
| Etiology |
| Viral | 136 | 14 | 0.655 | | | | | | |
| Non-viral | 39 | 5 | | | | | | | |
Immunohistochemical results
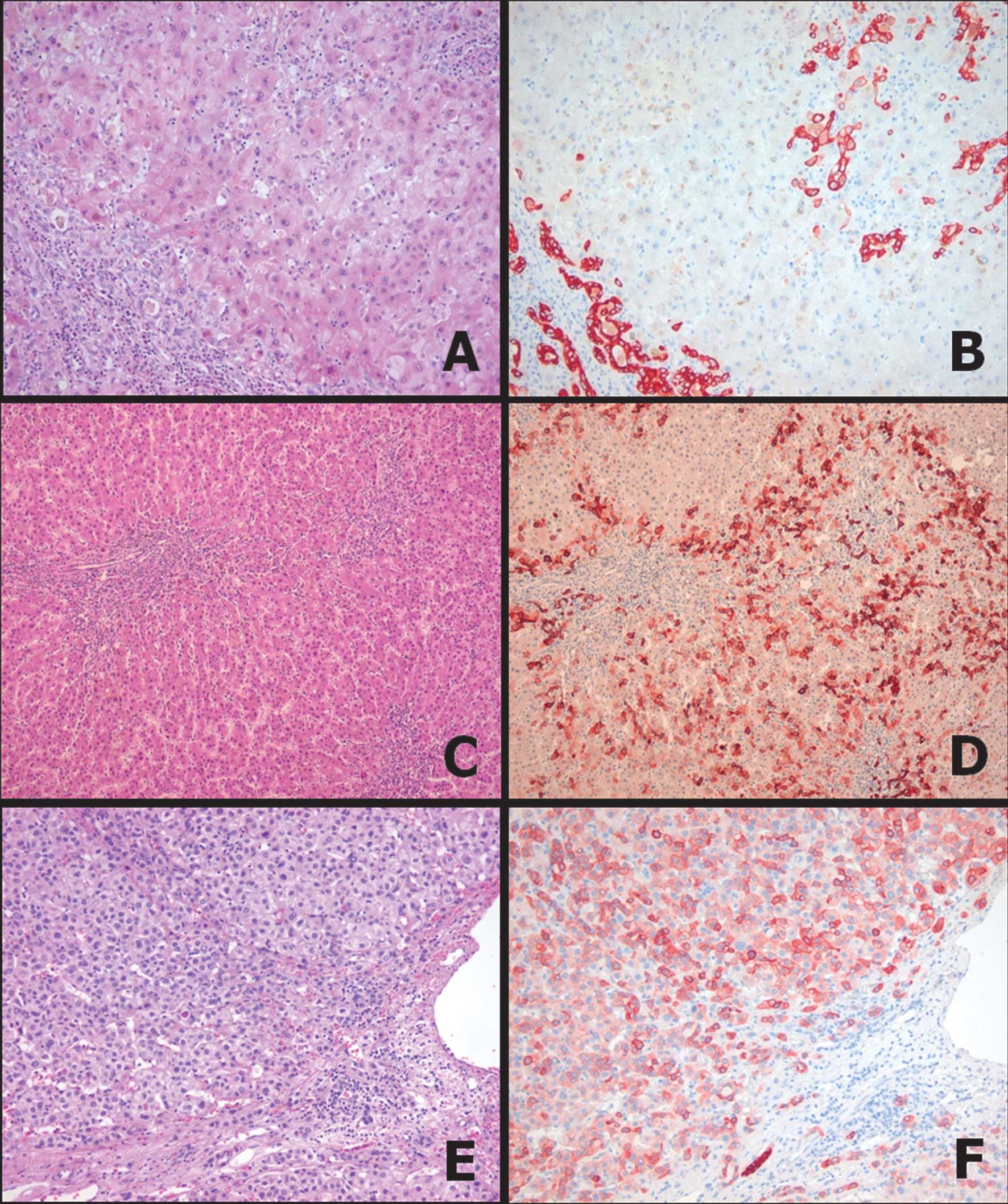
Reactive ductular cells surrounding an inflamed
portal tract were positive for K19 and/or K7 in cirrhotic livers
(Fig. 1A and B). Of 28 DNs, 24 were
K7−/K19− (86%), four were K7+/K19− (14%) and none were K7−/K19+ or
K7+/K19+. Of the four K7-positive DNs, three were LGDNs and one was
HGDN. The K7-positive DNs demonstrated a geographic staining
pattern (<30% of tumor cells) with accentuated staining in
intermediate hepatocyte-like cells around portal tracts in LGDNs
(Fig. 1C and D). These K7-positive
and K19-negative cells in DNs may be associated with intermediate
hepatocyte-like cells. Of the 79 whole HCC sections, K19 expression
was detected in 9 (11%) HCCs, including the diffuse pattern in 8
cases and the geographic pattern in 2 cases (Fig. 1E and F). Nineteen of the 79 (24%)
HCCs were positive for K7, the diffuse pattern was found in 5 cases
and the geographic pattern was found in 14 cases. Among 27 small
HCCs, 15 were K7−/K19− (56%), 7 were K7+/K19− (26%), one was
K7−/K19+ (4%) and four were K7+/K19+ (15%). Among the five
K19-positive small HCCs, four were distinctly nodular and one was
infiltrative type. No vaguely nodular HCC was positive for K19. Of
11 K7-positive small HCCs, two were vaguely nodular, eight were
distinct nodular and one was infiltrative type. In progressed HCC,
42 were K7−/K19− (81%), six were K7+/K19− (12%), two were K7−/K19+
(4%) and two were K7+/K19+ (4%). Similar to a previous study
(5), HCC cells reactive to K7
and/or K19 were mostly small or intermediate-sized cells, but
smaller than the non-neoplastic hepatocytes in the tissue
surrounding the tumor. The K19 expression pattern in HCC was more
homogeneous and diffuse compared with that of K7. Of the 132 TMA
samples, 116 were K7−/K19− (88%), five were K7+/K19− (4%), eight
