Introduction
Nasopharyngeal carcinoma (NPC), an Epstein-Barr
virus (EBV)-associated cancer, is highly prevalent in southeast
Asia, especially in the Cantonese region around Guangzhou in China
(1). NPC is distinct from other
cancers of the head and neck in terms of its epidemiology,
histopathology, clinical characteristics, methods of treatment and
patterns of failure (2). Although
patients benefit from multi-modal treatment, including
radiotherapy, chemotherapy and biological therapy, limited
improvement in 5-year survival has been achieved over the last few
decades (3). Cancer metastasis and
therapy failure account for the low survival rates in patients with
NPC (4,5). However, the molecular mechanism of the
development and progression of NPC remains poorly understood. It is
of great clinical value to understand the molecular mechanism of
this cancer and identify valuable predictive markers as well as
novel therapeutic strategies.
The Eph protein family constitutes the largest group
of transmembrane receptor tyrosine kinases (RTKs) identified in the
genome. Depending on sequence homology and binding affinity of the
two different types of membrane-anchored ephrin ligands, the Eph
family may be classified as EphA or EphB. Eph kinases have been
extensively studied for their roles in embryonic development where
they transduce key directional signals for cell positioning, axon
guidance, tissue border formation, vascular development and cancer
(6,7).
EphA2, a member of the Eph kinase family, originally
designated as epithelial cell kinase (Eck), is usually expressed at
low levels in adult epithelial tissues (8). Mounting evidence suggests EphA2
overexpression in cell transformation, primary tumor initiation,
progression, angiogenesis and metastasis in a variety of cancer
models (9,10). In non-transformed mammary epithelial
cells, the ectopic overexpression of EphA2 has been shown to result
in a malignant phenotype in in vitro and in vivo
contexts (11). Cancer cells that
overexpress EphA2 exhibit increased motility and invasive
properties, consistent with a pro-metastatic phenotype (12). EphA2 overexpression is prevalent in
numerous solid tumors, including ovarian (13,14),
lung (15), glioblastoma (16), prostate (17), and renal tumors (18). Moreover, a high EphA2 expression is
correlated with disease stage, increased tumor metastasis, and poor
patient survival, suggesting EphA2 to be a candidate prognostic
marker for a variety of human malignancies. However, few published
reports have evaluated the role of EphA2 expression in NPC,
especially its effects on the malignant behavior of NPC in
vitro.
To gain better insight into the clinical relevance
of EphA2 in NPC, the present study was carried out to investigate
EphA2 expression patterns in NPC tissue samples and assess whether
EphA2 expression is correlated with clinicopathological parameters
in patients with NPC. A lentiviral RNAi system was employed to
knock down the expression of EphA2 in NPC 5-8F cells. The roles of
EphA2 in NPC cell invasion and chemotherapy in vitro
conditions were investigated.
Materials and methods
Patients and tissue preparation
A total of 47 fresh undifferentiated NPC (WHO type
III) tissues and 21 cases of non-carcinoma epithelial tissues
(NCET) from the nasopharynx were obtained from the Department of
Otolaryngology of Xiangya Hospital, Central South University,
China, between October 2010 and March 2011. None of the patients
had any history of previous malignancies, or history of
radiotherapy or chemotherapy. Metastases were diagnosed by clinical
examination and imaging evaluation. The clinical stage of all the
patients was classified according to the 2008 NPC staging system of
China. Specimens were snap-frozen immediately and stored in liquid
nitrogen for total protein extraction. The study was approved by
the Research Ethics Committee of Central South University,
Changsha, China. Informed consent was obtained from all patients.
The specimens were handled and anonymized according to ethical and
legal standards. Clinical information for all the NPC samples is
described in Table I.
 | Table ICorrelation between the
clinicopathological characteristics and protein expression of
EphA2. |
Table I
Correlation between the
clinicopathological characteristics and protein expression of
EphA2.
| Characteristics | No. of samples | Relative protein
expression of EphA2 | t-value | P-value |
|---|
| Age (y) |
| <46 | 24 | 0.86±0.37 | 0.959 | 0.343 |
| ≥46 | 23 | 0.76±0.30 | | |
| Gender |
| Male | 37 | 0.79±0.33 | 0.571 | 0.571 |
| Female | 10 | 0.86±0.36 | | |
| Smoking history |
| Smoker | 29 | 0.86±0.35 | 1.423 | 0.162 |
| Nonsmoker | 18 | 0.72±0.29 | | |
| T classification |
| T1 + T2 | 20 | 0.68±0.28 | 2.272 | 0.028 |
| T3 + T4 | 27 | 0.90±0.34 | | |
| Clinical stage |
| I + II | 19 | 0.64±0.21 | 3.069 | 0.004 |
| III + IV | 28 | 0.92±0.36 | | |
| Metastasis |
| Yes | 27 | 0.91±0.34 | 2.616 | 0.012 |
| No | 20 | 0.67±0.28 | | |
| EB-virus
infection |
| Yes | 26 | 0.87±0.34 | 1.338 | 0.188 |
| No | 21 | 0.74±0.32 | | |
Western blotting
The western blot analyses were performed as
described in previous studies (19,20).
