Introduction
Fibrous dysplasia (FD) of the bone is a battery of
tumor-like lesions in which fibrous tissue proliferation replaces
normal bone architecture. FD accounts for only 5% of benign bone
tumors (1). The incidence of this
disease is 10–30 cases per million and accounts for 5.89% of benign
bone tumors in China. In 1891, FD was first described by Von
Recklinghausen (2), but it was
Lichtenstein (3) who labeled it
polyostotic FD (PFD) in 1938. Lichtenstein and Jaffe (4) initially described the spectrum of the
clinical, radiographic and histological symptoms. FD may involve a
single skeletal site with an isolated lesion [monostotic FD (MFD)]
or it may involve multiple bones [polyostotic FD (PFD)]. In
addition, it may occur in association with distinct café-au-lait
skin spots and/or a number of hyperfunctioning endocrinopathies
labeled as McCune-Albright syndrome (MAS). In addition, FD may
occur in association with intramuscular myxomas, which is referred
to as Mazabraud’s syndrome (5,6). It
has been reported that the monostotic form is 8–10 times more
common than the polyostotic form (7). Compared with the spine, ribs and
skull, FD is more common in the bones of the limbs. Following
puberty or epiphyseal closure, the asymptomatic lesions of the
majority of patients are restrictive and do not develop or
progress. However, development and progression may occasionally be
detected in lesions of patients with symptoms of pain, deformity
and/or pathological microfracture.
In the present study, we retrospectively examined
patient outcome following intramedullary nailing for FD of the
lower limbs via long-term clinical follow-up.
Patients and methods
Patients
We retrospectively reviewed patients with FD of the
lower limbs treated by intramedullary nailing between 2003 and
2010. A total of 39 patients participated in the study. The study
was approved by the ethics committee of Anhui Medical University.
Informed consent was obtained from the patients or the patient’s
family. The mean age of the patients was 31 years (range, 17–55),
of which 22 were male and 17 were female. The mean follow-up period
was 50 months (range, 4–93 months). Types of tumor included
monostotic (33 patients) and polyostotic (7 patients). Symptoms
included pain (15 patients), pathological fracture (10 patients),
swelling/deformity (13 patients), coxa vara (8 patients) and a limp
(1 patient). Two patients had already undergone several treatments.
One patient aged 12 years who presented with FD of the right
humerus had received conservative treatment. Curettage and grafts
of the left tibia were performed at 15 years old and were
pathologically confirmed to be FD. Pathological fracture of the
left tibia occurred at 17 years. Another patient presented with a
lesion and received conservative treatment in the neck of the left
femur aged 10 years following a two-year limp. The patient
underwent curettage due to deformity of the skull at 13 years old,
and coxa vara was diagnosed at 18 years. The sites of the lesions
were the femur (31 lesions), coxa vara (8 lesions), tibia (14
lesions), fibula (1 lesion), spine (2 lesions), bilateral lower
limbs (1 patient), ipsilateral femur and tibia (1 patient) and
ipsilateral femur and fibula (1 patient). The lesions located by
patient self-observation and clinical manifestations, were detected
by radiography, including plain films, computed tomography (CT) and
magnetic resonance imaging (MRI). Following surgery, pathology was
the ultimate diagnostic method. Patients characteristics are shown
in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient | Age (years) | Gender | Location | Symptoms | Return to full
activity (months) | Guillea | Guilleb | Neck shaft