were K7−/K19+ (6%) and three were K7+/CK19+ (2%). In the validation
study between the whole section and TMA samples, the concordance
rates for K7 and K19 staining in HCC were 81% (29 of 36) and 89%
(32 of 36), respectively. Five K7-positive HCCs in whole sections
changed to negative cases in TMA samples, while two K19-positive
cases changed to negative cases in TMA samples. The frequencies of
K19 and/or K7-positive HCC decreased in small HCC, large HCC and
TMA samples, respectively (Table
II).
 | Table IIExpression rate of K19 and K7 in DNs,
small HCC, large HCC and TMA samples. |
Table II
Expression rate of K19 and K7 in DNs,
small HCC, large HCC and TMA samples.
| Keratin | DN (%) | Small HCC (%) | Large HCC (%) | TMA section
(%) |
|---|
| 7 | 4/28 (14) | 11/27 (41) | 8/52 (15) | 8/132 (6) |
| 19 | 0/28 (0) | 5/27 (19) | 4/52 (8) | 10/132 (8) |
Correlation between immunohistochemical
results and clinicopathological characteristics
To elucidate the significance of K19 and K7 in HCCs,
we correlated their protein expression with major
clinicopathological variables (Table
I). The clinicopathological analysis demonstrated that
K19-positive HCC was significantly associated with high
histological grade (P=0.023), serum AFP level (P=0.001) and K7
expression (P=0.001). Other factors, including age, gender,
etiology, background liver disease, albumin level, presence of
intrahepatic metastasis, microvessel invasion and presence of
ascites were not correlated with K19 expression. No significant
differences were observed between K7-positive and K7-negative HCC
with regard to any clinicopathological parameters.
Patient outcomes
The follow-up intervals ranged from 1 to 142 months.
Sixty-one patients died during the follow-up period. The median
survival of patients with K19-positive HCC was 82.0 months. The
median survival of patients with K19-negative HCC was 87 months.
