Introduction
Lung cancer (LC) continues to be the leading cause
of cancer mortality worldwide. Non-small cell lung cancer (NSCLC)
is the most common type of LC, accounting for approximately 80% of
all cases, and is classified into adenocarcinoma, squamous cell
carcinoma and large cell carcinoma. Various therapeutic approaches
have been developed and applied in accordance with the disease
state of individual patients. Despite intensive studies of
treatment modalities for NSCLC, the prognosis of affected patients
remains poor (1).
Idiopathic interstitial pneumonia (IIP) is a group
of slowly progressive pulmonary diseases which lead to respiratory
insufficiency. IIP is a devastating parenchymal lung disease
characterized by alveolar destruction, excess matrix production and
varying levels of inflammation leading to impaired gas exchange.
IIP has a poor prognosis, with a median survival time of 3 to 5
years from the time of diagnosis (2–4). The
clinical course of IIP is usually chronic, but patients may
occasionally experience episodes of acute respiratory worsening.
Although these episodes may be secondary to special conditions,
including pneumonia, pulmonary embolism, pneumothorax or cardiac
failure, the term ‘acute exacerbation (AE) of IIP’ has been used
when a cause for the acute respiratory worsening cannot be
identified. AE of IIP is characterized by the acute or subacute
onset of dyspnea with or without other symptoms, including cough
and low-grade fever, and often progresses rapidly to respiratory
failure requiring hospitalization and mechanical ventilation. Since
no effective therapies are currently available, the prognosis of
patients with AE of IIP remains extremely poor (2–4).
IIP has been considered to be one of the risk
factors for LC. For example, Kawasaki et al reported that
IIP was observed in 7.5% of surgically resected cases of LC
(5). Although IIP patients show a
higher incidence of LC, with a relative risk of 7–14 (2–4,6,7),
no standard therapy for LC complicated by IIP (NSCLC-IIP) has yet
been established. In fact, patients with NSCLC-IIP are often
followed up without standard treatments such as chemotherapy or
radiotherapy, as there has been an underlying belief that
chemotherapy may cause AE of IIP in NSCLC-IIP patients, although no
concrete evidence has been reported. In clinical practice,
NSCLC-IIP has been carefully treated with chemotherapy. Since there
is limited information regarding the feasibility and efficacy of
chemotherapy for NSCLC-IIP, in the present study we conducted a
retrospective analysis of patients with NSCLC- IIP.
Materials and methods
NSCLC patients with or without IIP
We retrospectively examined LC patients with (n=57)
and without (n=488) IIP between 1999 and 2008 at Kurume University
Hospital (Kurume, Japan). This study was approved by the
Institutional Review Board of Kurume University. To focus on the
feasibility and efficacy of chemotherapy, we excluded NSCLC
patients with or without IIP who had received treatments other than
chemotherapy alone, including concurrent chemoradiotherapy,
epidermal growth factor receptor-tyrosine kinase inhibitors
(EGFR-TKIs) and surgery. Patients with an Eastern Cooperative
Oncology Group (ECOG) performance status (PS) of 3 or 4, who were
ineligible for chemotherapy, and those with small-cell lung cancers
were also excluded. A full explanation of the potential risks and
benefits was provided to 28 NSCLC-IIP patients; 22 received
chemotherapy and the remaining 6 selected best supportive care
(BSC). Tumors in the NSCLC-IIP patients receiving chemotherapy were
diagnosed histologically as adenocarcinoma in 11 patients, squamous
cell carcinoma in 7, large cell carcinoma in 2 and non-small cell
carcinoma in 2 on the basis of the World Health Organization (WHO)
criteria. The treatment regimens consisted of carboplatin (CBDCA)
and paclitaxel (TXL; n=19), cisplatin-vinorelbine (n=2) and
