Introduction
Esophageal carcinoma is a common cancer in China.
Traditional surgery or radiotherapy alone is not sufficiently
effective and the median survival period is 9 months. In 1992,
Herskovic et al (1) reported
a phase III clinical trial using radiotherapy (50 Gy/25 fx) and
concurrent chemotherapy (cisplatin combined with 5-fluorouracil) to
treat patients who had been diagnosed with local esophageal
carcinomas and who had not yet undergone surgery. It was shown that
the survival of patients in the radiotherapy plus chemotherapy
group was markedly longer compared with that of patients in the
radiotherapy group (1). al-Sarraf
et al (2) and Cooper et
al (3) further confirmed the
advantages of the radiotherapy plus chemotherapy in prolonging
survival by showing that the 3- to 10-year survival ratios of the
radiotherapy plus chemotherapy group were 20–30%. However, the
3-year survival ratio of the radiotherapy alone group was 0. To
further improve the efficacy of radiotherapy plus chemotherapy, a
phase II clinical trial was performed (4) using radiotherapy plus chemotherapy
with increased radiotherapy and chemotherapy doses and increased
chemotherapy cycles. Although certain patients did not complete the
treatments due to severe toxic effects, the results showed that no
significant efficacy improvements were found. In another phase III
trial (5), using the radiotherapy
plus chemotherapy regimen as the control, it was found that even
when the radiotherapy dose was increased to 64.8 Gy, the local
curing efficacy, median survival period and 2-year survival rate
were similar to those of the control group. Certain radiotherapy
plus chemotherapy regimens have been reported to be used to treat
esophageal carcinomas (6–17). However, these trials show that
efficacy cannot be improved by increasing the doses of radiotherapy
and cisplatin with 5-fluorouracil.
In this study, we performed radiotherapy in
combination with weekly nedaplatin plus docetaxel chemotherapy. The
results of this study showed that our chemotherapy regimen is able
to significantly increase the effect of radiotherapy and reduce the
toxicity effects of chemotherapy. Chemotherapy with platinum has
better curative effects, lower toxicity and largely increases the
effect of radiotherapy. The dose intensity-modulated radiotherapy
(IMRT) reduces the irradiation volume, so the target area is
smaller than in the conventional method.
Materials and methods
Patients
A total of 86 patients with esophageal carcinomas
were enrolled in this study and their clinical characteristics are
shown in Table I. Patient consent
was obtained, and the study was approved by the Medical Ethics
Committee of the Third Hospital of Wenzhou Medical College (Ruian,
China).
 | Table IComparison of clinical characteristics
of 86 patients with esophageal carcinomas. |
Table I
Comparison of clinical characteristics
of 86 patients with esophageal carcinomas.
| Characteristic | EX group (n=44) | CON group (n=42) |
|---|
| Age (years) |
| <60 | 9 (20) | 7 (17) |
| 60–65 | 25 (57) | 22 (52) |
| 66–70 | 10 (23) | 13 (31) |
| Gender |
| Female | 5 (11) | 4 (9) |
| Male | 39 (89) | 38 (91) |
| PS |
| 0–1 | 29 (65) | 31 (74) |
| 2 | 15 (35) | 11 (26) |
| Weight loss in 6
months |
| <5% | 11 (25) | 11 (26) |
| ≥5% | 33 (75) | 31 (74) |
| Difficulty in
swallowing |
| No | 3 (7) | 3 (7) |
| Mild to
moderate | 36 (82) | 35 (83) |
| Moderate | 5 (11) | 4 (10) |
| Lesion length
(cm) |
| <5 | 8 (17) | 7 (17) |
| 5–7 | 15 (35) | 15 (36) |
| 7–10 | 21 (48) | 20 (47) |
| Lesion sites |
| Neck | 7 (16) | 7 (17) |
| Upper thoracic | 12 (27) | 12 (29) |
| Middle thoracic | 21 (48) | 21 (49) |
| Lower thoracic | 4 (9) | 2 (5) |
| UICC/02 stage |
| T1N0M0 | 1 (2) | 2 (5) |
| T N0M0 | 7 (16) | 7 (17) |
| T4N0-1M0 | 11 (25) | 10 (24) |
| T1-3N1M0 | 25 (57) | 23 (54) |
| Type based on
X-ray |
| Medullary | 37 (83) | 35 (83) |
| Fungoid | 3 (7) | 3 (7) |
| Ulcerative | 2 (5) | 3 (7) |
| Other | 2 (5) | 1 (3) |
| CT examination |
| Symmetric
growth | 11 (25) | 12 (29) |
| Eccentric
growth | 33 (75) | 30 (71) |
| Tumor invasion |
| Invasion | 15 (35) | 15 (36) |
| No marked
invasion | 29 (65) | 27 (64) |
Therapeutic schedule of the experimental
group
IMRT was performed. During radiotherapy, nedaplatin
plus docetaxel chemotherapy was conducted once weekly using the CMS
radiotherapy treatment planning system (CMS Xio 4.40). The
radiation energy was 6 mV and the radiation dose was
intensity-modulated. The prescription dose was 95% of the planning
target volume (PTV1) DT 60 Gy/5 times/week (2 Gy/time). The
uniformity of the clinical target volume (CTV) dose was between 95
and 105%. PTV was in the 93 to 107% dose range. The chemotherapy
regimens were as follows: nedaplatin (25 mg/m2) and
docetaxel (20 mg/m2) were administered by intravenous
drip at 1, 8, 15, 22, 29 and 36 days following radiotherapy.