In brief, total protein (50 μg/sample) was extracted and separated
by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto PVDF membranes (Millipore,
Bedford, MA, USA). The blotted membranes were incubated with rabbit
poly-antibody against EphA2 (sc-924, dilution 1:400, Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and then secondary antibody
(Beyotime, China), respectively. β-actin protein was also
determined by using specific antibody (Beyotime) as a loading
control.
Cell culture and reagents
NPC 5-8F cells were maintained as monolayer cultures
in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 100
IU/ml penicillin and 100 IU/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2. Exponentially growing cells were
used for the subsequent experiments.
Lentiviral vectors mediated
EphA2-specific shRNA transfection
EphA2-specific shRNA lentiviral particles
(sc-29304-V, Santa Cruz) are pools of concentrated,
transduction-ready viral particles containing three target-specific
constructs that encode 19–25 nt (plus hairpin) shRNA designed to
knock down EphA2 gene expression in human NPC 5-8F cells. 5-8F
cells (2×104) were seeded into 24-well plates and
allowed to grow at 80% confluence. Medium containing EphA2 or
control shRNA lentiviral particles was added to these cells. Twelve
hours later, the original medium was replaced with fresh complete
medium and the cells were subjected to silencing efficiency assay
via western blotting 72 h post-infection.
Cell proliferation assay
A Cell Counting Kit-8 (Beyotime) was employed to
draw cell growth curves according to the manufacturer's
instructions. In brief, cells at a concentration of
3.0×103/well were cultured in triplicate in 96-well
plates with 10% FBS at 37°C in humidifed 5% CO2
atmosphere for various periods and exposed to fresh media every
other day. At 24, 48, 72 and 96 h post-transfection, cell
proliferation was measured by absorbance at an optical density of
490 nm.
Cell apoptosis and cell cycle analysis by
flow cytometry
Fluorescence-activated cell sorting analysis was
carried out. Following incubation in serum-free medium for 24 h,
the cells were washed with PBS at 4°C twice and their concentration
was altered to 1×106/ml. Annexin V/Cy5 and propidium
iodide were added for incubation for 30 min. Fluorescence-activated
cell sorting analysis was carried out using the FACS Calibur
instrument (Becton Dickinson, Franklin Lakes, NJ, USA).
Matrigel invasion assay
The invasiveness of the transfected cells was
evaluated in 24-well transfected chambers (Costar, Cambridge, MA,
USA) according to the manufacturer's instructions. Briefly,
transwell with an 8 μm diameter pore membrane was coated with 200
μl matrigel at 200 μg/ml and incubated overnight. Cells
(3×104) in 100 μl of serum-free medium were seeded into
the upper chamber of the transwell and the lower chamber was filled
with 0.8ml RPMI-1640 containing 12% FBS to induce chemotaxis.
Following 48 h of incubation at 37°C in a humidifed 5%
CO2 atmosphere, the cells were fixed in methanol and
stained with crystal violet, and the cells that invaded through the
pores to the lower surface of the filter were counted under a
microscope. Three invasion chambers were used per condition. The
values obtained were calculated by averaging the total number of
cells from three filters.
Cell viability assay
Cells were seeded in 96-well plates. Following
overnight culture, they were exposed to various concentrations of
paclitaxel (0.001, 0.01, 0.1, 1.5, 10, 20 and 30 nM) for 48 h in a
CO2 incubator. CCK8 assay was used to detect the
chemo-sensitivity of cells. Absorbance values were expressed as
percentages relative to the controls, and the concentrations
resulting in 50% inhibition of cell growth (IC50 values)
were calculated.
Statistical analysis
Statistical analysis was performed with SPSS 17.0
software. Results of quantitative data in this study were shown as
the mean ± SD. Statistical differences between groups were compared
using the two-tailed Student's t-test. P<0.05 was considered
statistically significant.
