Introduction
Low-grade gliomas (LGGs) have a relatively slow
growth rate. Adjuvant treatment following maximal safe surgical
resection for patients with LGG remains controversial due to the
favorable natural history of the disease. A survival benefit in
patients treated with aggressive surgical resection and
postoperative radiotherapy has not been demonstrated in prospective
clinical trials. The majority of retrospective studies demonstrated
improved outcomes in groups of patients with total or subtotal
resection (1–3). However, even with aggressive surgical
procedures, total resection is rarely achieved. This is due to the
tumor size, location in critical regions of the brain and the
diffusely infiltrative nature of these tumors.
In general, the majority of patients undergo surgery
at the time of symptom presentation. Consequently, it is difficult
to establish the histopathological diagnosis and grade, and to
determine further treatment strategies. Three phase III trials
investigated the role of radiotherapy in patients with LGGs who
underwent surgical resection. In the EORTC 22845
multi-institutional study, patients were randomized to receive
postoperative radiotherapy of 54.0 Gy, or radiotherapy at the time
of progression. A statistically significant improvement in median
progression-free survival (PFS), 5.3 versus 3.4 years
(p<0.0001), was found in the group of patients treated with
early radiotherapy compared to patients treated with radiotherapy
at progression. There was no difference in the median OS in the
study, 7.4 versus 7.2 years in the group of patients with early
versus late radiotherapy (p=0.8) (4). In the subsequent study (EORTC 22844),
the radiation dose was established in postoperative patients, who
were randomized to receive 45.0 or 59.4 Gy. There were no
statistically significant differences in 5-year OS, 58 versus 59%,
and PFS, 47 versus 50%, in patients who received 45.0 and 59.4 Gy,
respectively (5). In the third
study, patients were again randomized to radiotherapy at a low dose
of 50.4 Gy or a high dose of 64.8 Gy. There were no statistically
significant differences in OS and PFS, but grade 3–5 neurotoxicity
was observed in 5% of patients treated with a high-dose of
radiotherapy compared to 2.5% of patients in the low-dose regimen
(6). Several studies have been
conducted to identify prognostic factors in patients treated for
LGG (1,3,7,8). The
age at the time of diagnosis, performance status, histological
subtype, tumor site and size, type of symptoms at diagnosis, the
duration of disease symptoms prior to diagnosis and extent of
resection have been proposed as prognostic factors for PFS and OS.
However, in many of these retrospective studies, the small number
of patients and the heterogeneity of treatments limited the
analysis of prognostic factors.
Pignatti et al (9) conducted an analysis, which was based
on data from EORTC 22844 and 22845 studies, and presented a scoring
system to identify patients with a low and high risk. The
multivariate analysis showed that patients >40 years, with
astrocytoma histopathology, a tumor size of ≥6 cm, tumor crossing
the midline, as well as occurrence of neurological deficits, were
correlated with worse survival (9).
In our retrospective study, the treatment outcomes of patients with
LGG were presented. Potential prognostic factors were evaluated as
well as their impact on DFS and OS.
Patients and methods
Patients
A total of 30 patients who underwent tumor excision
and adjuvant radiotherapy due to diagnosis of brain tumor were
enrolled in this retrospective study. The patients underwent
radical radiotherapy between February 2008 and July 2011 at the
Radiotherapy Department of the Medical University of Lodz, Poland.