Therapeutic schedule of the control
group
Conventional radiotherapy was performed. During
radiotherapy, 5-fluorouracil plus cisplatin chemotherapy was
conducted. Radiotherapy regimens were conducted with 2 Gy
administered every day for 5 consecutive days in one week. The
regional radiotherapy dose was 30 Gy/5 times/week and the intensive
radiotherapy in the shrinking field was 20 Gy/5 times/week. The
total dose was 50 Gy/25 times/5 weeks. The dose gradient changes in
the treatment tissue were <10% and the maximum permissible dose
of the spinal cord (in accordance with 2 cm below the beam upper
bound) was 42.5 Gy. The dose in the normal lung tissues 2 cm
outside the gross tumor volume (GTV) did not exceed 25 Gy. The
chemotherapy regimens were as follows: 5-fluorouracil (1,000
mg/m2/day) was continuously intravenously injected with
Baxter pump on the first 4 days of the 1st, 5th, 8th and 11th
weeks; cisplatin (75 mg/m2) was delivered at 1 mg/min on
the first day of each course.
Therapeutic effects
The short-term effect evaluation was performed
according to the RECIST solid tumor efficacy evaluation standards,
including a comparison of the enhanced CT and the barium swallow
X-ray radiography prior to and following the treatments. Patients
with complete remission (CR) and partial remission (PR) were
required to receive esophageal biopsy. Both imaging and pathology
complete remission were classified as pathological complete
remission (pCR). Partial pathological remission (pPR) indicated
that imaging showed CR or PR, but the pathological examination
revealed residual tumors. The effective cases that had not received
histopathologic reexamination and the cases with pathological pPR
were classified as non-pathological complete remission (no-pCR).
The long-term effect was evaluated through the 1- and 2-year
survival rates and survival times.
Toxicity evaluation
The toxicity of the anticancer drugs was evaluated
according to the WHO acute toxicity criteria. Radiotherapy toxicity
was evaluated according to the RTOG acute radiation injury grade
standard.
Statistical analysis
Statistical analysis was performed using the
χ2 test for characteristics prior to treatments in the
two groups. The log-rank test was used to compare the survival
curves of the two groups. The relative risk estimation of mortality
was conducted using 95% confidence intervals (95% CIs). Survival
analysis software (SPSS 13.0, SPSS, Inc., Chicago, IL, USA) was
used.
Results
General data
The 86 patients who enrolled in the present study
were divided into two groups, the experimental and control groups
(Table I). The clinical data of
these patients are listed in Table
I and the clinical characteristics of the patients in the
experimental and control groups were comparable.
Toxicity reaction
In the two groups, no treatment-related mortalities
occurred. Grade III and IV toxicities, including radiation
gastritis, weakness and radioactive esophagitis, were found in both
groups. The ratio of the detected grade IV toxic reactions was 4.5%
(2/44) in the experimental group and 21.4% (9/42) in the control
group (χ2=5.49; P<0.05). The ratios of the detected
grade III and grade IV toxicity reaction were 22.7% (10/44) and
42.9% (18/42) in the two groups, respectively (χ2=3.97;
P<0.05). The ratios of life-threatening toxicities (hemorrhage,
perforation, mucosal necrosis and fistula) were 4.5 (2/44) and
23.5% (8/42) in the two groups, respectively (χ2=4.40;
P<0.05). The above data show that the incidence and severity of
the toxicities which occurred in the experimental group were
significantly lower than those in the control group.