The 5-year survival rate in patients with K19-positive HCC was
lower (52%) than that of patients with K19-negative HCC (55%). In a
univariate analysis, intrahepatic metastasis, serum albumin levels,
microvessel invasion and pT stage were significantly associated
with poor patient survival (P<0.001, P=0.010, P=0.030, P=0.031,
respectively). The multivariate analysis revealed that intrahepatic
metastasis and serum albumin levels were independent prognostic
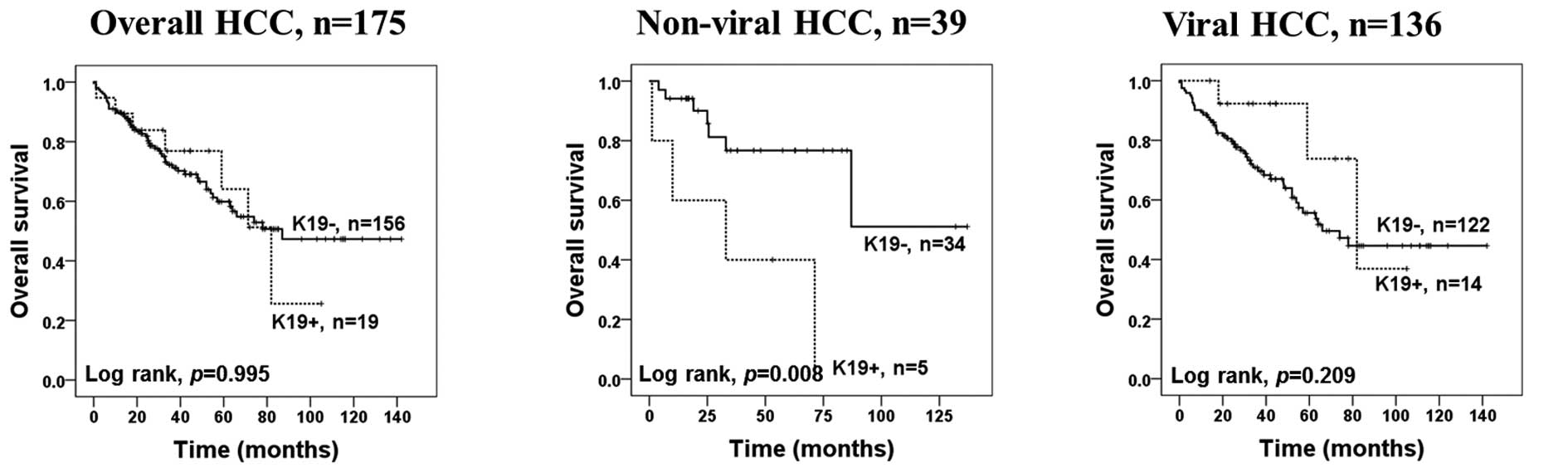
indicators (P=0.002, P=0.001, respectively) (Table III). In non-viral HCC patients,
the median survival of patients with K19-positive HCC was 33
months. K19 expression was significantly associated with patient
survival in non-viral HCC patients in univariate and multivariate
Cox survival analyses (P=0.028, P=0.003, respectively) (Table IV; Fig.
2). No survival difference was observed between patients with
K7-positive and K7-negative HCC.
 | Table IIICox proportional hazard analyses of
factors associated with HCC in 175 patients. |
Table III
Cox proportional hazard analyses of
factors associated with HCC in 175 patients.
| Univariate
model | Multivariate
modela |
|---|
|
|
|
|---|
| No. of patients
(%) | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Albumin (g/dl) |
| ≥3.5 | 151 (86.3) | 2.330 | 1.229–4.418 | 0.010 | 3.061 | 1.575–2.885 | 0.001 |
| <3.5 | 24 (13.7) | | | | | | |
| Intrahepatic
metastasis |
| Absence | 119 (68.0) | 2.690 | 1.627–4.499 | <0.001 | 2.503 | 1.407-4-453 | 0.002 |
| Presence | 56 (32.0) | | | | | | |
| Microvessel
invasion |
| Absence | 75 (42.9) | 1.823 | 1.060–3.136 | 0.030 | | | |
| Presence | 100 (57.1) | | | | | | |
| pT stage |
| 1 | 69 (39.4) | 0.031 | | | | | |
| 2 | 72 (41.1) | 2.214 | 1.206–4.065 | 0.010 | | | |
| 3 and 4 | 34 (19.5) | 2.026 | 1.000–4.107 | 0.050 | | | |
| K19 |
| Negative | 156 (89.1) | 0.997 | 0.454–2.193 | 0.995 | | | |
| Positive | 19 (10.9) | | | | | | |
| K7 |
| Negative | 148 (84.6) | 0.880 | 0.417–1.858 | 0.737 | | | |
| Positive | 27 (15.4) | | | | | | |
| Etiology |
| Viral | 136 (77.7) | 1.265 | 0.658–2.430 | 0.481 | | | |
| Non-viral | 39 (22.3) | | | | | | |
 | Table IVCox proportional hazard analyses of
factors associated with non-viral HCC in 39 patients. |
Table IV
Cox proportional hazard analyses of
factors associated with non-viral HCC in 39 patients.