anglea | Neck shaft
angleb |
|---|
| 1 | 35 | F | Femur (L) | Pain | 4.8 | | | | |
| 2 | 44 | M | Bilateral limb | Fracture | 14.1 | | | | |
| 3 | 42 | F | Femur (R) | Pain | 5.1 | | | | |
| 4 | 38 | M | Tibia (R) | Pain | 4.9 | | | | |
| 5 | 31 | F | Femur (R) | Pain | 4.2 | | | | |
| 6 | 20 | M | Tibia (R) | Fracture | 3.8 | | | | |
| 7 | 29 | M | Femur (L) | Fracture | 5.0 | | | | |
| 8 | 26 | M | Femur (L) | Deformity | 5.8 | 3 | 9 | 70 | 119 |
| 9 | 33 | M | Femur (R) | Pain | 3.9 | | | | |
| 10 | 41 | M | Femur (R) | Deformity | 4.7 | | | | |
| 11 | 35 | F | Tibia (R) | Pain | 4.0 | | | | |
| 12 | 17 | M | Tibia (R) | Fracture | 2.6 | | | | |
| 13 | 18 | F | Femur (R) +
skull | Deformity | 5.2 | 4 | 9 | 85 | 120 |
| 14 | 45 | M | Tibia (R) + L3 | Pain | 3.6 | | | | |
| 15 | 23 | M | Femur (L) | Deformity | 4.7 | | | | |
| 16 | 30 | M | Femur (L) | Fracture | 5.4 | | | | |
| 17 | 51 | F | Tibia (L) | Pain | 6.4 | | | | |
| 18 | 17 | M | Femur (L) | Pain | 4.4 | | | | |
| 19 | 18 | F | Femur (L) | Deformity | 3.5 | 5 | 10 | 104 | 130 |
| 20 | 55 | F | Femur (R) + C6C7 | Pain | 6.8 | | | | |
| 21 | 18 | M | Femur (R) + tibia
(R) | Deformity | 3.2 | 3 | 9 | 75 | 120 |
| 22 | 34 | M | Femur (L) | Deformity | 4.5 | 6 | 8 | 120 | 140 |
| 23 | 20 | F | Tibia (R) | Pain | 3.3 | | | | |
| 24 | 18 | M | Femur (L) | Deformity | 5.5 | 5 | 8 | 90 | 127 |
| 25 | 20 | M | Femur (R) | Fracture | 4.7 | | | | |
| 26 | 30 | M | Femur (L) | Deformity | 5.8 | | | | |
| 27 | 18 | F | Femur (L) | Limp | 4.2 | | | | |
| 28 | 48 | F | Femur (L) | Fracture | 6.0 | | | | |
| 29 | 54 | F | Femur (L + R) | Pain | 5.4 | | | | |
| 30 | 20 | M | Femur (L) | Fracture | 3.6 | | | | |
| 31 | 52 | F | Femur (R) | Deformity | 6.5 | | | | |
| 32 | 21 | F | Femur (L) + fibula
(L) | Pain | 3.2 | | | | |
| 33 | 19 | F | Femur (R) | Fracture | 2.4 | | | | |
| 34 | 47 | M | Tibia (L) | Pain | 3.1 | | | | |
| 35 | 21 | F | Tibia (R) | Pain | 2.6 | | | | |
| 36 | 33 | F | Femur (L) | Deformity | 4.0 | 4 | 7 | 87 | 120 |
| 37 | 43 | F | Femur (R) | Deformity | 5.5 | 5 | 6 | 92 | 125 |
| 38 | 23 | M | Tibia (L) | Deformity | 4.1 | | | | |
| 39 | 52 | M | Tibia (L) | Fracture | 5.7 | | | | |
Surgical technique
The patient was placed in the supine position under
an image intensifier. During surgery, a cortical fenestration
sufficiently large to be able to visualize the whole lesion was
made using an osteotome. The lesion was removed with curettes, and
the cavity was enlarged with a burr and irrigated with sterile
saline. The removed tissue was sent for pathological examination.
For varus of the hips, a lateral closing wedge osteotomy was
performed and a guide pin was inserted into the medullary canal
through the distant osteotomized site. This was then reamed, and
repeated removal of fibrous tissues of the intramedullary cavity
was performed. After reaming, an intramedullary nail was inserted
and then locked with proximal and distal screws. The cavity was
then irrigated with sterile saline followed by the placement of a
combination of autogenous cancellous bone and/or allograft
cancellous chips.
Rehabilitation
Following surgery, all patients were advised against
weight bearing but to mobilize joints depending on the location of
the lesion. Patients were then provided with crutches to use for
walking. Approximately two weeks after surgery, if there was
clinically significant restrictive motion, physiotherapy was
offered. Patients received a wound check and suture removal two
weeks after surgery. Partial weight bearing was allowed
approximately 2–3 months later. Time taken to return to normal
walking was determined by patients’ symptoms and follow-up
radiographical imaging of osteotomy sites.