There were 16 males (53%) and 14 females (47%). The male to female
ratio was 1.1:1. The age of patients fluctuated between 20 and 72
years [mean 41.4; median 40.5; 95% confidence interval (CI 95%)
36.9–45.9; standard deviation (SD) 12.0]. In the analyzed group,
the performance status of patients was evaluated using the
Karnofsky Performance Scale (KPS) index. There were three patients
(10%) classified at 100% KPS. Fourteen (47%) and 8 patients (27%)
were classified at 90 and 80% KPS, respectively. Five patients
(17%) were classified at 70% KPS. The duration of disease symptoms
prior to the start of treatment ranged from 1 to 107 days. The mean
duration of disease symptoms was 19.0±30.9 days (median 6.0, CI 95%
7.1–31.0). Tumor limited to one lobe was reported in 20 patients
(67%). In seven cases (23%) the tumor was localized within two or
three lobes. In two (7%) and one (3%) patients the tumor was
limited to the midline zone and cerebellum, respectively. The tumor
size (the largest diameter defined in millimeters, measured by
preoperative CT or MRI) ranged from 14 to 102 mm (mean 69.9±35.2;
median 85; CI 95% 56.5–83.3).
The tumor tissue collected during surgery allowed a
histopathological diagnosis to be determined in all patients, and
became a basis for planning the further treatment strategy.
According to tumor histology, there were 14 astrocytomas (47%), 3
oligodendrogliomas (10%), 8 mixed tumors (26%) and 5 gemistocytic
astrocytomas (17%). Five patients (17%) underwent a total tumor
excision. All patients were enrolled in the study after total
excision due to high risk of surgical treatment failure (patients
>40 years, astrocytoma histopathology, tumor size of ≥6 cm,
tumor crossing the midline as well as occurrence of neurological
deficits) (9). In total, 19 (63%)
and 6 patients (20%) underwent subtotal excision and biopsy,
respectively. The mean size of the residual tumor following
subtotal resection or biopsy was 51.5±18.6 mm (median 47.5; CI 95%
44.5–58.4). In the group of patients who underwent total and
subtotal tumor excision 3 recurrences (10%) and 3 cases of disease
progression (10%), respectively, were observed prior to the start
of adjuvant therapy. Four patients (13%) underwent tumor
re-excision, which was defined as the subtotal in all cases. No
adjuvant chemotherapy was administered following surgery. Patient
and tumor characteristics are shown in Table I.
 | Table IPatient characteristics and tumor
details. |
Table I
Patient characteristics and tumor
details.
| Analysed factors | N (%) |
|---|
| Gender |
| Males | 16 (53) |
| Females | 14 (47) |
| Age (years) |
| <40 | 14 (47) |
| ≥40 | 16 (53) |
| Performance
status |
| ≥90% | 17 (57) |
| <90% | 13 (43) |
| Duration of disease
symptoms (days) |
| ≥19 | 20 (67) |
| <19 | 10 (33) |
| Tumor site |
| One lobe | 20 (67) |
| Two or three
lobes | 7 (23) |
| Midline zone | 2 (7) |
| Cerebellum | 1 (3) |
| Tumor size (cm) |
| <3 | 3 (10) |
| 3–5 | 13 (43) |
| >5 | 14 (47) |
| Histopathology |
| Astrocytoma | 14 (47) |
|
Oligodendroglioma | 3 (10) |
| Mixed tumor | 8 (26) |
| Gemistocytic
astrocytoma | 5 (17) |
| Residual tumor |
| Yes | 25 (83) |
| No | 5 (17) |
| Response to
radiotherapy |
| Complete
remission | 10 (33) |
| Stabilization | 17 (57) |
| Disease
progression | 3 (10) |
The study was approved by the ethics committee of
the Medical University of Lodz. Consent was obtained from the
patients or from a family member.
Radiotherapy
External beam radiotherapy was delivered using a
linear accelerator (6 MV photons). Precise patient immobilization
and reproducibility of head positioning was achieved by using
thermoplatic masks. The treatment volumes as well as critical
organs (optic nerves and optic chiasm, lenses and brain stem) and
radiation dose were defined using the ‘Eclipse Varian’ computer
planning system. The tumor or tumor bed demonstrated on
preoperative T1- and T2-weighted MRI sequences with a margin of 1.5
cm, included in clinical target volume (CTV), were treated. The
three-dimensional conformal technique was used in each case.