| Univariate
model | Multivariate
modela |
|---|
|
|
|
|---|
| N (%) | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Albumin (g/dl) |
| ≥3.5 | 34 (87.2) | 2.108 | 0.437–10.173 | 0.353 | 9.148 | 1.201–69.698 | 0.033 |
| <3.5 | 5 (12.8) | | | | | | |
| Intrahepatic
metastasis |
| Absence | 30 (76.9) | 3.618 | 0.988–13.248 | 0.052 | 8.560 | 1.707–42.933 | 0.009 |
| Presence | 9 (23.1) | | | | | | |
| pT stage |
| 1 | 16 (41.0) | 0.938 | | | | | |
| 2 | 14 (35.9) | 0.772 | 0.183–3.261 | 0.725 | | | |
| 3 and 4 | 9 (23.1) | 0.958 | 0.221–4.181 | 0.958 | | | |
| K19 |
| Negative | 34 (87.2) | 4.713 | 1.327–16.738 | 0.028 | 10.047 | 2.218–45.506 | 0.003 |
| Positive | 5 (12.8) | | | | | | |
| K7 |
| Negative | 32 (82.1) | 2.257 | 0.564–9.038 | 0.250 | | | |
| Positive | 7 (17.9) | | | | | | |
Discussion
Keratins are cytoskeletal intermediate filaments
present in both normal and malignant epithelial cells (13). In normal livers, hepatocytes express
K8 and K18, while biliary cells also contain K7 and K19 (14,15).
As this keratin phenotype is considered to be preserved during
neoplastic transformation, HCC would be expected to express K8 and
K18, but not K7 or K19 (14,15).
K19 is a marker of biliary cells and hepatic progenitor cells,
while K7 is expressed in intermediate hepatocyte-like cells,
biliary cells and progenitor cells (16). Several studies have demonstrated
that K19 expression in HCCs and K19-positive HCCs have a high
recurrence and metastasis rates, which are associated with a poor
prognosis (5–8). Although the clinical significance of
K19-positive HCC appears to be established, the mechanism
underlying the development of K19-positive HCC remains unclear. The
presence of K19-positive tumor cells in HCC may be explained by two
distinct mechanisms: either the cell of origin is a progenitor cell
or the tumors dedifferentiated and acquired the K19 phenotype
during carcinogenesis. When progenitor cells are the cells of
origin of K19-positive HCCs, it is expected that the premalignant
precursor lesions consist of K19-positive cells and their
progeny.
We found for the first time that K19 expression was
extremely rare in DNs, but appeared in the distinctly nodular type
of small HCC. Small HCC is defined as a carcinoma that measures ≤2
cm in diameter. There are two types of small HCCs; vaguely nodular
and distinctly nodular. Vaguely nodular HCC is early HCC, and
distinctly nodular HCC is small progressed HCC (3,4).
Contrary to the hypothesis that K19-positive HCC originates from
hepatic progenitor cells, our results suggest that HCCs could
obtain the K19 phenotype during a small progressed stage of HCC.
Our findings are consistent with the results of Libbrecht et
al who reported the absence of K19 expression in small cell
dysplastic foci, the earliest premalignant lesions known thus far
in human HCC, although these authors suggested that differentiating
putative progenitor cells gives rise to small cell dysplasia foci
(16). The keratin expression
pattern in HCC might not always be preserved, and aberrant K19
and/or K7 expression is observed during HCC dedifferentiation
(17). Furthermore, in a study of
HCC cell lines with different metastatic potentials established
from the same parent cell line, K19 demonstrated a consistently
increased expression from a low metastatic to a high metastatic
cell line (18). Taken together,
our data suggest that K19 expression is likely an acquired feature
of carcinoma cells during HCC progression in certain HCCs, although
the presence of cancer stem cells may be another contributor to
K19-positive HCC.