Follow-up
Patients were reexamined using plain film
radiography following surgery. Radiography and clinical
examinations were repeated every month for the first six months,
and once every six months after that. Outcome criteria for the
study included: i) pain status at the last follow-up; ii) time
taken until return of full activity; iii) repeat/additional
surgical interventions. Pain was measured using a patient visual
analogue scale. Recovery time was measured between the date of
surgery and the date when full activity was resumed. The clinical
score was in accordance with the modified criteria of Guille et
al (8).
Results
Recovery time
None of the patients had infection, thromboembolism
or other notable complications. No loosening of screws or
refracture was detected in the follow-up period. However, one
patient with a lesion in the bilateral lower limbs, who underwent
an osteotomy of the femur, had an unrelated pathological fracture
on the other femur and was treated with further intramedullary
nailing surgery. Therefore, the time taken to return to full
activity was prolonged. One patient aged 12 years presented with a
pathological fracture of FD and was treated with an external
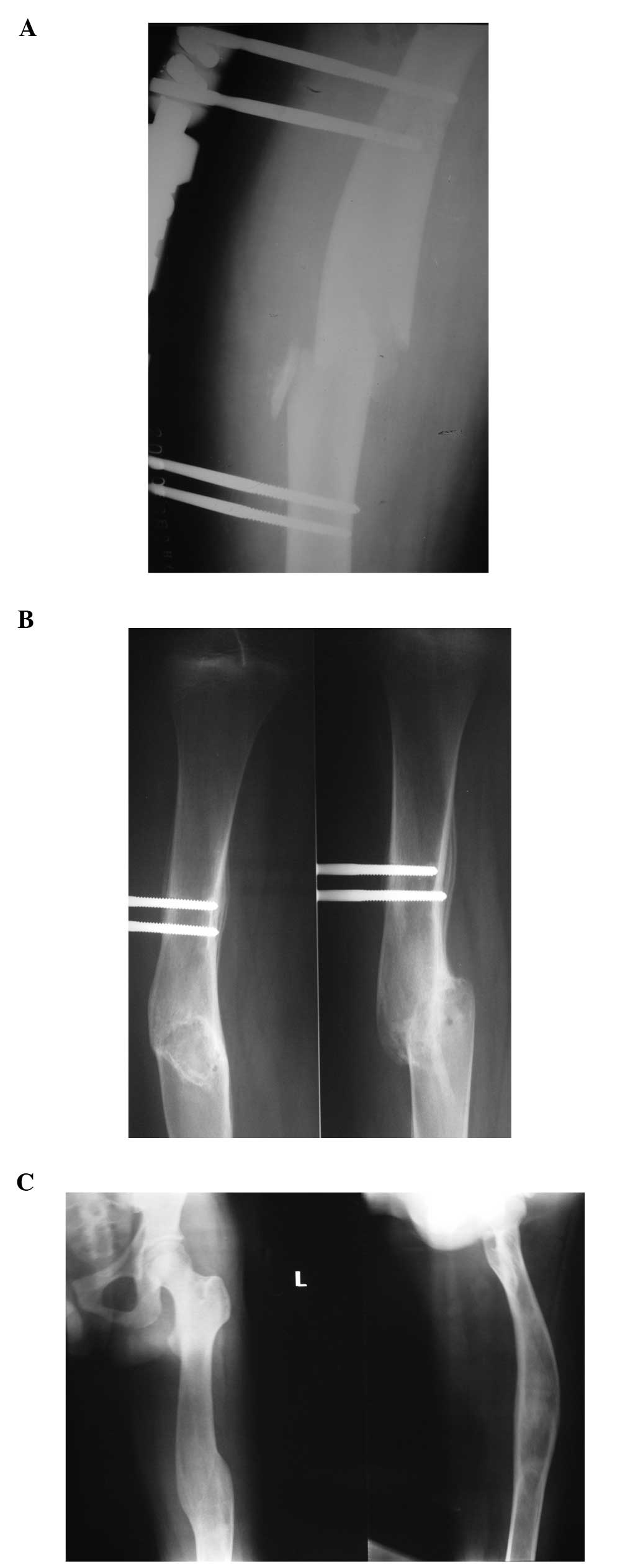
fixator (Fig. 1A). The fracture