Results
Overexpression of EphA2 protein in NPC
and its correlation with clinicopathological parameters
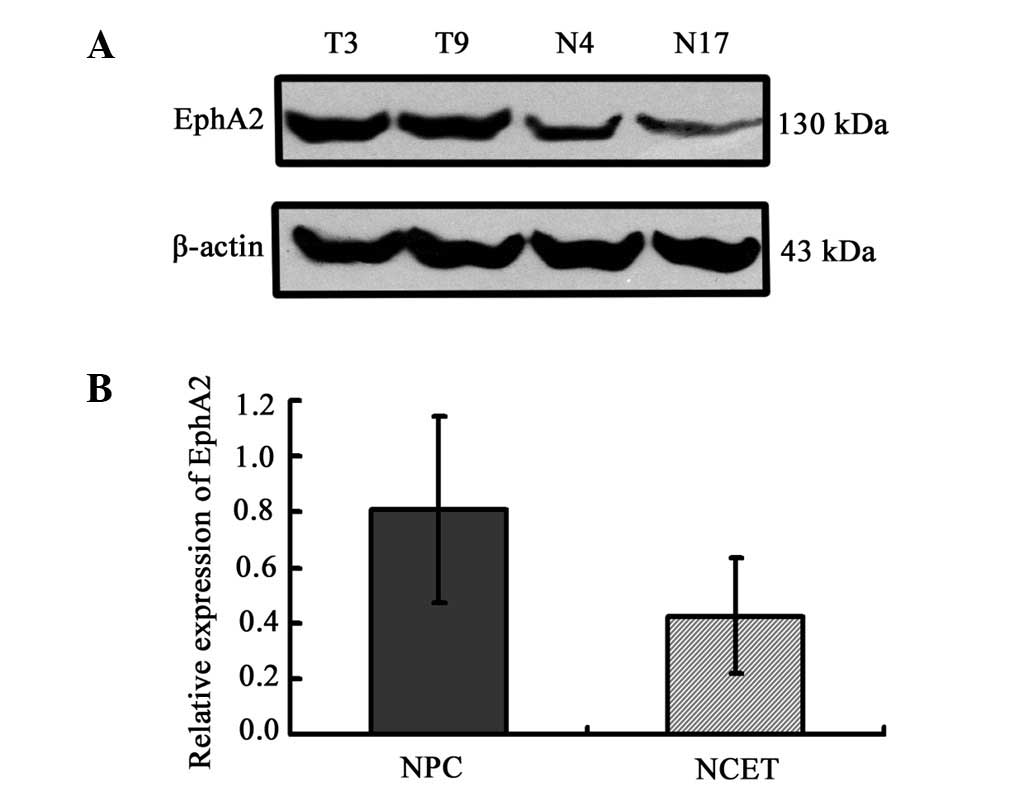
The expression pattern of EphA2 protein was
initially assayed in 47 fresh NPC specimens and 21 non-carcinoma
epithelial tissue (NCET) specimens. Western blot analysis clearly
revealed that the expression of EphA2 protein in NPC tissues was
significantly higher than that in NCET (0.81±0.33 vs. 0.43±0.21;
t=4.841, P<0.001) (Fig. 1).
Furthermore, the association between the expression of EphA2
protein and clinicopathological characteristics of NPC was explored
using the Student's t-test. EphA2 overexpression was significantly
associated with NPC T classification (P=0.028), advanced clinical
stages (P=0.004) and lymph node metastasis (P=0.012), respectively
(Table I). However, no significant
relationship existed between protein level of EphA2 and variables
such as age (P=0.343), gender (P=0.571), smoking history (P=0.162)
and the status of EB-virus infection (P=0.188).
