Introduction
The wild-type p53 gene (wt-p53), an important tumor
suppressor gene, is a key regulatory factor determining cell
survival during the cellular stress response to radiation-induced
DNA damage. DNA damage responses trigger a series of chemical
modifications, including p53 phosphorylation. Activated p53
transcription promotes or inhibits expression of downstream genes,
thereby causing cell cycle arrest, DNA repair and tumor cell
apoptosis through the regulation of target genes (1,2). To
date, dysfunction of tumor suppressor genes has been confirmed as
the most significant genetic damage in human cancers. Mutations or
deletions of p53 occur in 50–70% of non-small cell lung cancers
(NSCLCs) and are associated with poor prognosis of lung cancer
patients (3,4).
A previous study demonstrated that patients with
wt-p53 with loss of function were less sensitive to radiotherapy
(5). In vivo and in
vitro studies have revealed that wt-p53 transfection in
combination with radiotherapy (RT) may increase the sensitivity to
low linear energy transfer (LET) radiation (6–8);
although mutant p53 may respond well to high LET radiation
(9,10). Recombinant human adenovirus (Ad) p53
injection (rAd-p53; trade name, Gendicine) is a gene therapy
administered intratumorally, which uses type 5 Ad to carry the
recombinant human p53 gene. Transfection of Ad is able to introduce
the p53 gene into tumor cells to express wt-p53 protein, which may
execute its functions in inhibiting cell division and inducing
tumor cell apoptosis. Combination therapy of rAd-p53 and radiation
has demonstrated good efficacy for the treatment of multiple
tumors. It has also been approved as a treatment model for certain
head and neck malignancies, including nasopharyngeal carcinoma
(11). Previous studies have
revealed that the peak time for wt-p53 expression following
Gendicine treatment was 48–72 h after transfection (12–14).
Therefore, Gendicine administration twice a week helps to achieve a
stable expression platform of wt-p53 proteins. The time of rAd-p53
transfection may overlap with at least one of five-weekly standard
RT treatments. However, whether RT may affect the expression of
exogenous wt-p53 mediated by Ad, and what the optimal interval
between radiation and rAd-p53 treatment is remains unclear.
This study introduced rAd-p53 containing the human
wt-p53 gene into the human lung adenocarcinoma cell line A549. RT
was applied at various time points following gene transfection in
order to evaluate the effect of combination therapy on growth
inhibition, cell apoptosis and p53 protein expression. Our aim was
to explore the optimal interval time of RT following rAd-p53
transfection, in order to obtain maximum clinical effects.
Materials and methods
Materials
Human lung adenocarcinoma cell line A549 was
obtained from the Biological Testing Center of the Cancer Hospital
(Chinese Academy of Medical Sciences, Beijing, China), RPMI-1640
culture medium from Gibco (Carlsbad, CA, USA) and 15% fetal bovine
serum (FBS) from Hyclone Laboratories Inc., (Logan, UT, USA). The
MTT assay was obtained from Sigma (St. Louis, MO, USA), Hoechst
33342/propidium iodide (PI) double-staining kit from GenScript (USA
Inc., Piscataway, NJ, USA) and total protein extraction kit from,
Nanjing KeyGene Biotechnology Co., Ltd., (Nanjing, China). We
obtained the concentrated mouse anti-human p53 monoclonal antibody
(1:1000) from Abcam (Cambridge, MA, USA), the ready to use mouse
anti-human β-actin monoclonal antibody (1:1000) from Santa Cruz
Biotechnology Inc., (Santa Cruz, CA, USA), goat anti-mouse IgG
antibody (1:2000) from Beijing Zhongshan Golden Bridge
Biotechnology Co., (Beijing, China), human Ad type 5 from Vector
BioLabs (Philadelphia, PA, USA) and the recombinant human p53 Ad
injection (rAd-p53 injection; trade name, Gendicine; lot number,
20090514; 1012 viral particles/vial, 2 ml/vial) from
Shenzhen Sibiono Gene Technology Co., Ltd. (Shenzhen, China).
Plaque assay determined the titer as 1×10 plaque forming unit
(PFU)/vial.
Cell culture
RPMI-1640 culture medium containing 300 μg/ml
glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 15% FBS
was used. A549 cells were cultured in a 37°C, 5% CO2 and
95% O2 incubator and those at exponential growth phase
were selected for further study.