resulted in a malunion as demonstrated in the six-month and
one-year follow-up radiographs (Fig. 1B
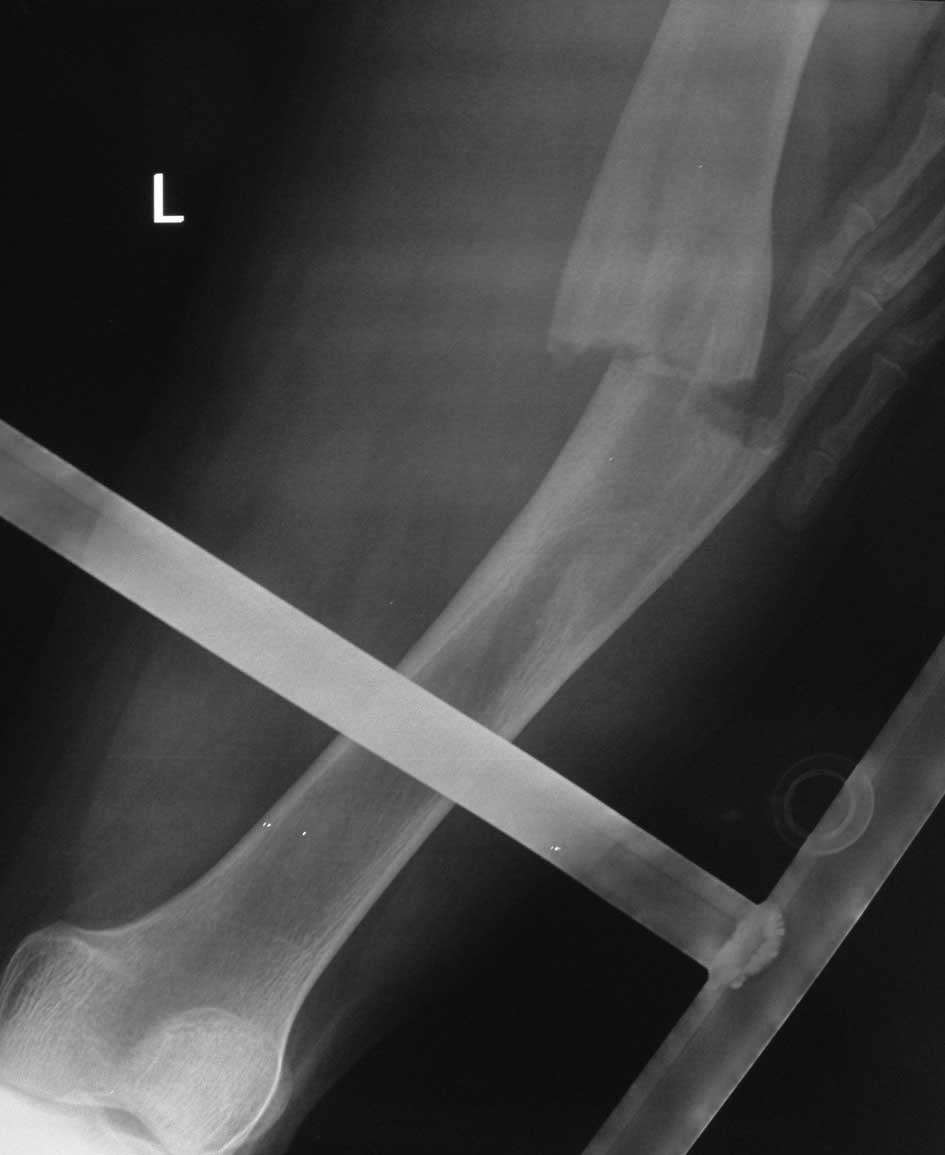
and C, respectively). A refracture of the lesion (Fig. 2) was detected and treated with
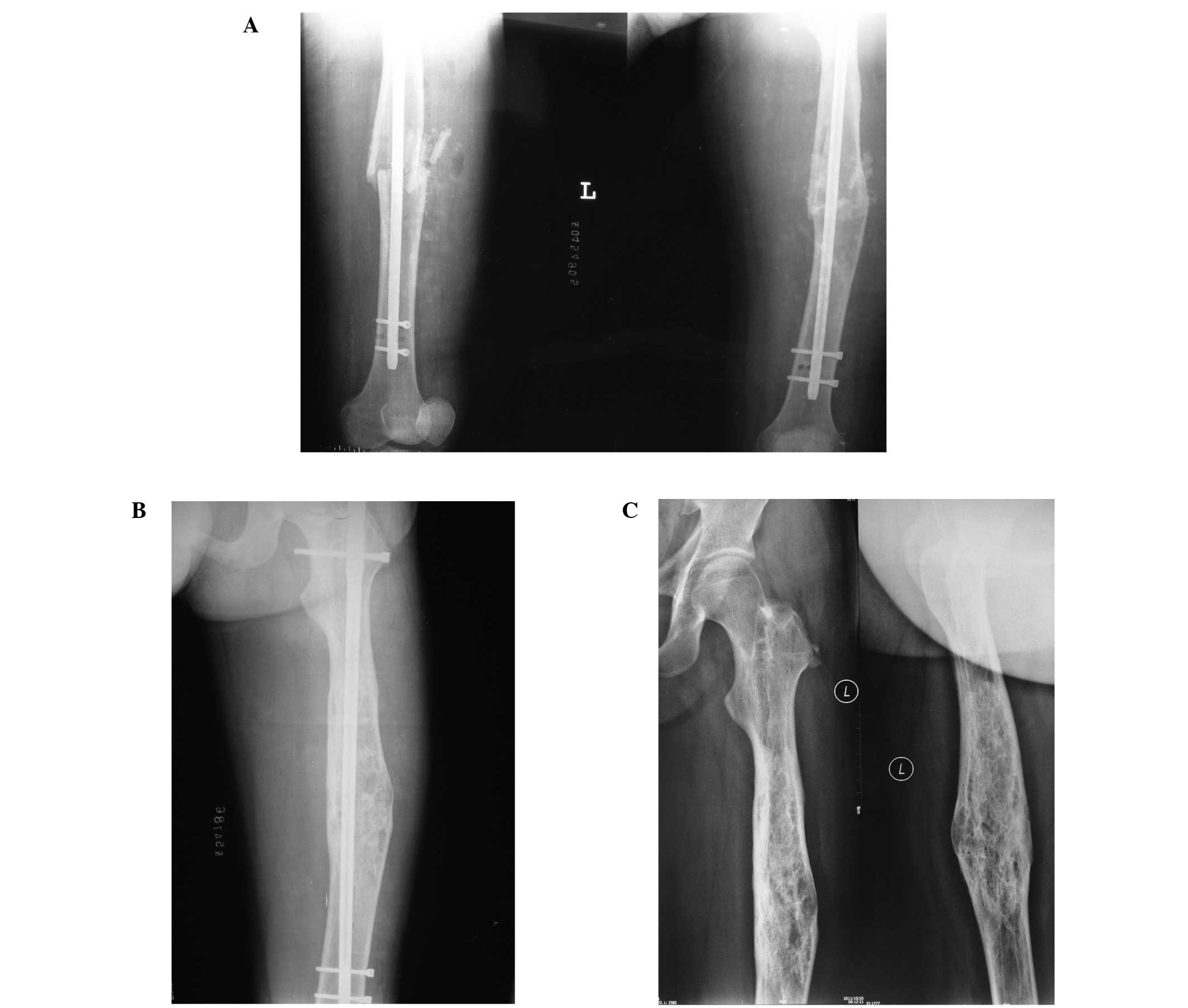
intramedullary nailing at two years follow-up (Fig. 3A). No loosening of screws or
refracture was detected in the follow-up period (Fig. 3B). The fixator was removed at three
years follow-up (Fig. 3C).
Patient recovery and symptoms
A total of 32 patients (82.1%) presented with no
pain and 7 patients (17.9%) had mild and occasional pain at the
last follow-up. All patients resumed full activity and function.