Efficacy evaluation
One month following chemotherapy, the 86 patients
were evaluated for short-term efficacy. The survival rates and the
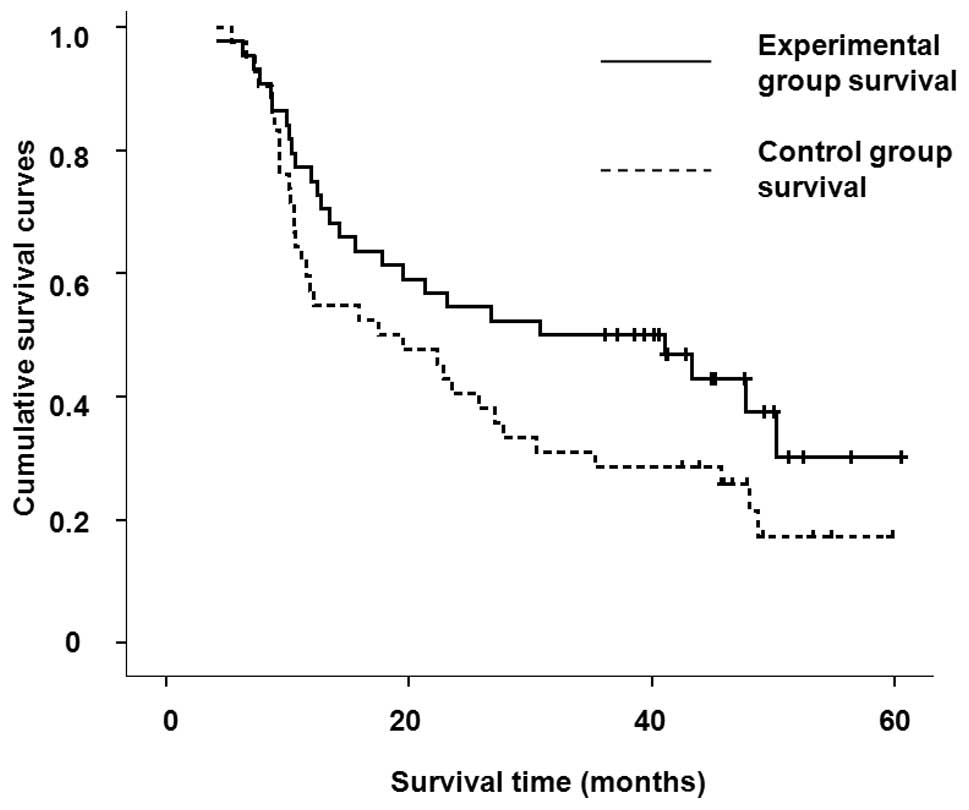
overall survival periods are shown in Table II and the cumulative survival
curves are shown in Fig. 1. The
complete remission rate (47.7 vs. 16.7%; P<0.005), the 1-year
survival rate (75 vs. 52.4%; P<0.05) and the 2-year survival
rate (56.8 vs. 33.3%; P<0.05) in the experimental group were
higher than those in the control group. However, a comparison of
the overall survival and the median survival times in the two
groups indicated that the relative mortality risk was 0.683 in the
experimental group (95% CI, 0.381–1.067). The survival rates in the
two groups were not significantly different. The total efficacy
(84.1 and 64.3%, respectively) in the two groups was not
significantly different (P>0.05).
 | Table IIComparison of curative effects in 86
patients with esophageal carcinomas. |
Table II
Comparison of curative effects in 86
patients with esophageal carcinomas.