cisplatin-docetaxel (n=1). As a control group, 276 NSCLC patients
without IIP who received chemotherapy alone were examined. The
tumor histology was as follows: 203 adenocarcinomas, 57 squamous
cell carcinomas, 9 large cell carcinomas, 3 adenosquamous cell
carcinomas and 4 non-small cell carcinomas (unclassified). The
treatment regimens consisted of CBDCA-based (n=192),
cisplatin-based (n=66) and non-platinum regimens [irinotecan +
ifosfamide (n=5), irinotecan + mitomycin C (n=1), gemcitabine +
vinorelbine (n=2), vinorelbine (n=10)]. The combination of CBDCA
and TXL was the most frequently used regimen (n=177). Details of
the demographics, treatments and follow-up characteristics of the
patients are shown in Table I. The
patients were followed up until the time of mortality or September
2010. All underwent plain chest X-ray examinations, computed
tomography scans of the chest and upper abdomen, bone scans and
magnetic resonance images of the brain prior to chemotherapy and at
least every 6 weeks during chemotherapy. Tumor response was
evaluated following chemotherapy according to the RECIST (Response
Evaluation Criteria for Solid Tumors).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | NSCLC with IIP
chemotherapy | NSCLC |
|---|
| Age (years), median
(range) | 70 (40–76) | 66 (35–84) |
| Gender, n |
| Male | 21 | 172 |
| Female | 1 | 104 |
| Histology, n |
| Adenocarcinoma | 11 | 203 |
| Squamous cell
carcinoma | 7 | 57 |
| Adenosquamous cell
carcinoma | 0 | 3 |
| Large cell
carcinoma | 2 | 9 |
| Non-small cell
carcinoma | 2 | 4 |
| Smoking status,
n |
| Never | 0 | 105 |
| Smoker | 22 | 171 |
| Performance status,
n |
| 0 | 12 | 186 |
| 1 | 10 | 60 |
| 2 | 0 | 30 |
| Stage, n |
| IIIA | 1 | 10 |
| IIIB | 6 | 28 |
| IV or recurrent | 15 | 238 |
| Regimen, n |
| CDDP-based
chemotherapy | 3 | 66 |
| CBDCA-based
chemotherapy | 19 | 192 |
| Others
(non-platinum) | 0 | 18 |
| Cycle, median
(range) | 3 (1–4) | 3 (1–6) |
Diagnosis of IIP and AE
IIP patients were diagnosed histologically when they
showed usual interstitial pneumonia (UIP) or non-specific
interstitial pneumonia (NSIP) by surgical lung biopsy. However,
without histological evidence, they were diagnosed as having
clinical idiopathic pulmonary fibrosis (IPF), a type of IIP, on the
basis of high-resolution computed tomography (HRCT) scans of chest
and/or clinical findings, including basal predominant subpleural
reticular abnormality with traction bronchiectasis and honeycomb
cysts and without atypical features of IPF, auscultation of fine
crackles, presence of clubbed fingers, results of pulmonary
function tests and results of blood examinations [i.e., lactate
dehydrogenase (LDH) and KL-6 levels]. Other diseases, including
connective tissue disease, infection and hypersensitivity
pneumonia, were excluded. The patients had been clinically stable
with no disease exacerbation for at least three months prior to
diagnosis. All the patients were diagnosed as having IIP by at
least three respirologists (M.O., M.T. and K.F.) in accordance with
the clinical criteria established by the American Thoracic Society
(ATS)/European Respiratory Society (ERS), as reported previously
(3).
A diagnosis of AE in IIP patients was made in
accordance with the criteria detailed in previous studies (8,9), as
follows: i) previous or concurrent diagnosis of IIP; ii) worsening
of dyspnea within days to weeks (generally <30 days); iii)
evidence of abnormal gas exchange as defined by a low partial
pressure of arterial oxygen (PaO2)/percentage of
inspired oxygen (FiO2) ratio or a decrease in
PaO2; iv) new radiographic opacities with new bilateral
ground-glass abnormality and/or consolidation superimposed on a
background reticular or honeycomb pattern consistent with IIP; and
v) an absence of an alternative explanation, such as pulmonary
infection, left heart failure, pulmonary embolism or an
identifiable cause of acute lung injury.