Treatment volumes were defined according to the 50 and 62
International Commission on Radiation Units and Measurements (ICRU)
reports. The target volume was covered by the 95% isodose. The
daily radiation dose was 1.8 Gy increasing to a total radiation
dose of 54.0 Gy in 30 fractions. In the group of patients with
gemistocytic astrocytoma histopathology, the total dose was
escalated to 60 Gy, with 2.0 Gy per fraction. Treatment was
administered five days per week for five weeks. The correctness of
treatment plan realization was checked on a simulator, where
treatment fields were transmitted on the patient’s skin, and saved
in the computer system simultaneously. Treatment field set-up
repeatability was evaluated through simulator and accelerator image
fusion and geometric error measurement.
Follow-up
Patients were followed up after the end of
radiotherapy. A general and neurological examination was evaluated
during every follow-up visit, as well as a brain MRI study.
Patients were evaluated for disease progression as well as
treatment-related toxicity. The treatment-related toxicity was
evaluated according to the RTOG/EORTC classification. The response
to radiotherapy was evaluated from six to eight weeks following the
end of treatment using MRI study. Complete remission was achieved
in 10 patients (33%). Disease progression and stabilization were
observed in 3 (10%) and 17 patients (57%), respectively. In 9
patients (30%), disease progression was reported during the whole
follow-up period. The disease progression was confirmed in a brain