Previous studies have demonstrated that epidermal
growth factor (EGF) and hepatocyte growth factor (HGF) are potent
inducers of the biliary phenotype in rat hepatocytes (19,20).
Yoneda et al have reported that activation of the EGF
receptor signaling pathway is associated with the development of
K19-positive HCC, and that the EGF-induced increase in the growth
abilities of HCC account for the poor patient prognosis (21). Transarterial chemoembolization
(TACE) induces a more aggressive type of HCC characterized by a
biliary phenotype (22,23). Nishihara et al have suggested
the HCC with biliary phenotype originates from the adaptive
transformation of the unaffected or TACE-resistant tumor cell
population (24). It was initially
reported that the cancer stem cell model is essentially synonymous
with the hierarchy model of carcinogenesis (25). However, stemness-related marker
expression exists as a functional phenotype in the stochastic
(dedifferentiation) model and could be demonstrated by any member
of the malignant population in the presence of the appropriate
endogenous and exogenous factors (26). Taken together, K19 expression might
be induced in specific HCCs after being stimulated by a certain
type of growth factor or under certain growth conditions,
accounting for the development of K19-positive HCCs.
This study has demonstrated that K19 and K7
expression were observed in 11 and 24% of our 79 patients with HCC,
respectively. This expression proportion was similar to earlier
studies reporting K19 or K7 in 10–50% of HCCs, despite geographic
differences, different carcinogens and genetic backgrounds
(5–7,9,27–29).
In this study, we found that the K19 and K7 positivity rate was
highest in small HCCs and decreased in advanced large HCCs. The
reason for the decreased K19 and/or K7 immunoreactivity in patients
with advanced HCC is unclear. The majority of the small HCCs could
be examined completely for immunohistochemical staining, while only
a small part of the large HCCs were examined, which may be a
possible explanation for this finding. The finding that a large
proportion of the K19 and/or K7-positive HCCs demonstrated a
heterogeneous or focal staining pattern supports this hypothesis.
Discrepancies in the immunohistochemical results for keratin
expression between representative whole tissue sections and TMA in
the present study may also have been caused by a similar reason. As
K19 and/or K7-positive HCC cells were not diffusely present in this
study, the expression frequencies in the 3 mm TMA core may have
been underestimated. This hypothesis was supported by a recent
study which demonstrated that K19 expression in biopsy specimens
for HCC taken prior to radiofrequency ablation is extremely low
(4.1%) (8). Assessment of a
biomarker in a small TMA tissue core may not accurately reflect the
assessment that would be obtained from a whole section analysis due
to intratumoral heterogeneity (30). Similar to a number of other solid
tumors, HCCs are characterized by a high degree of tumor cell
heterogeneity. Our results demonstrated that the intratumoral
heterogeneity and the size of tissue sections have a great impact
on the results of K19 and K7 expression in HCC.
We found that K19 expression in HCC was
significantly associated with the histological grade and serum AFP
level. These findings are consistent with observations reported by
other studies (7,18,28–30). A
correlation between K19 expression and high AFP levels and
high-grade HCC has been demonstrated by Yuan et al (29). Similarly, serum AFP concentration in
patients with K19-positive HCC increases along with K19
immunostaining grades, suggesting a correlation between serum AFP
and K19 expression (28). Uenishi
et al also demonstrated that K19 expression correlates with
poor differentiation of HCC (31).
Since K19 expression was associated with high tumor grade and high
AFP levels in this study, the results of K19 as a prognostic factor
for HCC is reasonable. The prognosis of patients with K19-positive
HCC is considered to be worse than those with pure HCC (9,21,28).
In this study, K19 expression in HCC was not an independent
predictor of the overall rate of survival in all patients with HCC.
However, we found that K19 expression was associated with poor
survival in patients with non-viral HCC. Based on the limited
number of cases, insufficient follow-up periods, different
etiologies and genetic backgrounds of HCC, a definite conclusion on
the effect of K19 on the prognosis of HCC patients could not be
reached. A longer term follow-up with a larger cohort and strictly
categorized tumors is required to adequately define the clinical
and biological behavior of this tumor.