Statistical methods
All values are presented as mean ± SD. The Fisher’s
exact and Wilcoxon tests were used to analyze the significance of
the associations between NSCLC-IIP with AE and without AE and other
patient characteristics. Progression-free survival (PFS) was
defined as the time between the start of chemotherapy and the date
when disease progression began. Patients without progression were
regarded as censored at the date of the last follow-up. Overall
survival (OS) was defined as the time between the onset of
chemotherapy and the date of mortality due to any cause. Patients
were regarded as censored if they were alive on the date of the
last follow-up. Curves for PFS and OS were estimated by the
Kaplan-Meier method, and the differences in survival functions were
compared using the log-rank test. The Cox proportional hazards
model was applied to examine the prognostic factors significantly
associated with PFS or OS after adjustment for other factors. All
tests were two-sided, and P<0.05 was considered to indicate a
statistically significant difference. All the statistical analyses
were conducted using JMP version 8 software (SAS Institute Inc.,
Cary, NC, USA).
Results
Characteristics of NSCLC-IIP patients
receiving chemotherapy
Table I shows the
characteristics of the 22 patients with NSCLC-IIP. A full
explanation regarding the potential risks and benefits was provided
to all the patients with NSCLC with IIP. As a result, 22 patients
received 1 to 4 cycles of chemotherapy (median, 3 cycles). The
treatment regimens consisted of CBDCA and TXL (n=19),
cisplatin-vinorelbine (n=2) and cisplatin-docetaxel (n=1). Tumor
response, evaluated by RECIST, was partial response (PR) in 8
patients, stable disease (SD) in 9 and progressive disease (PD) in
5. The response rate (PR and SD) was 72.3%. At the time of
analysis, the median follow-up time for NSCLC-IIP patients who had
received chemotherapy was 163 days (range, 46–589).
Poorer prognosis in NSCLC-IIP patients
compared with NSCLC patients without IIP following
chemotherapy
To clarify the consequences of concomitant IIP in
NSCLC patients receiving chemotherapy, we compared 22 NSCLC-IIP
patients with 276 NSCLC patients without IIP (172 males, 104
females) who had received chemotherapy. As shown in Table I, NSCLC patients without IIP
received 1 to 6 cycles of chemotherapy (median, 3 cycles)
comprising CBDCA-based treatment (n=192), cisplatin-based treatment
(n=66) and non-platinum regimens (n=18). Tumor response, evaluated
by RECIST, was complete response (CR) in 6 patients, PR in 90, SD
in 95 and PD in 85. The response rate (PR and SD) was 69.2%. At the
time of analysis, the median follow-up times for NSCLC patients
without and with IIP were 400 (range, 14–3,424) and 163 days
(range, 46–589), respectively. Univariate Cox analysis was carried
out to identify the factors that were significantly associated with