| Factor | EX group (n=44) | CON group (n=42) | χ2
value | P-value |
|---|
| Complete remission
(CR) | 47.7% (21/44) | 16.7% (7/42) | 9.44 | <0.005 |
| Partial pathological
remission (CR+PR) | 84.1% (37/44) | 64.3% (30/42) | 2.0 | >0.05 |
| 1-year survival
rate | 75.0% (33/44) | 52.4% (22/42) | 4.77 | <0.05 |
| 2-year survival
rate | 56.8% (25/44) | 33.3% (14/42) | 4.86 | <0.05 |
| Median survival time
(months) | 24.9 (4.1–60.6) | 14.1 (5.4–59.9) | 2.996 | 0.083 |
Of the 86 cases, there were 69 effective cases
(CR+PR), 73.9% (51/69) of which accepted the esophageal endoscopic
biopsy pathology review. Moreover, the curative effects of 25.6%
(22/86) of the patients were pCR and at least 33.7% (29/86) were
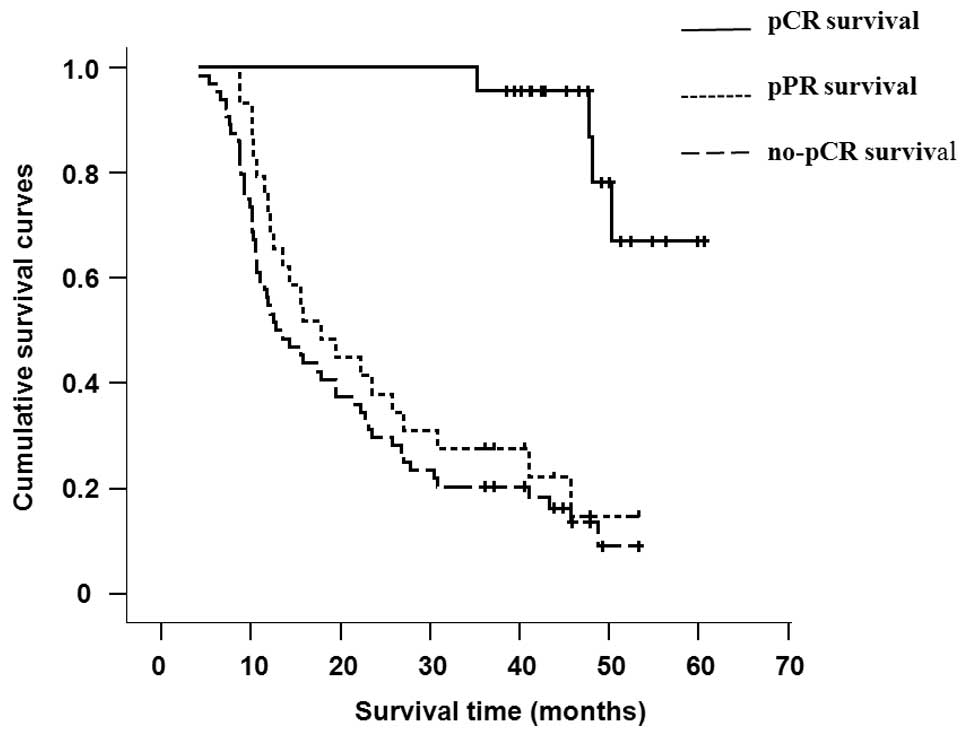
pPR. The survival period of the patients who did not reach clinical
CR or PR was less than 24 months (range, 4.1–23.1). The efficacy
comparison of the pCR and pPR or no-pCR patients (including the 18
patients who had not accepted the pathology review) in the two
groups are shown in Table III and
the cumulative survival curves are shown in Fig. 2. The mean survival period of the pCR
patients was 47.8 months (range, 35.3–60.6), which was markedly
longer than that of the pPR patients (mean, 17.8 months; range,
8.7–53.3 months). Furthermore, the relative risk of pCR mortality
was lower than that of the no-pCR patients, which were 0.088 (95%
CI, 0.029–0.265) and 0.08 (95% CI, 0.028–0.225), respectively. The
above results suggest that the pCR patients, when compared with the
pPR patients (or no-pCR patients), had a significant survival
advantage.
 | Table IIIThe curative effect comparison of
pathological complete or partial remission in patients with
esophageal cancers. |
Table III
The curative effect comparison of
pathological complete or partial remission in patients with
esophageal cancers.