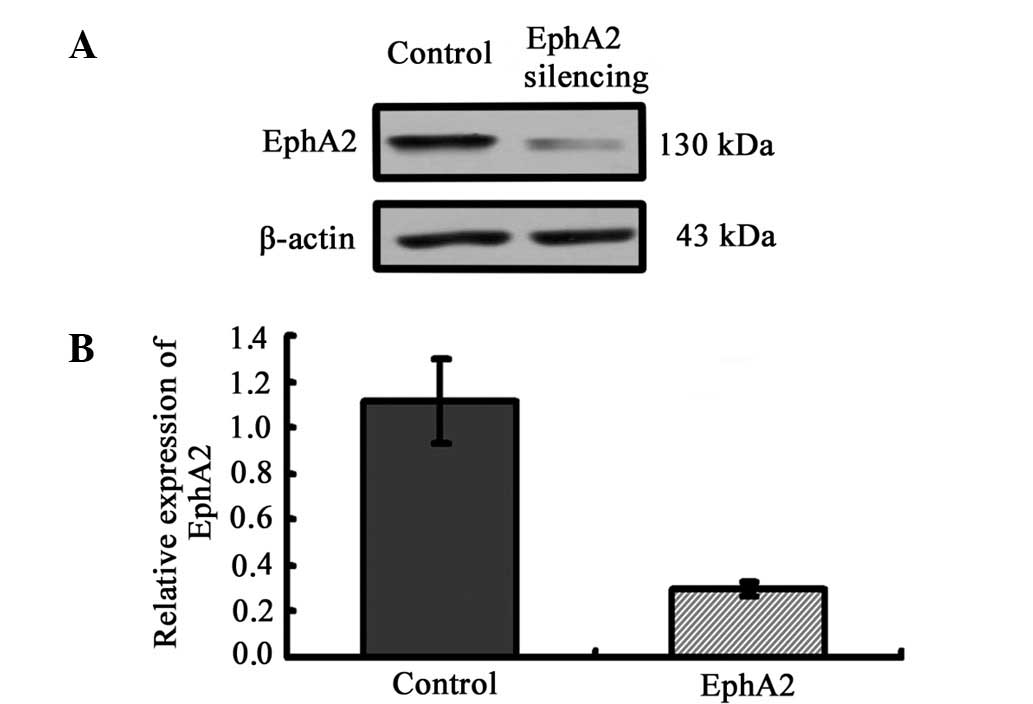
EphA2 knockdown by EphA2 shRNA lentiviral
particles in NPC 5-8F cells
To clarify the correlation of the expression of
EphA2 protein and the biological process of NPC, we employed the
lentivirus-delivered EphA2 or control shRNA to inhibit the
expression of EphA2 in NPC 5-8F cells. Western blotting was carried
out to assess the inhibition efficiency of EphA2 protein at 72 h
following infection. Lentivirus-delivered EphA2 shRNA successfully
resulted in >70% inhibition efficiency in protein expression
(Fig. 2).
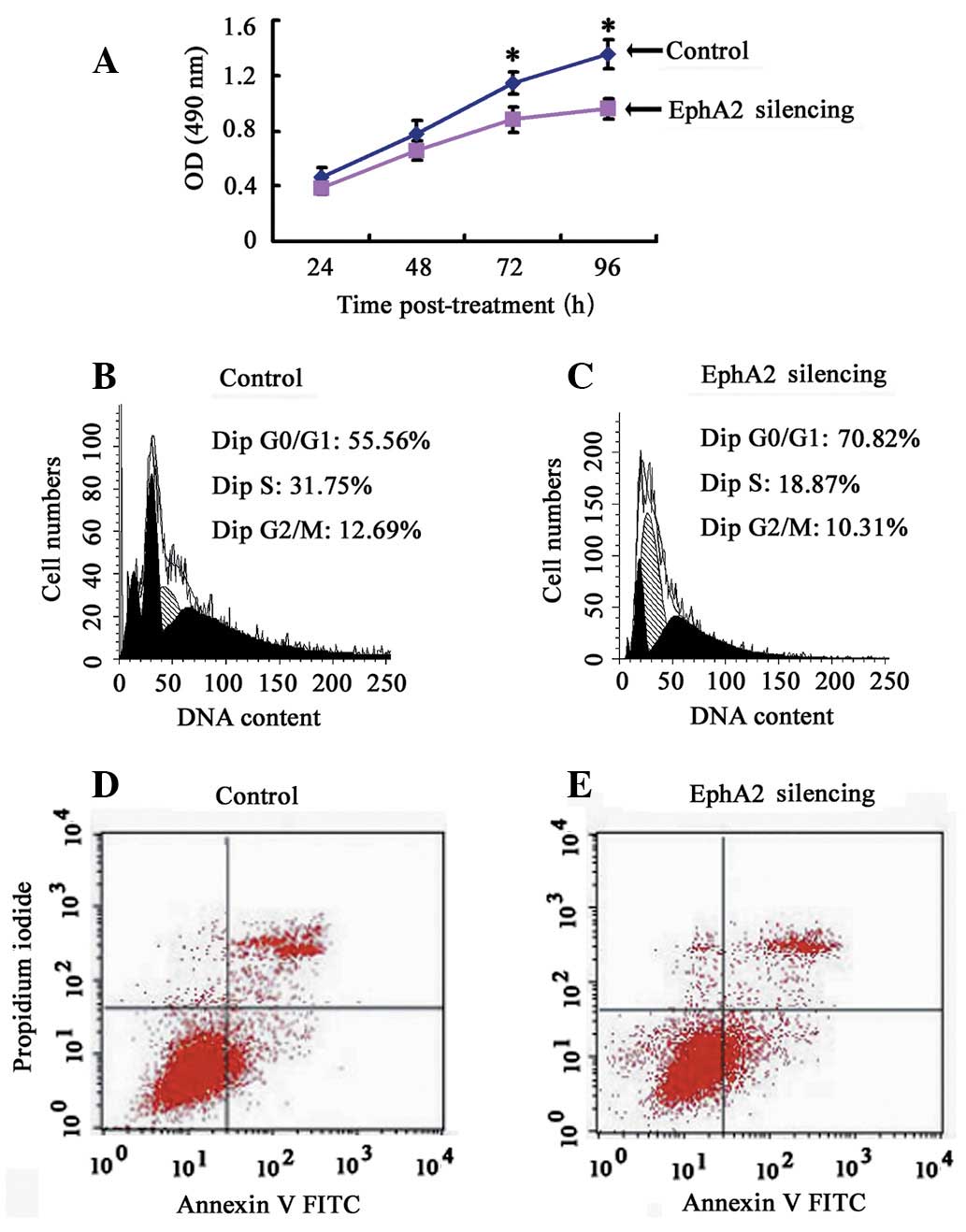
EphA2 knockdown inhibits the
proliferation of NPC 5-8F cells by inducing cell cycle arrest in
the G0/G1 phase in vitro
To clarify the effect of EphA2 on the proliferation
of NPC cells in vitro, a CCK-8 assay was performed and cell
growth curve was generated. EphA2 knockdown inhibited the
proliferation of NPC 5-8F cells in vitro, indicating that
the expression of EphA2 affects the growth of NPC cells (Fig. 3A). To elucidate the mechanism of
EphA2-mediated cell growth inhibition in NPC cells, flow cytometry
was carried out to monitor cell cycle and apoptotic changes. The
results demonstrated that, when compared with the control group,