In conclusion, our study indicates that K19
expression is rare in DNs, well-known precancerous HCC lesions, but
occurs predominantly in distinctly nodular small HCC. Although we
cannot conclude whether the K19-positive HCCs originated from
pre-existing cancer stem cells in HCC or from dedifferentiation of
HCC cells, the present results suggest that the K19 phenotype might
be an acquired feature of carcinoma cells during HCC
progression.
Acknowledgements
This study was supported by the National Research
Foundation of Korea Grant funded by the Korea government (No.
2011-0028223) and by the grant of Post-Doc. Program, Chonbuk
National University (2011).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park YN: Update on precursor and early
lesions of hepatocellular carcinomas. Arch Pathol Lab Med.
135:704–715. 2011.PubMed/NCBI
|
|
4
|
Theise ND, Curado MP, Franceschi S,
Hytiroglou P, Kudo M, Park YN, Sakamoto M, Torbenson M and Wee A:
Hepatocellular carcinoma. WHO Classification Of Tumours Of The
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND:
4th edition. IARC Press; Lyon: pp. 205–216. 2010
|
|
5
|
Durnez A, Verslype C, Nevens F, Fevery J,
Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V and Roskams
T: The clinicopathological and prognostic relevance of cytokeratin
7 and 19 expression in hepatocellular carcinoma. A possible
progenitor cell origin. Histopathology. 49:138–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuang PY, Zhang JB, Zhu XD, Zhang W, Wu
WZ, Tan YS, Hou J, Tang ZY, Qin LX and Sun HC: Two pathologic types
of hepatocellular carcinoma with lymph node metastasis with
distinct prognosis on the basis of CK19 expression in tumor.
Cancer. 112:2740–2748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee
JE, Cho JY, Yoo JE, Choi JS and Park YN: Human hepatocellular
carcinomas with ‘Stemness’-related marker expression: keratin 19
expression and a poor prognosis. Hepatology. 54:1707–1717.
2011.
|
|
8
|
Tsuchiya K, Komuta M, Yasui Y, Tamaki N,
Hosokawa T, Ueda K, Kuzuya T, Itakura J, Nakanishi H, Takahashi Y,
Kurosaki M, et al: Expression of keratin 19 is related to high
recurrence of hepatocellular carcinoma after radiofrequency
ablation. Oncology. 80:278–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi
GM, Zhang BH, Wu WZ, Shi YH, Wu B, Yang GH, Ji Y and Fan J: High
expression levels of putative hepatic stem/progenitor cell
biomarkers related to tumour angiogenesis and poor prognosis of
hepatocellular carcinoma. Gut. 59:953–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersen JB, Loi R, Perra A, Factor VM,
Ledda-Columbano GM, Columbano A and Thorgeirsson SS:
Progenitor-derived hepatocellular carcinoma model in the rat.
Hepatology. 51:1401–1409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: 2010
|
|
12
|
Choi HN, Bae JS, Jamiyandorj U, Noh SJ,
Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG and Moon WS: Expression
and role of SIRT1 in hepatocellular carcinoma. Oncol Rep.
26:503–510. 2011.PubMed/NCBI
|
|
13
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai YS, Thung SN, Gerber MA, Chen ML and
Schaffner F: Expression of cytokeratins in normal and diseased
livers and in primary liver carcinomas. Arch Pathol Lab Med.
113:134–138. 1989.PubMed/NCBI
|
|
15
|
Johnson DE, Herndier BG, Medeiros LJ,
Warnke RA and Rouse RV: The diagnostic utility of the keratin
profiles of hepatocellular carcinoma and cholangiocarcinoma. Am J
Surg Pathol. 12:187–197. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Libbrecht L, Desmet V, Van Damme B and
Roskams T: The immunohistochemical phenotype of dysplastic foci in
human liver: correlation with putative progenitor cells. J Hepatol.
33:76–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Eyken P, Sciot R, Paterson A, Callea
F, Kew MC and Desmet VJ: Cytokeratin expression in hepatocellular
carcinoma: an immunohistochemical study. Hum Pathol. 19:562–568.
1988.PubMed/NCBI
|
|
18
|
Ding SJ, Li Y, Tan YX, Jiang MR, Tian B,
Liu YK, Shao XX, Ye SL, Wu JR, Zeng R, Wang HY, Tang ZY and Xia QC:
From proteomic analysis to clinical significance: overexpression of
cytokeratin 19 correlates with hepatocellular carcinoma metastasis.