Irradiation
A 6 MeV medical linear accelerator (Siemens Primus,
Erlangen, German) was used with a 100 cm source skin distance, 0.5
cm tissue equivalent compensator, 300 cGy/min dose rate and a 4 Gy
single dose.
Treatment and grouping
A549 cells at exponential growth phase were cultured
in serum-free culture medium for 12 h for cell cycle
synchronization, and cultured for a further 24 h. Following cell
attachment, 1×1010 VP/well rAd-p53 injection was added
into each group (to infect cells at 1000 PFU/cell), and an equal
volume of culture medium was added into the control group.
According to the time between rAd-53 transfection and RT, cells
were divided into 5 groups: radiation administered immediately
following transfection (0 h-RT) group, after 3 h group (3 h-RT),
after 6 h group (6 h-RT), after 24 h group (24 h-RT) and after 48 h
group (48 h-RT). Cells with rAd-p53 transfection alone (Ad-p53) or
with the empty Ad vector transfection (Ad group) were included as
the two control groups. Cells of all groups were cultured until 72
h following transfection and were then subjected to
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide (MTT)
assay, flow cytometry and western blot analysis, respectively
(Fig. 1).
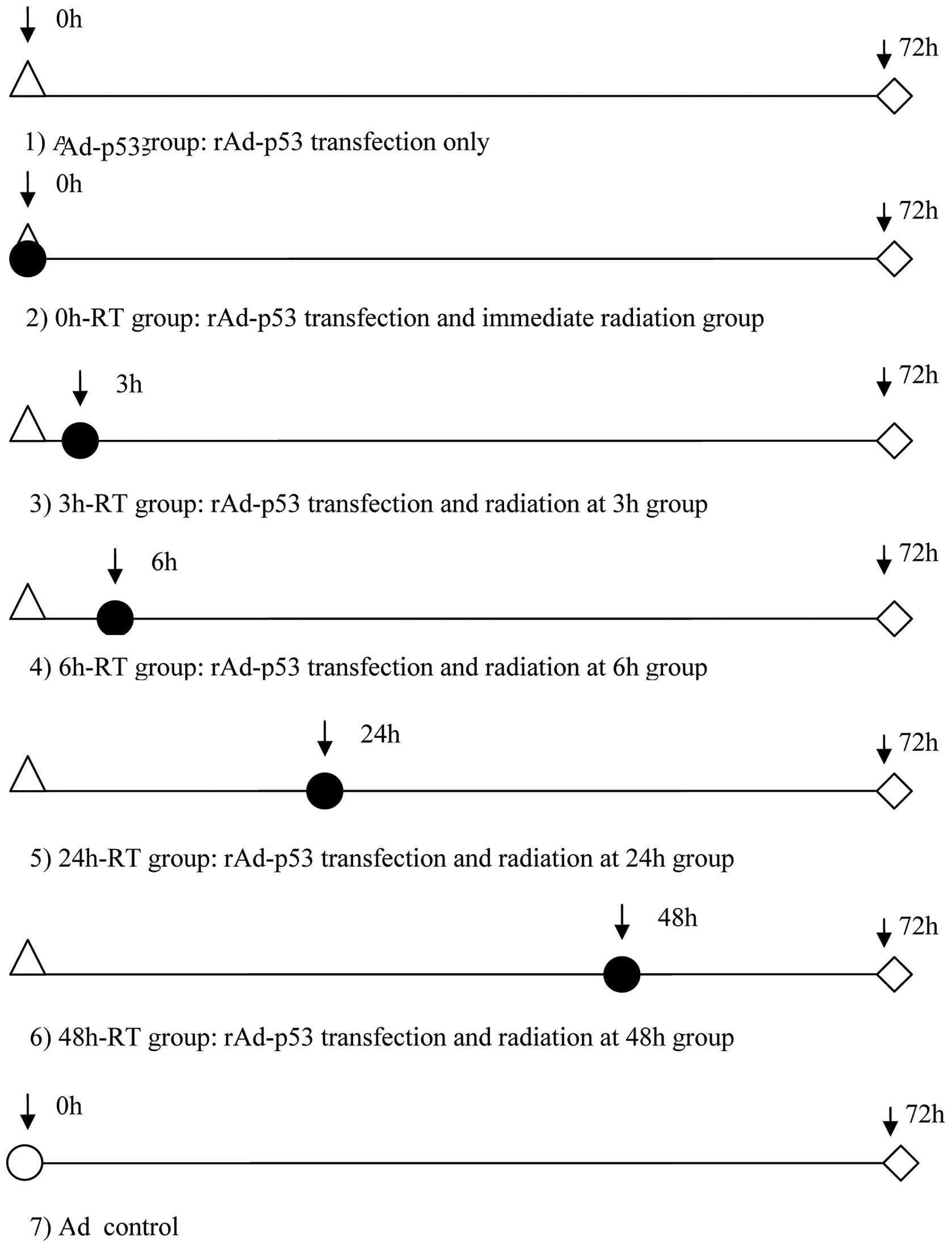 | Figure 1Experimental groups based on various
time points between rAd-p53 transfection and RT. △, time points of
rAd-p53 transfection; ●, time points of 4 Gy radiation; ⋄, time
points of conducting MTT, flow cytometry and p53 protein in western
blot analysis; ○, time points of PBS buffer addition. rAd-p53,
recombinant human adenovirus p53; RT, radiotherapy; MTT,
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide; PBS,
phosphate buffered saline. |
MTT assay
Following corresponding treatments, cells
(2×103/well) were plated in 96-well culture plates and
MTT (5 g/l) solution was added into each well and cultured for a
further 4 h. The supernatant was discarded from each well and 200
μl dimethyl sulfoxide was added. Following 10 min of agitation,
absorbance at 490 nm was measured on a microplate reader and the
growth inhibition rate was calculated as follows: Growth inhibition
rate = [(A value of the control group - A value of the experimental
group)/A value of the control group] × 100%. Results are
demonstrated as the mean ± standard deviation (SD).