MRI study. The tumor size after progression ranged from 20 to 106
mm (mean 90.8±27.2; median 85; CI 95% 80.3–101.2). Three patients
with disease progression underwent re-excision defined as subtotal
resections.
The histological progression of malignancy to grade
III (anaplastic variants), according to WHO scale, was reported in
two patients following re-excision. One patient qualified for
stereotactic radiosurgery as salvage retreatment. A dose of 12.0 Gy
radiation in 80% isodose was delivered. Chemotherapy as a salvage
treatment was used in a group of six patients with disease
progression. Three patients received temozolomide (TMZ), and the
remaining three patients received the PCV regimen (procarbazine,
lomustine and vincristine). Of 5 patients treated with
chemotherapy, only one did not receive six cycles. One patient
treated with the PCV regimen received 4 cycles due to general and
neurological deterioration. In four patients treated with
chemotherapy, further progression was reported in imaging studies.
Partial and complete regression were reported in two patients.
Statistical analysis
In the present retrospective study, OS and DFS for
all patients have been estimated. The Kaplan-Meier analysis was
used for OS and DFS estimation. The end-point of the analysis was
mortality as a result of any cause. The Chi-square test was used to
determine whether the distribution of prognostic variables was
significantly different. Comparisons of survival curves were made
using the log-rank test. Clinical prognostic factors including age,
performance status, duration of the disease symptoms, tumor site,
postoperative margin status, tumor size, histopathological tumor
type, recurrence after surgery, response to adjuvant radiotherapy
and use of chemotherapy were included in the statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Disease progression and
clinicopathological characteristics
Within a median follow-up of 21.8 months (range,
3.9–52.6) there were 9 patients (30%) reported to have disease
progression. In the analyzed group of patients, the 2- and 5-year
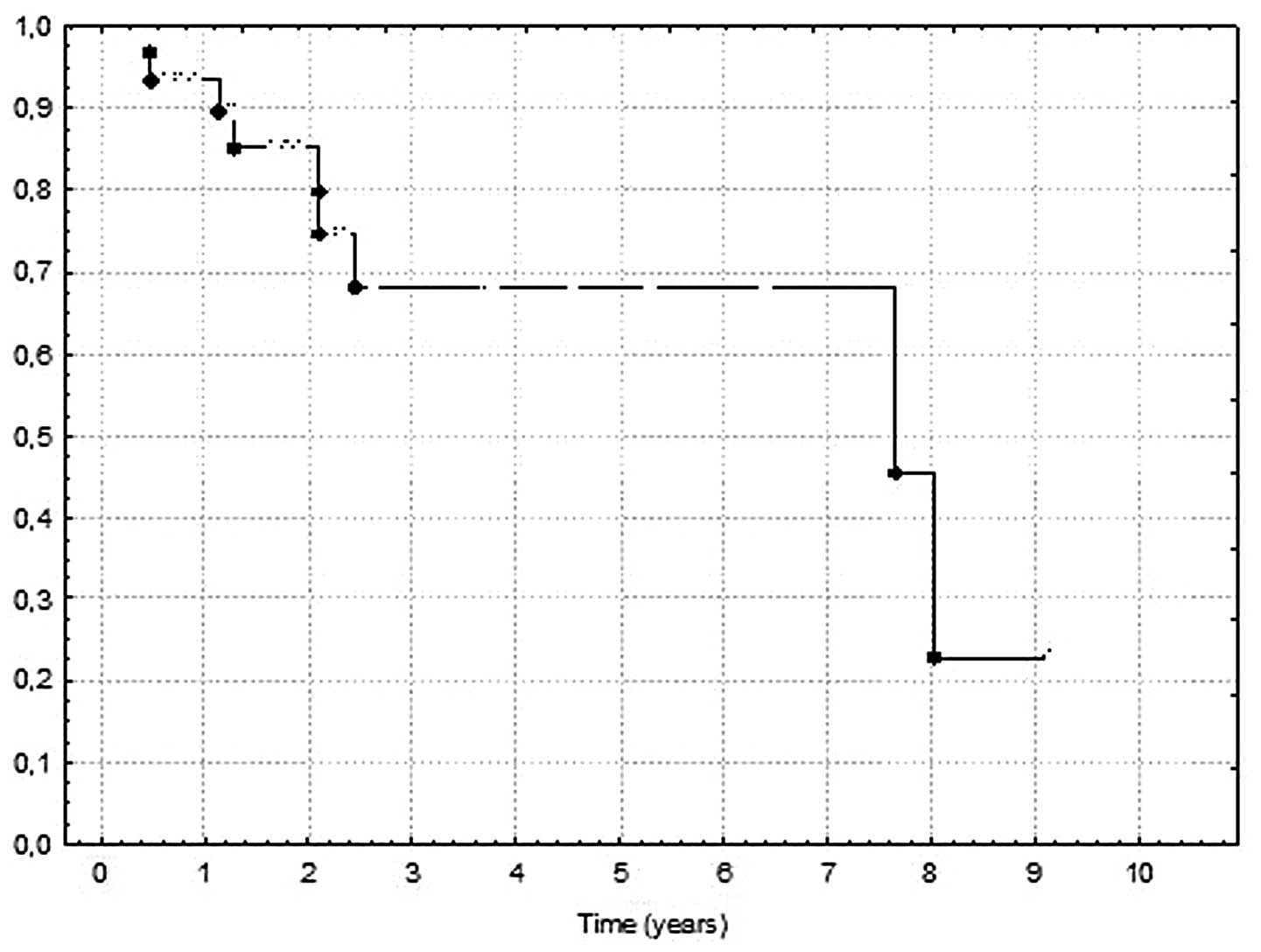
DFS was 85.2 and 68.3%, respectively. The mean DFS was 33.6 months
(range, 5.6–107.5; median 24.8; 95% CI 23.3–43.9; SD 27.6). The 2-
and 5-year OS in the analyzed group of patients was 84.3 and 63.4%,
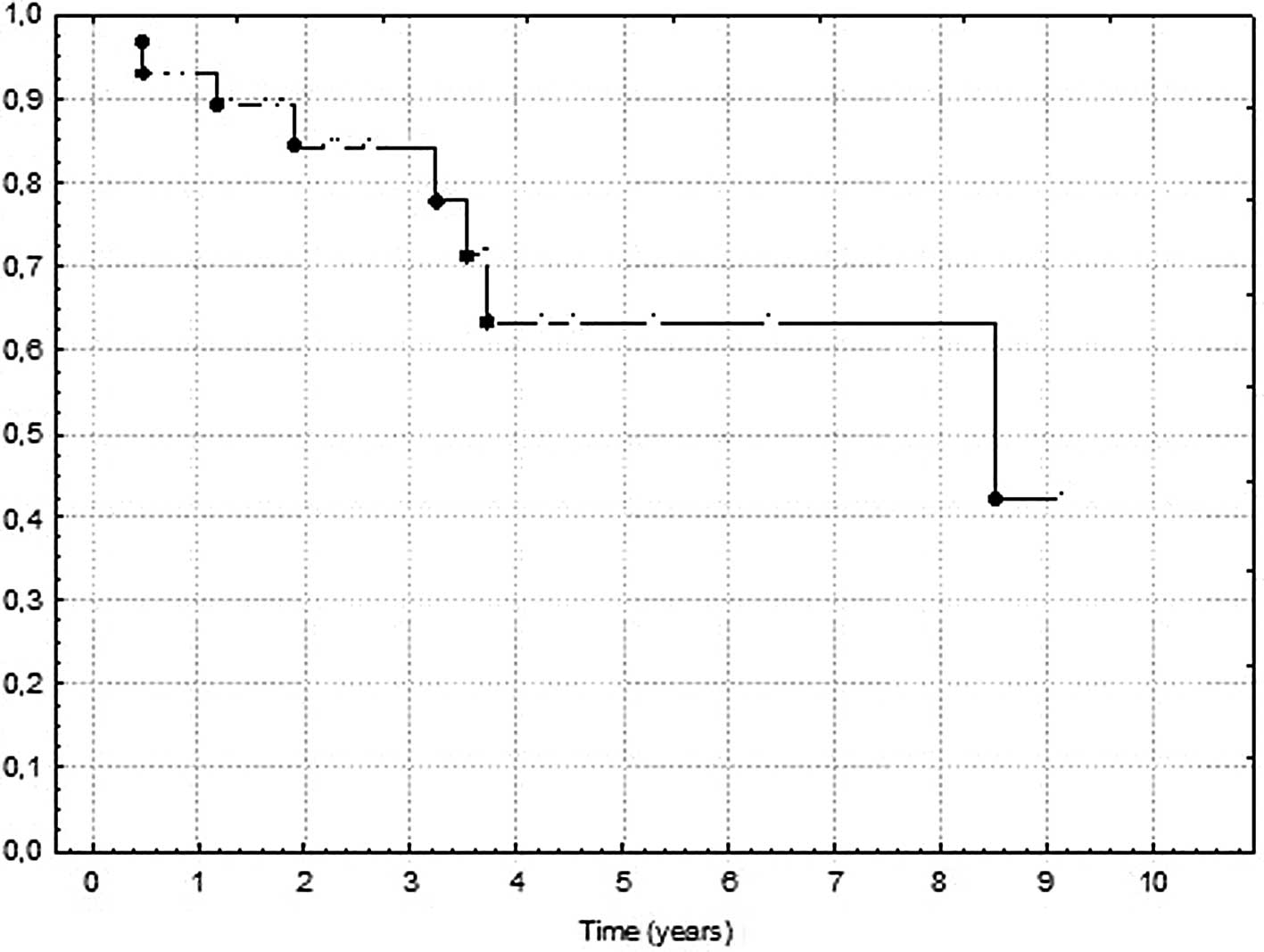
respectively. The mean OS was 36.2 months (range, 5.6–107.5; median
26.9; 95% CI 25.4–46.8; SD 28.6). The 2- and 5-year DFS and OS are
shown in Figs. 1 and 2, respectively. In the present analyzed
group of patients, the age, performance status, duration of disease
symptoms and tumor site, as well as tumor size and
histopathological tumor type, did not have any impact on DFS and
OS. The residual tumor following surgery did not show any negative
values in respect to OS (p=0.7), but we noted a trend towards
statistically significant longer OS in the group of patients who
underwent re-excision due to recurrence before radiotherapy had
been initiated (p=0.03).