Mol Cell Proteomics. 3:73–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Limaye PB, Bowen WC, Orr AV, Luo J, Tseng
GC and Michalopoulos GK: Mechanisms of hepatocyte growth
factor-mediated and epidermal growth factor-mediated signaling in
transdifferentiation of rat hepatocytes to biliary epithelium.
Hepatology. 47:1702–1713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishikawa Y, Doi Y, Watanabe H, Tokairin
T, Omori Y, Su M, Yoshioka T and Enomoto K: Transdifferentiation of
mature rat hepatocytes into bile duct-like cells in vitro. Am J
Pathol. 166:1077–1088. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoneda N, Sato Y, Kitao A, Ikeda H,
Sawada-Kitamura S, Miyakoshi M, Harada K, Sasaki M, Matsui O and
Nakanuma Y: Epidermal growth factor induces cytokeratin 19
expression accompanied by increased growth abilities in human
hepatocellular carcinoma. Lab Invest. 91:262–272. 2011. View Article : Google Scholar
|
|
22
|
Zen C, Zen Y, Mitry RR, Corbeil D,
Karbanová J, O’Grady J, Karani J, Kane P, Heaton N, Portmann BC and
Quaglia A: Mixed phenotype hepatocellular carcinoma after
transarterial chemoembolization and liver transplantation. Liver
Transpl. 17:943–954. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ravaioli M, Grazi GL, Ercolani G,
Fiorentino M, Cescon M, Golfieri R, Trevisani F, Grigioni WF,
Bolondi L and Pinna AD: Partial necrosis on hepatocellular
carcinoma nodules facilitates tumor recurrence after liver
transplantation. Transplantation. 78:1780–1786. 2004. View Article : Google Scholar
|
|
24
|
Nishihara Y, Aishima S, Kuroda Y, Iguchi
T, Taguchi K, Asayama Y, Taketomi A, Kinukawa N, Honda H and
Tsuneyoshi M: Biliary phenotype of hepatocellular carcinoma after
preoperative transcatheter arterial chemoembolization. J
Gastroenterol Hepatol. 23:1860–1868. 2008. View Article : Google Scholar
|
|
25
|
Shackleton M, Quintana E, Fearon ER and
Morrison SJ: Heterogeneity in cancer: cancer stem cells versus
clonal evolution. Cell. 138:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bomken S, Fiser K, Heidenreich O and
Vormoor J: Understanding the cancer stem cell. Br J Cancer.
103:439–445. 2010. View Article : Google Scholar
|
|
27
|
Aishima S, Nishihara Y, Kuroda Y, Taguchi
K, Iguchi T, Taketomi A, Maehara Y and Tsuneyoshi M: Histologic
characteristics and prognostic significance in small hepatocellular
carcinoma with biliary differentiation: subdivision and comparison
with ordinary hepatocellular carcinoma. Am J Surg Pathol.
31:783–91. 2007. View Article : Google Scholar
|
|
28
|
Lu XY, Xi T, Lau WY, Dong H, Zhu Z, Shen
F, Wu MC and Cong WM: Hepatocellular carcinoma expressing
cholangiocyte phenotype is a novel subtype with highly aggressive
behavior. Ann Surg Oncol. 18:2210–2217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan RH, Jeng YM, Hu RH, Lai PL, Lee PH,
Cheng CC and Hsu HC: Role of p53 and β-catenin mutations in
conjunction with CK19 expression on early tumor recurrence and
prognosis of hepatocellular carcinoma. J Gastrointest Surg.
15:321–329. 2011.
|
|
30
|
Simon R and Sauter G: Tissue microarrays
for miniaturized high-throughput molecular profiling of tumors. Exp
Hematol. 30:1365–1372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uenishi T, Kubo S, Yamamoto T, Shuto T,
Ogawa M, Tanaka H, Tanaka S, Kaneda K and Hirohashi K: Cytokeratin
19 expression in hepatocellular carcinoma predicts early
postoperative recurrence. Cancer Sci. 94:851–857. 2003. View Article : Google Scholar : PubMed/NCBI
|