Flow cytometry
Following corresponding treatments, cells were
harvested, adjusted to a cell density of 1×106 cells/ml
and incubated with Hoechst 33342 dye at 37°C for 10 min. Cells were
centrifuged at 1000 rpm for 5 min at 4°C and then the supernatant
was discarded. Following this, cells were incubated with PI at room
temperature for 10 min and the stained cells were immediately
analyzed using flow cytometry with UV/488 nm dual excitation. The
fluorescence emission of Hoechst 33342 at 460 nm and PI at 640 nm
emission were measured.
Western blot analysis
Following corresponding treatments, cells in each
group were homogenized at 4°C and a total protein extraction kit
was used for protein extraction. A Coomassie brilliant Blue assay
was used to determine the protein concentration to enable us to
adjust the extracted protein from each group into the same
concentration. The protein was then subjected to 10% denaturing
polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene fluoride membrane blocked using 50 g/l non-fat milk
at room temperature for 1 h. The proteins were then incubated with
mouse anti-human p53 monoclonal antibody and concentrated rabbit
anti-human β-actin monoclonal antibody, respectively, at 4°C
overnight, followed by horseradish peroxidase-labeled goat
anti-mouse IgG antibody at room temperature for 1 h. Protein bands
were visualized using a chemiluminescent kit and images were
captured.
Statistical analysis
Data shown are the average of three experiments and
are demonstrated as the mean ± SD. SPSS software (version 16.0 for
Windows, Chicago, IL, USA) was used for statistical analysis. Data
were analyzed using one-way ANOVA, followed by a Student's
two-tailed paired t-test for comparison between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of rAd-p53 and radiation at
various time points on cell viability
Our results demonstrated that a combination of
rAd-p53 and radiation inhibited A549 cell growth in all groups, and
cell viability suppression rates gradually increased from 0 h-RT to
48 h-RT (Table I). The cell
viability suppression rates in the 6 h-RT, 24 h-RT and 48 h-RT
groups were 56.7±5.4, 60.8±6.0 and 68.9±6.6%, respectively, which
were statistically significantly higher than that of the Ad-p53
(40.8±4.7), 0 h-RT (45.0±3.5) and 3 h-RT groups (47.0±4.3).