the percentage of NPC cells in the EphA2 silencing group in the
G0/G1 phase was significantly increased (EphA2 silencing group:
74.38±3.71 vs. the Control group: 53.81±2.19) (P<0.05), whereas
the percentage of cells in the S phase (EphA2 silencing group:
17.89±2.21 vs. the Control group: 30.41±1.55) and G2/M phase (EphA2
silencing group: 7.72±2.24 vs. the Control group: 15.80±3.72) were
obviously decreased (P<0.05) (Fig.
3B and C). However, there was no significant difference in
apoptotic rate (EphA2 silencing group: 7.01±1.06 vs. the Control
group: 5.78±1.14,) (P>0.05) (Fig. 3D
and E). These results demonstrate that knockdown of EphA2
induces G0/G1 phase arrest in NPC 5-8F cells, resulting in the
growth inhibitory properties of NPC cells.
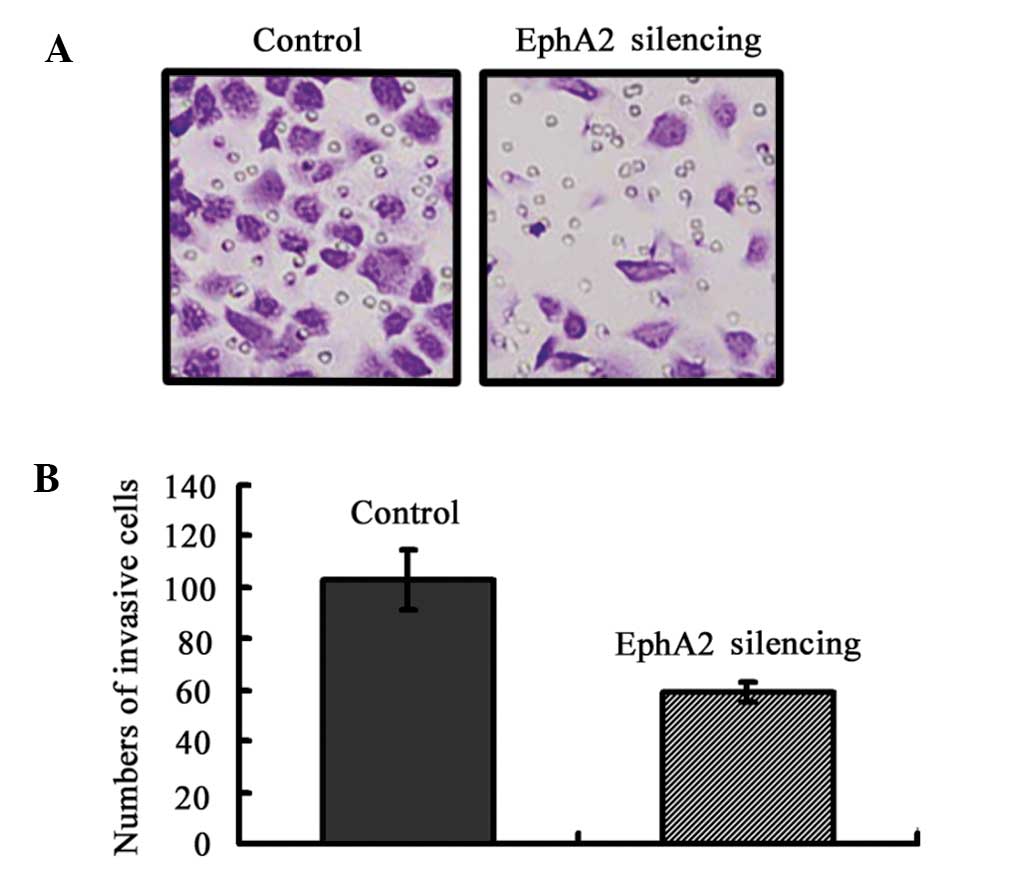
EphA2 knockdown inhibits the invasion of
NPC 5-8F cells in vitro
To evaluate the function of EphA2 on NPC cell
invasion, Matrigel invasion chambers were utilized. Inhibited EphA2
expression led to a significantly decreased invasive ability of NPC
5-8F cells (EphA2 silencing group: 59±4 vs. the Control group
103±12, P=0.004) (Fig. 4). These
results clearly demonstrate that EphA2 are crucial in mediating
cell invasion in NPC, which is a key determinant of NPC malignant
progression and metastasis.