One patient took longer to return to full activity due to
pathological fracture of another limb. The clinical score was in
accordance with the modified criteria described by Guille et
al (8). The clinical score
improved from an average of 4.4 (range, 3–6) points prior to
surgery to an average of 8 (range, 6–10) points at the last
follow-up. The neck shaft angle of the femur was corrected from an
average of 90˚ (range, 70–120˚) prior to surgery to an average of
125˚ (range, 119–140˚).
Discussion
FD of the bone is a type of benign bone lesion. A
single skeletal site with an isolated lesion is involved in the
majority of patients, but the disease may be polyostotic. Previous
studies have not reported indicators for FD, although there are
several methods of treatment, including conservative treatment
(medications and braces) and surgical procedures (curettage,
curettage and graft, and internal fixation). Kusano et al
(9) revealed that the majority of
lesions of FD usually cease to progress or develop following
adolescence, with the exception of McCune-Albright syndrome.
Therefore, conservative treatment is recommended for adolescents.
The mass of the lesion may not require surgery, unless ongoing
pain, lesion expansion or bone percentage predisposes the bone to
pathological fracture or a fracture has already occurred (10–15).
Pain is associated with lesion expansion. Generally, curettage of
the lesion leaves a cavity which predisposes the bone to
destabilization or even pathological fracture. Therefore, an
external or internal fixation is performed for stabilization. In
PFD patients, particularly those with shepherd’s crook deformity,
curettage and bone grafting are not advised due to reabsorption of
bone graft and lesion expansion following surgery. In this case,
internal fixation and correction are recommended for patients with
distinct deformity and/or pathological fracture, which result in
poor quality of life and complications due to bed rest.
In the case of large lesions (more than two-thirds
of the bone) or upon enlargement of the lesion, the bone becomes
unstable and predisposed to fracture when a cavity is left
following curettage. Muscle atrophy, thromboembolism, ankylosis or
other notable complications may occur in the long-term external
fixation period. However, it is difficult to provide sufficient
stability with plates and screws in the vicinity of the weakened
bones, and fracture or refracture may occur due to the
stress-shielding effect of the distal part of the plate. Guille
et al (8) suggested that a
refracture may easily occur after the plate and screws are removed
in patients with a lesion involving a large area. O’Sullivan and
Zacharin (16) reported that
intramedullary nailing and bisphosphonate treatment of 10 femurs
with McCune-Albright syndrome prevented fractures and resulted in
improved walking. Thus, we consider that intramedullary nailing may
be used in other anatomical sites, with the exception of proximal
femoral lesions of the lower limbs. This approach results in fewer
sequelae at the site of the lesion or fracture than when using
plates and screws, and provides sufficient stability with fixation
in the normal distal bones. Particularly in cases with a lesion
covering a large area, the incision has to be enlarged to fit a
long metal plate due to the vicinity of weakened bones, and loss of
fixation, delayed union or non-union of the fracture may occur more
frequently. When a valgus osteotomy is performed, overcorrection of
the neck shaft angle is recommended (more than 130˚) in
anticipation of postoperative loss of valgus in lesions of the
proximal femur, including shepherd’s crook deformity. However, a
satisfactory clinical result can be expected when the neck shaft
angle is at least 90˚ (8). We
recommended overcorrection of the neck shaft angle in anticipation
of loss of valgus angle after surgery, which is limited by muscle
contracture between the greater trochanteric area and the ilium.
This approach is prevented in adolescents due to epiphyseal
injuries. However, intramedullary nailing is performed on the
occurrence of epiphysis fusion or marked changes of the
epiphysis.
During surgery, the cortical fenestration should be
sufficiently large to allow visualization of the whole lesion with
an osteotome. Due to the internal fixation, the bone was stable and
the cortical fenestration did not result in pathological fracture
during or following surgery. We recommend removal of the lesion
using curettes and enlargement of the cavity using a burr. However,
normal trabecular bone reduction due to repeated curettes may cause
delayed union or non-union of the fracture.
A number of authors have recommended that the defect
should be filled with bone grafts or substitutes following
curettage. Autogenous bone grafting is recommended due to the
advantages of lack of immunological reaction and successful bone
induction. However, obtaining sufficient bone for large cavities
may be difficult and sequelae may be generated at the donor site.