Additionally, no statistically significant differences were
detected between the cell viability suppression rates of the 6
h-RT, 24 h-RT and 48 h-RT groups (P>0.05).
 | Table IEffects of rAd-p53 transfection and
radiation at various time points in A549 cells. |
Table I
Effects of rAd-p53 transfection and
radiation at various time points in A549 cells.
| Cell viability
suppression rate (%) | Cell apoptosis
(%) | p53 expression
level |
|---|
|
|
|
|
|---|
| Mean | SD | Mean | SD | Mean | SD |
|---|
| 0 h-RT | 45.00a | 3.5 | 30.10a | 5.4 | 0.509 | 0.105 |
| 3 h-RT | 47.00a | 4.3 | 32.50a | 4.9 | 0.643 | 0.089 |
| 6 h-RT | 56.70a,b,c | 5.4 | 39.70a,b,c | 5.4 | 0.856b,d | 0.092 |
| 24 h-RT | 60.80a,b | 6 | 42.90a,b | 6.6 | 1.193b | 0.202 |
| 48 h-RT | 68.90a,b | 6.6 | 52.00a,b | 8.9 | 1.590b,d | 0.211 |
| Ad-p53 | 40.80 | 4.7 | 9.20 | 2.7 | 1.625b,d,c | 0.172 |