Overall survival in respect to response
to radiotherapy
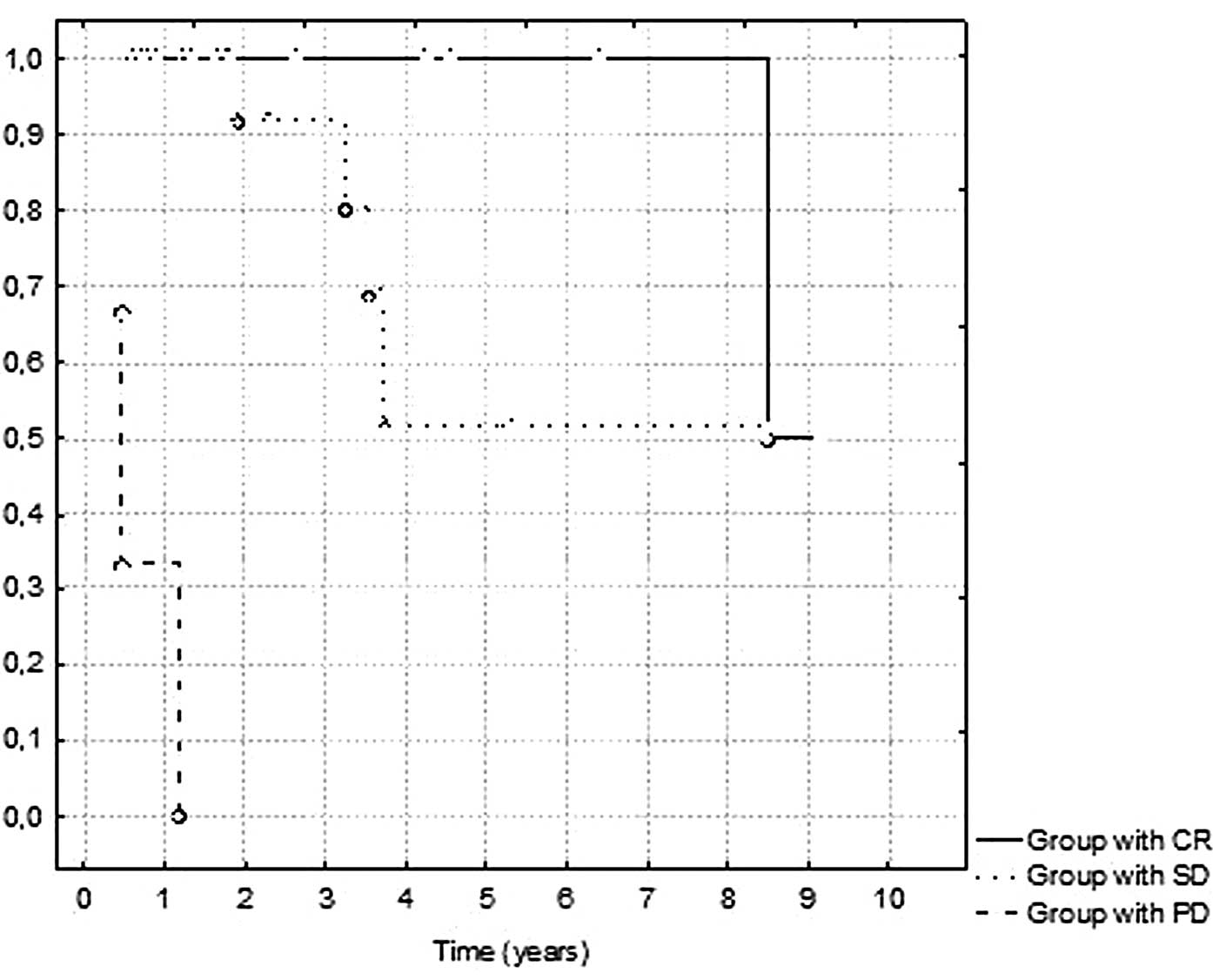
In the statistical analysis, we reported that
response to radiotherapy evaluated from 6 to 8 weeks after
treatment termination was highly correlated with OS. The mean OS in
the group of patients with noted complete remission was 4.03 years
compared to 2.9 years in the group with disease stabilization. The
mean survival in the group of patients with progression in the
period of 6–8 weeks following radiotherapy termination was 8.3
months. The p-value in the log-rank analysis was <0.0001. The OS
in responders and non-responders is shown in Fig. 3. In the presented analysis we
observed a statistically significant higher OS in patients with
disease progression after the end of radiotherapy treated with
salvage chemotherapy (p=0.08). The mean OS of patients treated with
salvage chemotherapy was 3.8 years compared to 2.8 years in the
group of patients without the treatment. Late toxicity was not
assessed in our group of patients due to the retrospective nature
of the study. For those patients who were alive without any active
tumor at the last follow-up, the quality of life was determined to
be extremely good, without evidence of postradiation necrosis in
imaging studies.
Discussion
The prognosis for patients with LGG is favorable,
and long survival is expected in this group of patients. In the
three largest randomized trials, EORTC 22844, EORTC 22845 and the
NCCTG trial, the 5-year OS ranged from 58 to 72%. In the present
study, the 5-year OS and DFS was 63.4 and 68.3%, respectively. The
treatment results are in agreement with previously reported studies
(4–6). The role of the extent of surgical
excision in the treatment of patients with LGG is controversial.
Extent of surgical excision was identified as a prognostic factor
in a number of studies (1,3,4–6,10,11).
However, it is worth noting that some authors reported that the
extent of surgical excision was not correlated with DFS and OS
(12,13). It was postulated that extensive
resection was possible to conduct due to the limited size and
superficial localization of the tumor. However, our results showed
the extent of surgical excision did not have any impact on DFS and
OS rates. LGGs are infiltrative tumors with microscopic invasion