| Factor | pCR (n=22) | pPR (n=29) | No-pCR (n=64) | χ2
value | P-value |
|---|
| 1-year survival
rate | 100 (22/22) | 72.4 (21/29) | 54.7 (35/64) | 7.20a | <0.01a |
| | | | 15.04b | <0.005b |
| 2-year survival
rate | 100 (22/22) | 37.9 (11/29) | 29.7 (19/64) | 21.10a | <0.005a |
| | | | 32.45b | <0.005b |
| Median survival time
(months) | 47.8 (35.3–60.6) | 17.8 (8.7–53.3) | 12.7 (4.1–53.3) | 24.08a | <0.005a |
| | | | 31.54b | <0.005b |
Discussion
Nedaplatin is a new platinum derivative. It has a
similar efficacy to cisplatin while its toxicity to the renal and
gastrointestinal tissues are low. The results of a phase II
clinical trial have shown that the nedaplatin treatment of
esophageal carcinoma has an efficiency of 42.9% and a low toxicity
(18). Docetaxel is another drug
used to treat esophageal cancers. Osaka et al (19) treated 28 cases of recurrent
esophageal carcinoma with nedaplatin (40 mg/m2) and
docetaxel (30 mg/m2) at intervals of 2 weeks and found
that 60.7% of the cases completed the treatment. The results
indicate that the CR rate was 3.6%, PR rate was 35.7%, the median
survival time was 8.5 months and the 1-year survival rate was
15.9%. Additionally, the most common toxicities were leukopenia and
anemia. Non-hematological toxicities were low grade with no
treatment-related mortalities. Matsumoto et al (17) used nedaplatin (30 mg/m2)
and docetaxel (30 mg/m2) to treat 11 patients with
relapsed or refractory esophageal carcinomas. There were no
treatment-related mortalities or grade IV toxicities. Bone marrow
toxicity appeared in three cases, however hematological toxicity
was not observed.
Previous studies (20) have shown that the efficacy of
concurrent chemoradiotherapy methods is not sufficient.
Radiotherapy controls the local diseases, while chemotherapy
affects the lesion outside the radiation field. Chemotherapy plays
a role in radiotherapy-resistant cell populations such as hypoxia
cells and enhances the radiotherapy sensitivity. Radiotherapy and
chemotherapy can mutually reinforce function at the molecular level
to improve survival outcome. Chemotherapy inhibits tumor
proliferation following radiotherapy. No studies concerning the
treatment of early esophageal carcinoma with a weekly dose of
nedaplatin and docetaxel synchronous IMRT were found. Our results
show that, compared with the control group, the complete remission
rate and the 1- and 2-year survival rates were higher in the
experimental group (P<0.05). Moreover, the treatment compliance
in the experimental group was better (P<0.05) and severe and
life-threatening toxicities were significantly reduced (P<0.05).
However, the total efficiency and the median survival time in the
two groups were not statistically different. As shown in the
cumulative survival curve results in Fig. 1, the survival curves of the two
groups are different and the survival rates of the experimental
group were better than those of the control group. However, no
statistically significant difference was found between the two
groups, which may result from the small sample size. The 1- and
2-year survival rates and the median survival time were markedly
increased in patients with complete pathology remission
(P<0.05). Therefore, pCR is a key factor for obtaining better
curative effects. Our findings suggest that patients who are newly
diagnosed with esophageal squamous cell carcinomas that are
confined in the chest may be treated with IMRT and a concurrent
weekly dose of nedaplatin plus docetaxel combination chemotherapy.
Furthermore, the method used in this study has improved the
curative effects and lower toxicity compared with other
regimens.
References
|
1
|
Herskovic A, Martz LK, al-Sarraf M, et al:
Combined chemotherapy and radiotherapy compared with radiotherapy
alone in patients with cancer of the esophagus. N Engl J Med.
326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
al-Sarraf M, Martz K and Herskovic A:
Progress report of combined chemoradiotherapy versus radiotherapy
alone in patients with esophageal cancer: an intergroup study. J
Clin Oncol. 15:277–284. 1997.PubMed/NCBI
|
|
3
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85–01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999.PubMed/NCBI
|
|
4
|
Minsky BD, Neuberg D, Kelsen DP, et al:
Final report of intergroup trial 0122 (ECOG PE-289, RTOG 90–12):
Phase II trial of neoadjuvant chemotherapy plus concurrent
chemotherapy and high-dose radiation for squamous cell carcinoma of
the esophagus. Int J Radiat Oncol Biol Phys. 43:517–523.
1999.PubMed/NCBI
|
|
5
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT O123 (Radiation Therapy Oncology Group 94–05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002.
|
|
6
|
Tanaka Y, Yoshida K, Osada S, Yamaguchi K
and Takahashi T: Docetaxel, nedaplatin, and S-1 (DGS) chemotherapy
for advanced esophageal carcinoma: a phase I dose-escalation study.