Effects of rAd-p53 and radiation at
various time points on cell apoptosis
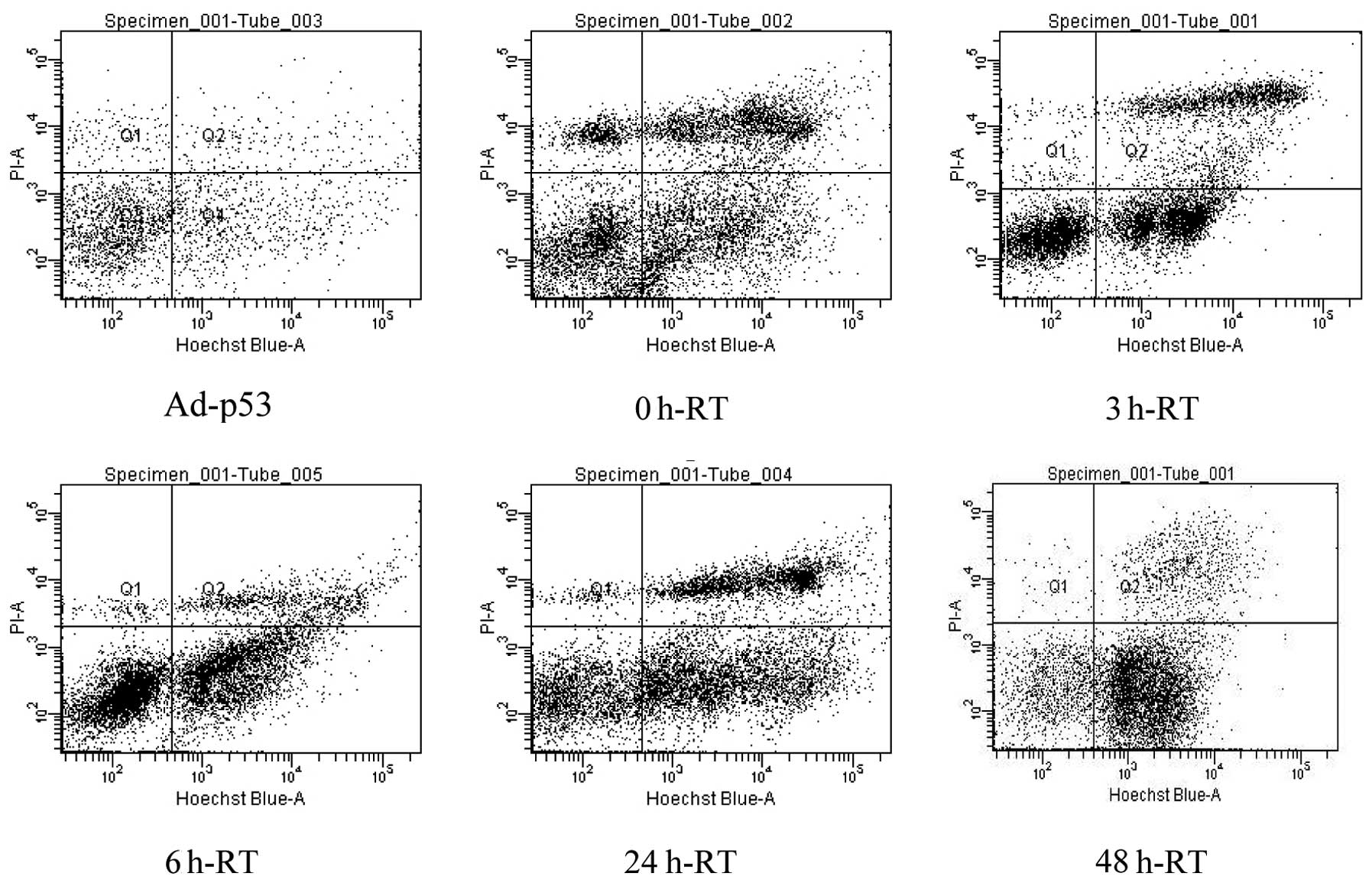
Flow cytometry analysis revealed that the percentage
of apoptotic cells gradually increased from 0 h-RT to 48 h-RT
(Table I; Fig. 2). The percentage of apoptotic cells
in the 6 h-RT, 24 h-RT and 48 h-RT groups were 39.7±5.4, 42.9±6.6
and 52.0±8.9%, respectively, which were statistically significantly
higher than that of Ad-p53 (9.2±2.7), 0 h-RT, (30.1±5.4%) and 3
h-RT groups (32.5±4.9). Additionally, no statistically significant
differences were detected in the percentage of apoptotic cells
among the 6 h-RT, 24 h-RT and 48 h-RT groups (P>0.05).
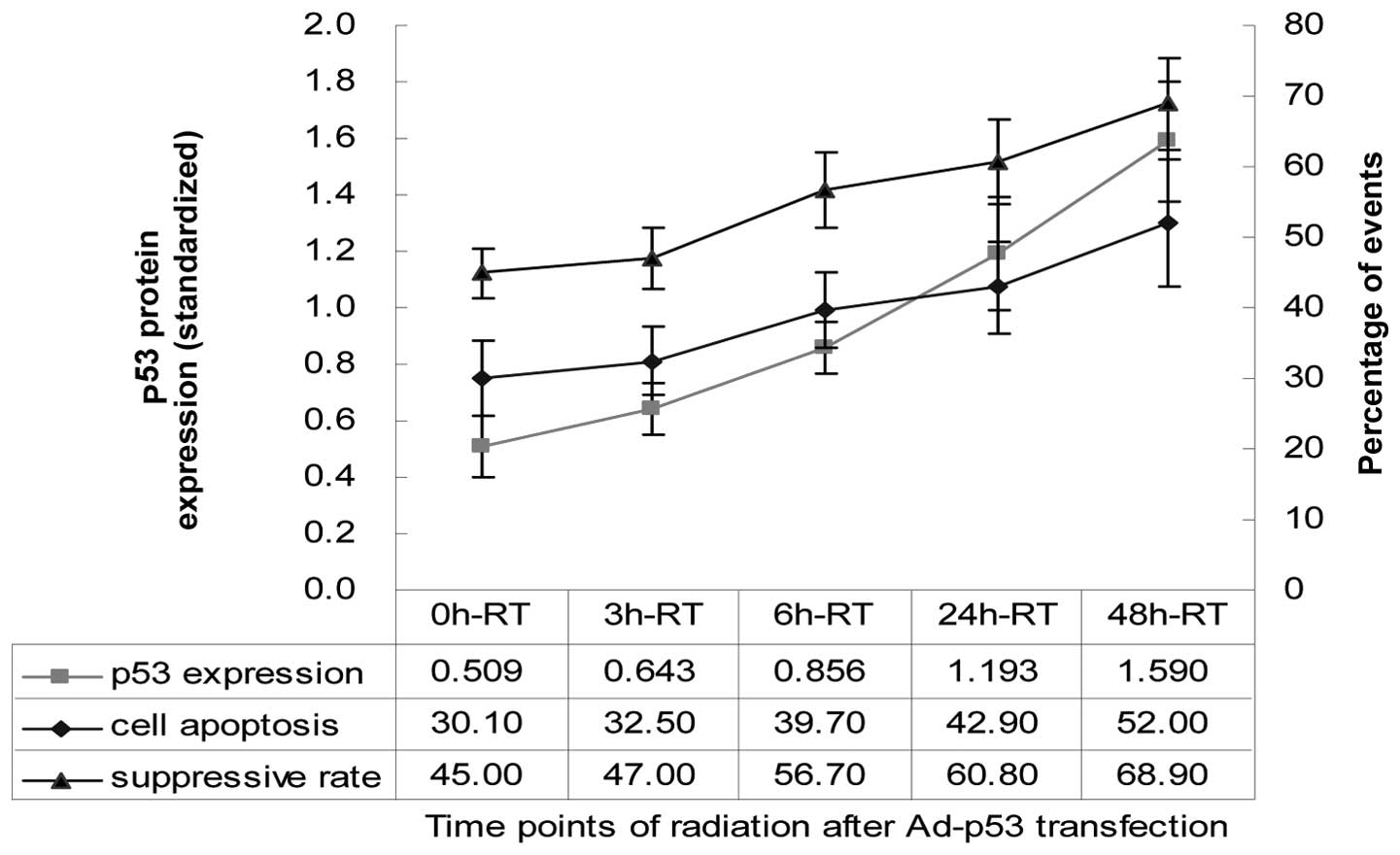
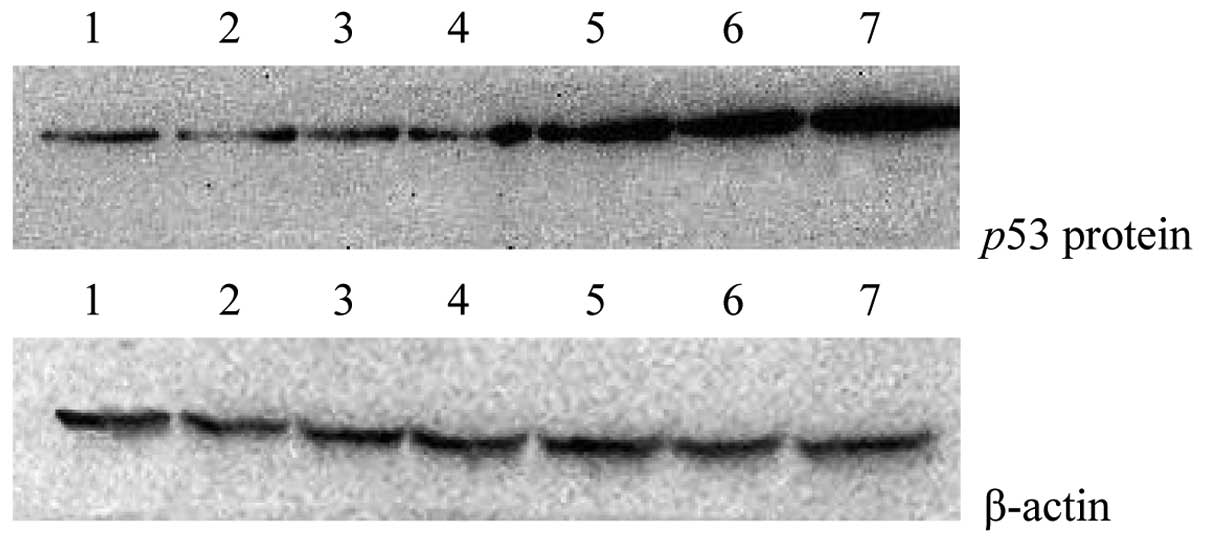
Effects of rAd-p53 and radiation at
various time points on p53 protein expression
From 0 h-RT to 48 h-RT the p53 protein expression
gradually increased (Fig. 3). The
highest p53 protein expression level was detected in the Ad-p53
control group (1.625±0.172), but it was not significantly different
from that of the 48 h-RT group (1.590±0.211) (P>0.05). The p53
protein expression level detected in the 6 h-RT group (0.856±0.092)
was higher than that in 3 h-RT group (0.643±0.089) (t=2.882;
P=0.045), but not significantly different from that of the 24 h-RT
group (1.193±0.202) (P>0.05). A higher p53 expression was
demonstrated in the Ad control group compared to that of the 0 h-RT
(0.509±0.105) and 3 h-RT groups, however there was no statistical
difference (P>0.05) (Table I;
Fig. 4). The A549 cell viability
suppression rates of and percentage of apoptotic cells were
positively correlated with p53 protein expression (P=0.012, r=0.87;
P=0.015, r=0.85, respectively) (Fig.