PFS and OS in all the NSCLC patients receiving chemotherapy,
including those with and without IIP (Table II). Poor PS (P=0.004) and
concurrent IIP (P<0.001) were negative predictors of PFS. For
OS, age (P=0.028), gender (P<0.001), smoking (P<0.001), PS
(P<0.001) and concurrent IIP (P<0.001) were prognostic. None
of the other factors examined were significantly correlated with
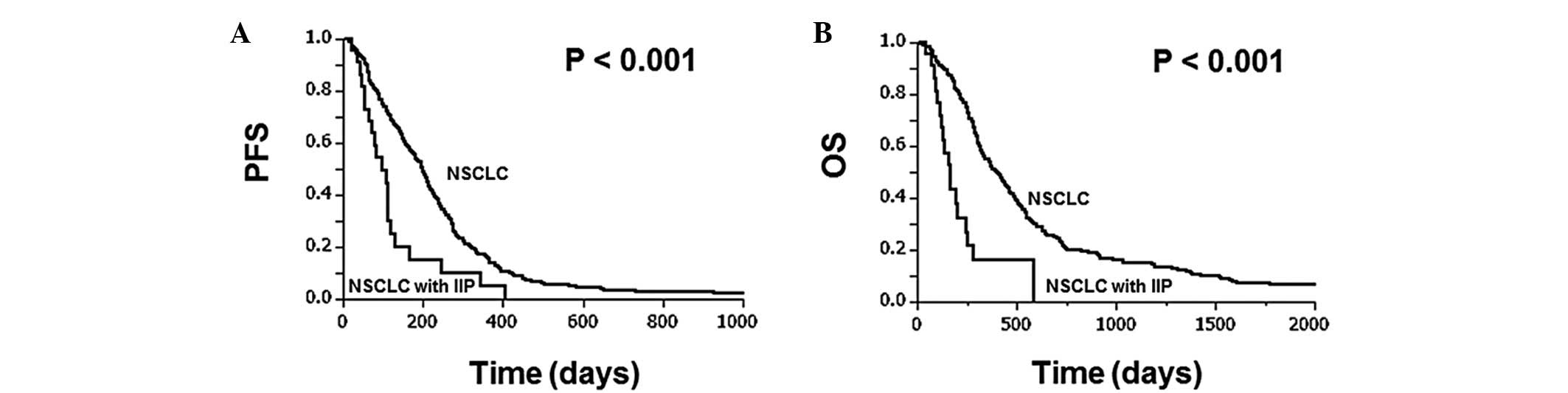
PFS or OS. Fig. 1 shows the
Kaplan-Meier survival curves for NSCLC patients with and without
IIP. NSCLC patients with IIP had a significantly shorter median PFS
(95.0 vs. 199.5 days, P<0.001) and OS (163.0 vs. 400.0 days,
P<0.001) than those without IIP. In addition, the factors that
were significantly associated with PFS or OS in NSCLC patients were
evaluated by applying Cox regression models adjusting for possible
confounding factors. The factors that were of potentially
prognostic significance in the univariate analysis were entered
into the Cox proportional hazards model: performance status and
concomitant IIP for PFS; age, gender, smoking, performance status
and concurrent IIP for OS. Table
III shows the correlation between incidence of AE and various
clinical characteristics, including gender, histology, smoking
status, performance status, stage, treatment modality, LDH, KL-6
and %VC. However, none of the other factors were associated with
incidence of AE.
 | Table IIFactors associated with PFS and
OS. |
Table II
Factors associated with PFS and
OS.
| Factor | n | Median PFS
(days) | P-valuea | Multivariate
analysis | Median OS (days) | P-valuea | Multivariate
analysis |
|---|
|
|
|---|
| Hazard ratio (95%
CI) | P-valueb | Hazard ratio (95%
CI) | P-valueb |
|---|
| Age (years) |
| High (>66) | 161 | 192.0 | 0.648 | | | 353.5 | 0.028 | 1.000 | 0.2398 |
| Low (<66) | 137 | 182.5 | | | | 379.0 | | 1.161
(0.905–1.491) | |
| Gender |
| Female | 105 | 181.5 | 0.536 | | | 475.5 | <0.001 | 1.000 | 0.109 |
| Male | 193 | 191.5 | | | | 319.5 | | 1.477
(0.917–2.374) | |
| Histology |
| Adenocarcinoma | 214 | 182.5 | 0.203 | | | 374.0 | 0.572 | | |
| Non-adenoc | 84 | 201.5 | | | | 360.0 | | | |
| Smoking |
| Never | 105 | 180.5 | 0.363 | | | 466.5 | <0.001 | 1.000 | 0.9441 |
| Smoker | 193 | 191.5 | | | | 320.0 | | 1.017
(0.634–1.646) | |
| Performance
status |
| 0 | 198 | 211.0 | 0.004 | 1.000 | 0.003 | 441.0 | <0.001 | 1.000 | <0.001 |
| 1 or 2 | 100 | 135.5 | | 1.462
(1.140–1.862) | | 258.0 | | 1.823
(1.414–2.367) | |
| Stage |
| III | 45 | 221.5 | 0.133 | | | 411.0 | 0.874 | | |
| IV or recurrent | 253 | 181.5 | | | | 367.5 | | | |
| NSCLC |
| NSCLC | 276 | 197.5 | <0.001 | 1.000 | 0.001 | 394.0 | <0.001 | 1.000 | <0.001 |
| NSCLC with
IIP | 22 | 95.0 | | 2.335
(1.443–3.577) | | 163.0 | | 2.874
(1.675–4.654) | |
 | Table IIIFactors associated with AE in NSCLC
complicated by IIP. |
Table III
Factors associated with AE in NSCLC
complicated by IIP.