Anticancer Res. 31:4589–4597. 2011.PubMed/NCBI
|
|
7
|
Matsutani T, Uchida E, Yoshida H, et al: A
case of advanced esophageal carcinoma with nephrotic syndrome
completely responding to chemotherapy of docetaxel, nedaplatin and
5-fluorouracil. Gan To Kagaku Ryoho. 38:439–441. 2011.(In
Japanese).
|
|
8
|
Guo JF, Zhang B, Wu F, et al: A phase II
trial of docetaxel plus nedaplatin and 5-fluorouracil in treating
advanced esophageal carcinoma. Chin J Cancer. 29:321–324. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tokairin Y, Kumagai Y and Yamazaki S: A
case of postoperative liver metastasis of esophageal cancer remains
in progression free after successfully resected. Gan To Kagaku
Ryoho. 36:2462–2464. 2009.(In Japanese).
|
|
10
|
Noda S, Kubo N, Tanaka H, et al: A case of
advanced esophageal cancer with para-aortic lymph node metastasis
with complete response (CR) to combination chemotherapy including
nedaplatin. Gan To Kagaku Ryoho. 36:2451–2453. 2009.(In
Japanese).
|
|
11
|
Matsutani T, Sasajima K, Maruyama H, et
al: A case of the oldest old patient with advanced esophageal
cancer responding completely to the combination chemotherapy of
docetaxel/5-fluorouracil/nedaplatin with radiation. Nihon
Shokakibyo Gakkai Zasshi. 106:1026–1030. 2009.(In Japanese).
|
|
12
|
Jin J, Xu X, Wang F, et al: Second-line
combination chemotherapy with docetaxel and nedaplatin for
Cisplatin-pretreated refractory metastatic/recurrent esophageal
squamous cell carcinoma. J Thorac Oncol. 4:1017–1021. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsutani T, Sasajima K, Kobayashi Y, et
al: A case of double advanced cancer with esophageal and
hypopharyngeal carcinoma responding completely to combination
chemotherapy of docetaxel/5-fluorouracil and nedaplatin with
radiation. Gan To Kagaku Ryoho. 36:835–838. 2009.(In Japanese).
|
|
14
|
Nakajima Y, Suzuki T, Haruki S, et al: A
pilot trial of docetaxel and nedaplatin in cisplatin-pretreated
relapsed or refractory esophageal squamous cell cancer.
Hepatogastroenterology. 55:1631–1635. 2008.PubMed/NCBI
|
|
15
|
Fujita Y, Hiramatsu M, Kawai M, Sumiyoshi
K, Nishimura H and Tanigawa N: Evaluation of combined docetaxel and
nedaplatin chemotherapy for recurrent esophageal cancer compared
with conventional chemotherapy using cisplatin and 5-fluorouracil:
a retrospective study. Dis Esophagus. 21:496–501. 2008. View Article : Google Scholar
|
|
16
|
Yamazaki K, Hironaka S, Boku N, et al: A
retrospective study of second-line chemotherapy for unresectable or
recurrent squamous cell carcinoma of the esophagus refractory to
chemotherapy with 5-fluorouracil plus platinum. Int J Clin Oncol.
13:150–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsumoto H, Hirai T, Hirabayashi Y, et
al: A feasible study of docetaxel/nedaplatin combined chemotherapy
for relapsed or refractory esophageal cancer patients as a 2nd-line
chemotherapy. Gan To Kagaku Ryoho. 34:725–728. 2007.(In
Japanese).
|
|
18
|
Taguchi T, Wakui A, Nabeya K, et al: A
phase II clinical study of cis-diammine glycolato platinum, 254-S,
for gastro-intestinal cancers. 254-S Gastrointestinal Cancer Study
Group. Gan To Kagaku Ryoho. 19:483–488. 1992.PubMed/NCBI
|
|
19
|
Osaka Y, Takagi Y, Hoshino S, et al:
Combination chemotherapy with docetaxel and nedaplatin for
recurrent esophageal cancer in an outpatient setting. Dis
Esophagus. 19:473–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kleinberg L and Forastiere A:
Chemoradiation in the management of esophageal cancer. J Clin
Oncol. 25:4110–4117. 2007. View Article : Google Scholar : PubMed/NCBI
|