4). We did not detect the highest peak of p53 protein
expression, which may be demonstrated 72 h following
transfection.
Discussion
In the absence of DNA damaging factors, p53 protein
expression is extremely low in cells, which maintains a wt-p53
protein half-life of 10–20 min through high turnover rate (15). The C-terminus of the wt-p53 protein
is capable of identifying and binding to damaged DNA bases or
radiation-induced double-strand DNA breaks. This stabilizes the p53
protein and accumulates in the cells; wt-p53 half-life is extended
to 1–2 h (16,17). Expression of the wt-p53 protein in
tumor cells following Ad transfection may take a certain amount of
time. Studies have demonstrated that p53 protein expression is
initiated 3 h following rAd-p53 transfection, with an expression
rate of approximately 50% at 12 h and a peak expression at 48–72 h
(12–14). Based on these results, we selected a
72-h time point to detect all indicators following rAd-p53
infection. The present study revealed that p53 protein expression
in the 0 h-RT and 3 h-RT groups was significantly lower than that
of the 6 h-RT, 24 h-RT and 48 h-RT groups (P<0.05), suggesting
that radiation may affect p53 expression within a short period of
time following rAd-p53 administration. This may be due to the
decreased transfection power and/or the loss of the bystander
killing effect of certain infected tumor cells, which were
inhibited or killed by irradiation. Although the 48 h-RT group had
the highest level of p53 protein expression, it was not
statistically different from that of the 6 h-RT and 24 h-RT groups
(P>0.05). This also indicates that radiation greater than 6 h
following rAd-53 transfection may have minimal effects on p53
protein expression.
Wt-p53 transfection may not only directly inhibit
cell division and induce tumor cell apoptosis, but also has a
bystander killing effect on tumor cells. Thus, wt-p53 gene therapy
may also increase the sensitivity of tumor cells to radiotherapy
and increase tumor cell susceptibility to apoptosis (18). This study demonstrated that in
addition to the effect on p53 protein expression, radiation also
affects p53-induced tumor cell apoptosis and cytotoxicity. In the 0
h-RT to 48 h-RT groups, the apoptotic rate of A549 cells gradually
increased with prolonged rAd-p53 action and interval of
radiotherapy. The A549 cell viability suppression rates of the 6
h-RT, 24 h-RT and 48 h-RT groups were not significantly different,
but were significantly higher than that of the immediate radiation
and 3 h-RT groups. This suggests that a combination of RT within 3
h of rAd-p53 administration is ineffective in causing sufficient
synergistic cytotoxic effects on cancer cells. The A549 cell growth
inhibition rate was positively correlated with p53 protein
expression. The main mechanism of A549 cell growth inhibition in
the 0 h-RT and 3 h-RT groups compared with the 6 h-RT, 24 h-RT and
48 h-RT groups may be that radiation reduces wt-p53 protein
expression and decreases the radiation-sensitizing effect.
Therefore, despite the above reasons leading to the decreased p53
protein expression and suppression of cell viability, a combination
of rAd-p53 transfection into lung cancer cells and RT can be
selected at 6–48 h following rAd-p53 transfection, rather than
immediate to 3 h. Otherwise, radiation may inhibit or damage p53
protein expression carried by the Ad and reduce its ability to
directly kill tumor cells or indirectly sensitize them to
irradiation.
In conclusion, radiation at various time points is
able to affect p53 protein expression and cytotoxic effects of
rAd-p53, and administration of RT at 6–48 h following rAd-p53
transfection may maximize the synergistic killing effect of these
two treatments on tumor cells. The single high dose of radiation
used in in vitro experiments may not accurately reflect the
molecular biological changes caused by the conventional
fractionated 2 Gy dose used in clinics. However, this study
provides a theoretical basis for the reasonable arrangement of
rAd-p53 transfection and radiotherapy interval, and further
investigation is required.
Acknowledgements
This study was supported in part by a grant from the
National Natural Science Foundation of China (#30700979) and
Science and Technology Project of Shenyang (#110492) to Dr Cheng-Bo
Han.