beyond the radiographic margins, thus, gross total resection is
rarely achieved. In total, 25 patients included in our study
received immediate radiotherapy with a total dose of 54.0 Gy in 30
fractions, and the remaining five patients with gemistocytic
astrocytoma histopathology received a total dose of 60 Gy. The
EORTC 22845 study compared immediate radiotherapy with treatment
after recurrence. OS and time to progression were the primary end
points. In this study, immediate postoperative irradiation
increased the median PFS, but not OS (4). The EORTC study conducted a detailed
analysis of prognostic factors for OS based on the 22844 and 22845
studies. Factors that negatively affected survival were age >40
years, astrocytoma histology, maximum tumor diameter ≥6 cm, tumor
crossing the corpus callosum and the presence of a neurological
deficit prior to surgery (4,5).
In the present retrospective study, the patients had
indications for radiotherapy according to the negative prognostic
factors that were defined in the EORTC studies. These factors were
included in the statistical analysis. The majority of studies found
some correlation between survival and signs and symptoms at the
time of presentation. A number of studies found that the presence
of neurological deficits as well as the poor performance status of
the patients were negative prognostic factors (3,7,10,11).
In one study, the authors noted the favorable outcomes in a group
of patients presenting with seizures as the only symptom of disease
(3). In the present analysis
patients’ KPS, and the duration of disease symptoms were evaluated.
Our results did not show an association between these values and
treatment outcomes. These three factors (performance status,
presence of seizures and neurological deficits) are connected. When
neurological or other functional deficits are observed, a decrease
in the performance status is reported. Pignatti et al
revealed a close relationship between the presence of seizures and
presence of other neurological symptoms (9). This association was also observed in a
study in which epilepsy was associated with a better outcome if
this was the only symptom at presentation. When other symptoms were
presented, seizures were no longer a positive prognostic factor
(14).