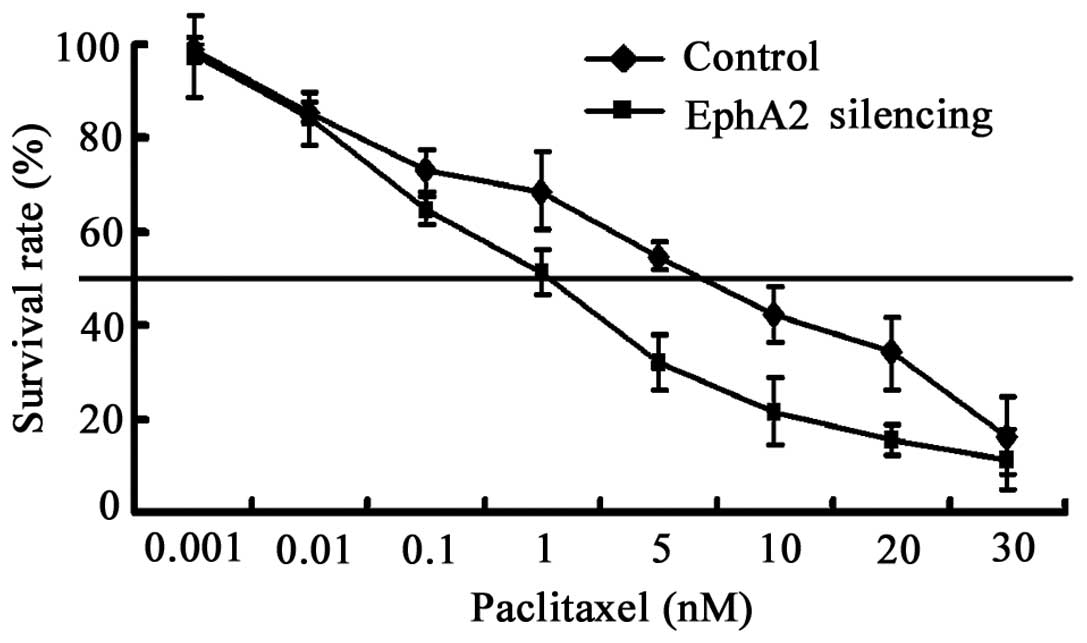
EphA2 knockdown sensitizes NPC 5-8F cells
to paclitaxel in vitro
Chemotherapy is vital for the treatment of NPC,
particularly neoadjuvant chemotherapy (21). In this study, we tested whether the
downregulation of EphA2 was capable of sensitizing the NPC cell
line 5-8F to paclitaxel, a widely used chemotherapeutic agent in
NPC. 5-8F cells were exposed to paclitaxel following transfection
with EphA2-shRNA for 48 h. Cell viability was evaluated using CCK-8
assays. The IC50 value of cells transfected with
EphA2-shRNA and control-shRNA were 1.6 and 5.6 nM, respectively
(Fig. 5). The results show that the
sensitivity of 5-8F to paclitaxel is enhanced by inhibiting the
expression of EphA2 3.5-fold.
Discussion
Our previous investigations have clearly
demonstrated EphA2 overexpression in human laryngeal and
hypopharyngeal squamous cell carcinoma, and EphA2 regulation of the
growth and cervical lymph node metastasis of human laryngeal and
hypopharyngeal squamous cell carcinoma in vitro and in
vivo (20,22). Although NPC is also classified as a
subtype of head and neck squamous cell carcinoma, it possesses
unique and distinct epidemiology, clinical characteristics,
etiology and histopathology (2).