| NSCLC patients with
IIP | |
|---|
|
| |
|---|
|
Characteristics | AE (−) n=19 | AE (+) n=3 | P-value |
|---|
| Age (years), median
(range) | 70 (45–76) | 66 (66–75) | |
| Gender, n |
| Male | 18 | 3 | 1.000a |
| Female | 1 | 0 | |
| Histology, n |
|
Adenocarcinoma | 9 | 2 | 1.000a |
|
Non-adenocarcinoma | 10 | 1 | |
| Smoking status,
n |
| Never | 1 | 0 | 1.000a |
| Smoker | 18 | 3 | |
| Perfomance status,
n |
| 0 | 12 | 0 | 0.779a |
| 1 | 7 | 3 | |
| Stage, n |
| III | 7 | 0 | 0.5227a |
| IV or
recurrent | 12 | 3 | |
| Regimen, n |
| CDDP-based
chemotherapy | 3 | 0 | 1.000a |
| CBDCA-based
chemotherapy | 16 | 3 | |
| LDH, mean ± SD | 280.6±105.6 | 374±308.8 | 0.9238b |
| KL-6, mean ±
SD | 1373.8±1073.3 | 950±298.3 | 0.6323b |
| %VC, mean ± SD | 89±18.9 | 76.1±21.4 | 0.3379b |
Risk of AE in NSCLC-IIP patients
The incidence of AE was 13.6% (3/22) among NSCLC-IIP
patients who received chemotherapy. Table IV shows the clinical
characteristics of the NSCLC-IIP patients who developed AE
following chemotherapy. All the patients with AE had been treated
with the CBDCA and TXL combination.
 | Table IVCharacteristics of the three patients
with AE. |
Table IV
Characteristics of the three patients
with AE.
| No. | Age (years) | Gender | PS | Smoking
(packs/year) | Histology | Stage | Regimen | Clinical
symptoms | Onset (days from
chemotherapy) | Survival
(days) |
|---|
| 1 | 73 | M | 1 | 80 | Non-small | IV | CBDCA + PTX | Fever | 132 | 169 |
| 2 | 67 | M | 1 | 100 | Adenocarcinoma | IV | CBDCA + PTX | Dyspnea | 53 | 138 |
| 3 | 66 | M | 1 | 40 | Adenocarcinoma | IV | CBDCA + PTX | Dyspnea | 52 | 130 |
Discussion
Patients with LC are known to be frequently
complicated by IIP. However, there has been little information
regarding the optimal treatment approach for advanced NSCLC-IIP. In
the present study, to investigate the feasibility and efficacy of
chemotherapy for NSCLC-IIP patients, we retrospectively examined 22
NSCLC-IIP patients who received chemotherapy. Consistent with our
findings, Minegishi et al also recently reported the
efficacy of chemotherapy with CBDCA and TXL in 18 NSCLC-IIP
patients, who showed a response rate of 61%, a median PFS of 5.3
months and a median OS of 10.6 months (10). Based on these findings, chemotherapy
may be recommended as a feasible option for NSCLC-IIP patients, if
the risks of adverse effects are acceptable.
It has been speculated that NSCLC-IIP patients who
receive chemotherapy would have a higher risk of AE, which is the
most serious adverse event associated with IIP. However, there has
been little information concerning AE of IIP following
chemotherapy. AE is a well-known phenomenon that develops during
the natural course of IIP in 14–21% of affected patients. Kim et
al demonstrated that the incidence rate of AE in IPF patients
was 8.5% within 1 year of diagnosis and 9.6% within 2 years
(11). In the present study, 13.6%
of IIP patients with NSCLC (3 out of 22) developed AE. Similarly,
Minegishi et al demonstrated that the incidence rate of AE
was 5.6 and 18% following the first- and second-line chemotherapy
with CBDCA and TXL, respectively (10). Kenmotsu et al also reported
that the incidence rate of AE was 13% in CBDCA and 1% in TXL
(12). Taken together, these
results suggest that chemotherapy, particularly that with the
combination of CBDCA and TXL, may be of acceptable toxicity and
feasible for patients with NSCLC-IIP who have good performance
status.