References
|
1
|
Vousden KH and Lu X: Live or let die: the
cell's response to p53. Nat Rev Cancer. 2:594–604. 2002. View Article : Google Scholar
|
|
2
|
Hirao A, Kong YY, Matsuoka S, Wakeham A,
Ruland J, Yoshida H, Liu D, Elledge SJ and Mak TW: DNA
damage-induced activation of p53 by the checkpoint kinase Chk2.
Science. 287:1824–1827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang CL, Yokomise H and Miyatake A:
Clinical significance of the p53 pathway and associated gene
therapy in non-small cell lung cancers. Future Oncol. 3:83–93.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kandioler D, Stamatis G, Eberhardt W,
Kappel S, Zöchbauer-Müller S, Kührer I, Mittlböck M, Zwrtek R,
Aigner C, Bichler C, et al: Growing clinical evidence for the
interaction of the p53 genotype and response to induction
chemotherapy in advanced non-small cell lung cancer. J Thorac
Cardiovasc Surg. 135:1036–1041. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kandioler D, Zwrtek R, Ludwig C, Janschek
E, Ploner M, Hofbauer F, Kührer I, Kappel S, Wrba F, Horvath M, et
al: TP53 genotype but not p53 immunohistochemical result predicts
response to preoperative short-term radiotherapy in rectal cancer.
Ann Surg. 235:493–498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shiomitsu K, Sajo E, Xia X, Hunley DW,
Mauldin GE, Li S and Mauldin GN: Radiosensitivity of canine
osteosarcoma cells transfected with wild-type p53 in vitro. Vet
Comp Oncol. 6:193–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gudkov AV and Komarova EA: The role of p53
in determining sensitivity to radiotherapy. Nat Rev Cancer.
3:117–129. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuddihy AR and Bristow RG: The p53 protein
family and radiation sensitivity: yes or no? Cancer Metastasis Rev.
23:237–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi T, Fukawa T, Hirayama R, Yoshida
Y, Musha A, Furusawa Y, Ando K and Nakano T: In vitro interaction
of high-LET heavy-ion irradiation and chemotherapeutic agents in
two cell lines with different radiosensitivities and different p53
status. Anticancer Res. 30:1961–1967. 2010.PubMed/NCBI
|
|
10
|
Takahashi A, Matsumoto H, Yuki K, Yasumoto
J, Kajiwara A, Aoki M, Furusawa Y, Ohnishi K and Ohnishi T:
High-LET radiation enhanced apoptosis but not necrosis regardless
of p53 status. Int J Radiat Oncol Biol Phys. 60:591–597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan JJ, Zhang SW, Chen CB, Xiao SW, Sun Y,
Liu CQ, Su X, Li DM, Xu G, Xu B and Lu YY: Effect of recombinant
adenovirus-p53 combined with radiotherapy on long-term prognosis of
advanced nasopharyngeal carcinoma. J Clin Oncol. 27:799–804. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roth JA: Adenovirus p53 gene therapy.
Expert Opin Biol Ther. 6:55–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma JT, Zheng W and Zou HW:
Radiosensitivity enhanced by recombinant adenovirus p53 in lung
adenocarcinoma. Journal of China Medical University. 36:424–426.
2007.
|
|
14
|
Ma G, Kawamura K, Li Q, Suzuki N, Liang M,
Namba M, Shimada H and Tagawa M: Cytotoxicity of adenoviruses
expressing the wild-type p53 gene to esophageal carcinoma cells is
linked with the CAR expression level and indirectly with the
endogenous p53 status. Cancer Gene Ther. 16:832–840. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashcroft M, Kubbutat MH and Vousden KH:
Regulation of p53 function and stability by phosphorylation. Mol
Cell Biol. 19:1751–1758. 1999.PubMed/NCBI
|
|
16
|
Reed M, Woelker B, Wang P, Wang Y,
Anderson ME and Tegtmeyer P: The C-terminal domain of p53
recognizes DNA damaged by ionizing radiation. Proc Natl Acad Sci
USA. 92:9455–9459. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lakin ND and Jackson SP: Regulation of p53
in response to DNA damage. Oncogene. 18:7644–7655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gudkov AV and Komarova EA: Prospective
therapeutic applications of p53 inhibitors. Biochem Biophys Res
Commun. 331:726–736. 2005. View Article : Google Scholar : PubMed/NCBI
|