Therefore, the association between EphA2 expression and
clinicopathological parameters in patients with NPC, and its role
in growth, invasion and paclitaxel chemotherapy of NPC were
investigated for the first time in the present study.
Results of the present study initially revealed that
the expression level of EphA2 protein was significantly higher in
NPC samples than that in non-carcinoma tissues obtained from the
nasopharynx. Morever, the results of 47 NPC specimens assayed by
western blotting further demonstrated that a high protein
expression of EphA2 was significantly associated with T
classification, clinical stage and cervical lymph nodes metastasis,
which was consistent with recent publications on NPC (23) and other reports in urinary bladder
cancer (24), ovarian cancer
(13) and lung cancer (25,26),
indicating the importance of EphA2 in the malignant progression of
patients with NPC.
Metastasis at the early stages is one of the most
frequent clinical features in patients with NPC. Approximately
70–80% of new patients with NPC present with cervical lymph node
metastasis, and approximately 4.2% of those patients have distant
metastasis to the bone, lung, liver, and central nervous system
(27). Metastasis severely reduces
the possibility of successful treatment and overall survival time
(28). Therefore, identification of
molecular markers for metastasis would be beneficial in designing
optimized and individualized therapeutic regimens for patients with
NPC. The present study revealed the association of EphA2 with lymph
node metastasis in NPC, suggesting that EphA2 could be considered
as an indicator for the propensity to metastasize. Furthermore,
EphA2 inhibition was able to reduce the invasive ability of NPC
5-8F cells in vitro, supporting the theory that EphA2 is
involved in the process of invasion and metastasis in patients with
NPC.
Since acquired resistance to chemotherapy negatively
affects the outcome of patients with NPC (29) and the finding that EphA2 knockdown
led to inhibition of NPC proliferation in vitro, the
potential therapeutic function of EphA2 in combination with a
chemotherapeutic drug was further explored in the current study.
Paclitaxel is a tubulin-stabilizing anti-cancer agent and now
widely used in the therapy of patients with NPC. We found that
knockdown of the EphA2 protein significantly enhanced the
cytotoxicity of paclitaxel in human NPC 5-8F cells, which is a
novel finding of the present study. Our results reveal that
paclitaxel chemotherapy may be much more efficacious in human NPC
when administered in combination with EphA2 gene inhibition. This
result was consistent with a recent publication on ovarian cancer,
in which EphA2 knockdown greatly improved the anticancer efficacy
of paclitaxel in ovarian cancer in vitro and in vivo
(30). However, the combined effect
with paclitaxel chemotherapy needs to be further investigated in
vivo.
In conclusion, the present study reveals that the
EphA2 protein is overexpressed in NPC specimens and is associated
with the clinical progression of patients with NPC. Moreover, the
knockdown of EphA2 may significantly inhibit cell growth, invasion,
and enhance the sensitivity to paclitaxel in vitro,
indicating that EphA2 may be a valuable therapeutic target for
patients with NPC. However, a deeper understanding of the precise
mechanisms by which EphA2 regulates the above-mentioned processes
is necessary.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81172558, 81071757, 30872852
and 30901664), the Key Program of Natural Science Foundation of
Hunan Province (2010TP4012-1), the Research Fund for the Doctoral
Program of Higher Education of China (20100162110036,
20090162110065) and the Freedom Explore Program of Central South
University (No. 2012QNZT099).
References
|
1
|
Fahraeus R, Fu HL, Ernberg I, et al:
Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal
carcinoma. Int J Cancer. 42:329–338. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shanmugaratnam K: Nasopharyngeal
carcinoma: epidemiology, histopathology and aetiology. Ann Acad Med
Singapore. 9:289–295. 1980.PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
4
|
Teo P, Shiu W, Leung SF and Lee WY:
Prognostic factors in nasopharyngeal carcinoma investigated by
computer tomography - an analysis of 659 patients. Radiother Oncol.
23:79–93. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan EH, Khoo KS, Wee J, et al: Phase II
trial of a paclitaxel and carboplatin combination in Asian patients
with metastatic nasopharyngeal carcinoma. Ann Oncol. 10:235–237.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasquale EB: Eph receptors and ephrins in
cancer: bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sulman EP, Tang XX, Allen C, et al: ECK, a
human EPH-related gene, maps to 1p36.1, a common region of
alteration in human cancers. Genomics. 40:371–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brantley-Sieders DM, Zhuang G, Hicks D, et
al: The receptor tyrosine kinase EphA2 promotes mammary
adenocarcinoma tumorigenesis and metastatic progression in mice by
amplifying ErbB2 signaling. J Clin Invest. 118:64–78. 2008.