In addition, certain authors have recommended curettage of benign
bone tumors without grafts (17).
We recommend curettage and grafting with autogenous bone from the
ilium. However, large defects could alternatively be filled with
substitutes including cement, hydroxyapatite or tricalcium
phosphate, as bone grafts are difficult to obtain.
In conclusion, indicators for FD of the bone have
not yet been established due to low incidence and small sample
sizes. However, we propose an approach which allows patients to
return to full, painless activity quickly in the majority of cases.
The approach used in this study is recommended as a reliable and
effective surgery for FD of the lower limbs.
References
|
1
|
Campanacci M: Bone and Soft Tissue Tumors:
Clinical Features, Imaging, Pathology, and Treatment. 2nd edition.
Springer; New York, NY: 1999, View Article : Google Scholar
|
|
2
|
Von Recklinghausen F: Die Fibrose oder
deformierende Ostitis, die Osteomalacie und die oesteoplastische
carcinose in ihren gegenseitigen Beziehungen. In: Festschrift
Rudolf Virchow zum 13; Oktober; Berlin. 1891, PubMed/NCBI
|
|
3
|
Lichtenstein L: Polyostotic fibrous
dysplasia. Arch Surg. 36:874–898. 1938. View Article : Google Scholar
|
|
4
|
Lichtenstein L and Jaffe HL: Fibrous
dysplasia of bone: a condition affecting one, several or many
bones, graver cases of which may persent abnormal pigmentation of
skin, premature sexual development, hyperthyroidism or still other
extraskeletal abnormalities. Arch Pathol. 33:777–816. 1942.
|
|
5
|
Wirth WA, Leavitt D and Enzinger FM:
Multiple intramuscular myxomas. Another extraskeletal manifestation
of fibrous dysplasia. Cancer. 27:1167–1173. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blasier RD, Ryan JR and Schaldenbrand MF:
Multiple myxomata of soft tissue associated with polyostotic
fibrous dysplasia. A case report. Clin Orthop Relat Res.
206:211–214. 1986.PubMed/NCBI
|
|
7
|
Dorfman HD and Czerniak B: Bone Tumors.
Mosby; St. Louis, MO: 1998
|
|
8
|
Guille JT, Kumar SJ and MacEwen GD:
Fibrous dysplasia of the proximal part of the femur. Long-term
results of curettage and bone-grafting and mechanical realignment.
J Bone Joint Surg Am. 80:648–658. 1998.PubMed/NCBI
|
|
9
|
Kusano T, Hirabayashi S, Eguchi T and
Sugawara Y: Treatment strategies for fibrous dysplasia. J Craniofac
Surg. 20:768–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DiCaprio MR and Enneking WF: Fibrous
dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint
Surg Am. 87:1848–1864. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Easley ME and Kneisl JS: Pathological
fractures through large nonossifying fibromas: is prophylactic
treatment warranted? J Pediatr Orthop. 17:808–813. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arata MA, Peterson HA and Dahlin DC:
Pathological fractures through non-ossifying fibromas. Review of
the Mayo Clinic experience. J Bone Joint Surg Am. 63:980–988.
1981.PubMed/NCBI
|
|
13
|
Drennan DB, Maylahn DJ and Fahey JJ:
Fractures through large non-ossifying fibromas. Clin Orthop Relat
Res. 103:82–88. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahn JI and Park JS: Pathological fractures
secondary to unicameral bone cysts. Int Orthop. 18:20–22.
1994.PubMed/NCBI
|
|
15
|
Betsy M, Kupersmith LM and Springfield DS:
Metaphyseal fibrous defects. J Am Acad Orthop Surg. 12:89–95.
2004.
|
|
16
|
O’Sullivan M and Zacharin M:
Intramedullary rodding and bisphosphonate treatment of polyostotic
fibrous dysplasia associated with the McCune-Albright syndrome. J
Pediatr Orthop. 22:255–260. 2002.PubMed/NCBI
|
|
17
|
Yanagawa T, Watanabe H, Shinozaki T and
Takagishi K: Curettage of benign bone tumors without grafts gives
sufficient bone strength. Acta Orthop. 80:9–13. 2009. View Article : Google Scholar : PubMed/NCBI
|