Since the clinical outcomes of NSCLC-IIP have not
been well studied, it has been controversial whether concurrent IIP
would affect the prognosis of NSCLC. It has been previously
reported that the outcome of LC patients with IIP was worse than
that of patients without IIP (13).
By contrast, another study has shown that the survival of patients
with IIP and LC did not differ significantly from that of patients
with IIP or LC alone (7). Since
there has been no clear conclusion, we examined the effect of
concurrent IIP in the outcomes of NSCLC patients in the present
study. Multivariate analysis demonstrated that in NSCLC patients
receiving chemotherapy, IIP was a significantly unfavorable factor
for PFS and OS. Nevertheless, considering that the response rates
to chemotherapy were similar between NSCLC patients with and
without IIP (72.3 vs. 69.2%), the poor prognosis of NSCLC-IIP
patients may, at least in part, be due to the natural course of
IIP, rather than to poorer response to treatments.
In summary, the present findings suggest that
chemotherapy is a feasible option for NSCLC-IIP. Nevertheless, it
should be noted that there are some limitations in the present
study. First, the number of NSCLC-IIP patients was relatively
small, and the population was heterogeneous. Second, the
retrospective nature did not allow for a standardized measure of
PFS. Therefore, a larger-scale prospective randomized control study
employing homogeneous standard regimens is required in order to
evaluate more precisely the feasibility and efficacy of
chemotherapy for patients with NSCLC-IIP.
Acknowledgements
We thank Hisamichi Aizawa for useful advice.
References
|
1
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000: the global picture. Eur J Cancer. 37(Suppl
8): S4–S66. 2001.PubMed/NCBI
|
|
2
|
Turner-Warwick M, Lebowits M, Burrows B
and Johnson A: Cryptogenic fibrosing alveolitis and lung cancer.
Thorax. 35:496–499. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Thoracic Society/European
Respiratory Society. American Thoracic Society/European Respiratory
Society international multidisciplinary consensus classification of
the idiopathic interstitial pneumonias. This joint statement of the
American Thoracic Society (ATS), and the European Respiratory
Society (ERS) was adopted by the ATS board of directors, June 2001
and by the ERS Executive Committee, June 2001. Am J Respir Crit
Care Med. 165:277–304. 2002.
|
|
4
|
Katzenstein AL and Myers JL: Idiopathic
pulmonary fibrosis. clinical relevance of pathologic
classification. Am J Respir Crit Care Med. 157:1301–1315. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawasaki H, Nagai K, Yokose T, et al:
Clinicopathological characteristics of surgically resected lung
cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol.
76:53–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park J, Kim DS, Shim TS, et al: Lung
cancer in patients with idiopathic pulmonary fibrosis. Eur Respir
J. 17:1216–1219. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aubry MC, Myers JL, Douglas WW, et al:
Primary pulmonary carcinoma in patients with idiopathic pulmonary
fibrosis. Mayo Clin Proc. 77:763–770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collard HR, Moore BB, Flaherty KR, et al:
Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir
Crit Care Med. 176:636–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwal R and Jindal SK: Acute
exacerbation of idiopathic pulmonary fibrosis: a systematic review.
Eur J Intern Med. 19:227–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minegishi Y, Sudoh J, Kuribayashi H, et
al: The safety and efficacy of weekly paclitaxel in combination
with carboplatin for advanced non-small cell lung cancer with
idiopathic interstitial pneumonias. Lung Cancer. 71:70–74. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DS, Park JH, Park BK, Lee JS,
Nicholson AG and Colby T: Acute exacerbation of idiopathic
pulmonary fibrosis: frequency and clinical features. Eur Respir J.
27:143–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kenmotsu H, Naito T, Kimura M, et al: The
risk of cytotoxic chemotherapy-related exacerbation of interstitial
lung disease with lung cancer. J Thorac Oncol. 6:1242–1246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyazaki K, Kurishima K, Kagohashi K,
Kawaguchi M, Ishikawa H, Satoh H and Hizawa N: Serum KL-6 levels in
lung cancer patients with or without interstitial lung disease. J
Clin Lab Anal. 24:295–299. 2010. View Article : Google Scholar : PubMed/NCBI
|