View Article : Google Scholar
|
|
10
|
Lu C, Shahzad MM, Wang H, et al: EphA2
overexpression promotes ovarian cancer growth. Cancer Biol Ther.
7:1098–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zelinski DP, Zantek ND, Stewart JC,
Irizarry AR and Kinch MS: EphA2 overexpression causes tumorigenesis
of mammary epithelial cells. Cancer Res. 61:2301–2306.
2001.PubMed/NCBI
|
|
12
|
Zantek ND, Azimi M, Fedor-Chaiken M, Wang
B, Brackenbury R and Kinch MS: E-cadherin regulates the function of
the EphA2 receptor tyrosine kinase. Cell Growth Differ. 10:629–638.
1999.PubMed/NCBI
|
|
13
|
Lin YG, Han LY, Kamat AA, et al: EphA2
overexpression is associated with angiogenesis in ovarian cancer.
Cancer. 109:332–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han L, Dong Z, Qiao Y, et al: The clinical
significance of EphA2 and Ephrin A-1 in epithelial ovarian
carcinomas. Gynecol Oncol. 99:278–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brannan JM, Dong W, Prudkin L, et al:
Expression of the receptor tyrosine kinase EphA2 is increased in
smokers and predicts poor survival in non-small cell lung cancer.
Clin Cancer Res. 15:4423–4430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu F, Park PJ, Lai W, et al: A
genome-wide screen reveals functional gene clusters in the cancer
genome and identifies EphA2 as a mitogen in glioblastoma. Cancer
Res. 66:10815–10823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taddei ML, Parri M, Angelucci A, et al:
EphA2 induces metastatic growth regulating amoeboid motility and
clonogenic potential in prostate carcinoma cells. Mol Cancer Res.
9:149–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herrem CJ, Tatsumi T, Olson KS, et al:
Expression of EphA2 is prognostic of disease-free interval and
overall survival in surgically treated patients with renal cell
carcinoma. Clin Cancer Res. 11:226–231. 2005.PubMed/NCBI
|
|
19
|
Liu Y, Xie C, Zhang X, et al: Elevated
expression of HMGB1 in squamous-cell carcinoma of the head and neck
and its clinical significance. Eur J Cancer. 46:3007–3015. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Zhang X, Qiu Y, et al: Clinical
significance of EphA2 expression in squamous-cell carcinoma of the
head and neck. J Cancer Res Clin Oncol. 137:761–769. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson FM, Garden AS, Palmer JL, et al: A
phase I/II study of neoadjuvant chemotherapy followed by radiation
with boost chemotherapy for advanced T-stage nasopharyngeal
carcinoma. Int J Radiat Oncol Biol Phys. 63:717–724. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Yu C, Qiu Y, et al: Downregulation
of EphA2 expression suppresses the growth and metastasis in
squamous-cell carcinoma of the head and neck in vitro and in vivo.
J Cancer Res Clin Oncol. 138:195–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu G, Xiao D, Chen Q and Zhu Y: The
expression and its potentially clinical significance of EphA2 in
nasopharyngeal carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke
Za Zhi. 25:827–829. 8332011.(In Chinese).
|
|
24
|
Abraham S, Knapp DW, Cheng L, et al:
Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary
bladder. Clin Cancer Res. 12:353–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brannan JM, Sen B, Saigal B, et al: EphA2
in the early pathogenesis and progression of non-small cell lung
cancer. Cancer Prev Res (Phila). 2:1039–1049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kinch MS, Moore MB and Harpole DH Jr:
Predictive value of the EphA2 receptor tyrosine kinase in lung
cancer recurrence and survival. Clin Cancer Res. 9:613–618.
2003.PubMed/NCBI
|
|
27
|
King AD, Ahuja AT, Leung SF, et al: Neck
node metastases from nasopharyngeal carcinoma: MR imaging of
patterns of disease. Head Neck. 22:275–281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leung SF, Teo PM, Shiu WW, Tsao SY and
Leung TW: Clinical features and management of distant metastases of
nasopharyngeal carcinoma. J Otolaryngol. 20:27–29. 1991.PubMed/NCBI
|
|
29
|
Ciuleanu TE, Fountzilas G, Ciuleanu E,
Plataniotis M, Todor N and Ghilezan N: Paclitaxel and carboplatin
in relapsed or metastatic nasopharyngeal carcinoma: a multicenter
phase II study. J BUON. 9:161–165. 2004.PubMed/NCBI
|
|
30
|
Landen CN Jr, Chavez-Reyes A, Bucana C, et
al: Therapeutic EphA2 gene targeting in vivo using neutral
liposomal small interfering RNA delivery. Cancer Res. 65:6910–6918.
2005. View Article : Google Scholar : PubMed/NCBI
|