Age is the fundamental prognostic factor for
survival of patients with LGGs (3–5,7,10,11,13–15).
The elderly patients had a worse prognosis. In one study, a linear
relationship between age and patient prognosis was reported
(7). A cut-off point of 40 years
was selected in the majority of the abovementioned studies. In the
present group of patients the mean age was 41.4 years, median 40.5
and 95% CI was 36.9–45.9. Our results did not confirm a significant
association between age and prognosis of patients with LGGs. This
finding was probably due to the small number of patients in the
analysis, and the non-parametric distribution of this value. We
also did not find a significant correlation between tumor histology
subtype and DFS or OS. This was probably due to the small number of
patients included in the statistical analysis and the unequal
distribution of histological subgroups.
In the majority of studies, patients with
oligodendrogliomas or mixed oligoastrocytic tumors had a more
favorable prognosis than patients with pure astrocytic histology
(3–5,8,9). In
the present study, the response to radiotherapy following treatment
termination was highly correlated with higher OS rate. The mean OS
in the group of patients with complete remission was 4.03 years
compared to 2.9 years in the group with disease stabilization. The
mean survival in the group of patients with progression in the
period of 6–8 weeks following radiotherapy termination was 8.3
months. The p-value in the log-rank analysis was <0.0001. Few
data are available in the literature describing the correlation
between response of LGGs to radiotherapy and treatment outcomes.
Bauman et al (16) analyzed
symptomatic improvement and PFS as a function of radiographic
response in a group of 21 patients with LGGs treated with
postoperative radiotherapy. The authors reported clinical
improvement and a ≥50% decrease in the maximum tumor
cross-sectional area in 11 patients (52%). More partial responders
improved symptomatically than non-responders, but there was no
statistically significant correlation between radiographic response
and PFS. The median time to maximum response was 2.8 months
following treatment termination. The median time to progression
measured from the start of radiotherapy was 4.8 years, and the
5-year PFS rate was 43% (16). The
results of this study suggest that LGGs are moderately
radioresponsive tumors. These results were confirmed by other
authors (17,18). Eyre et al reported an 80%
rate of response to radiotherapy (17), whereas results of another study
found the rate of response to radiotherapy to be 46% (18). In the EORTC 22845 and EORTC 22844
studies postoperative computed tomography scans were not routinely
performed, thus, the residual amount of tumor was not assessed, and
the response to radiotherapy was not evaluated (4,5). For
patients with LGGs suffering from tumor progression, the optimal
management is unclear. In the present study, we observed a
statistically significant higher OS in patients treated with
salvage chemotherapy due to disease progression after the end of
radiotherapy. In high-risk patients, the adjuvant chemotherapy
after postoperative radiotherapy has been explored in a large
randomized RTOG trial. Patients were randomized to postoperative
radiotherapy with or without adjuvant PCV chemotherapy. Patients
were stratified by age, histology, performance status and
presence/absence of contrast enhancement on postoperative MRI
studies. The initial analysis following a median follow-up of >4
years showed that adjuvant chemotherapy did not translate into
improved outcome in high-risk patients (19). Response to treatment and prognosis
may vary in patients with LGGs.
Several phase II studies of PCV or TMZ chemotherapy
regimens in the treatment of new, progressive or recurrent LGGs
have been performed (20–26). A similar response rate was observed
for astrocytomas and oligodendrogliomas (20,25,26).
Chemotherapy was effective in previously treated and untreated
patients (21,22,26).
Although oligodendrogliomas with deletion of chromosome 1p and 19q
may be more sensitive to treatment (23,28),
tumors without these deletions were also responsive to chemotherapy
(27). The natural history of
oligodendroglial tumors is more protracted compared with astrocytic
tumors. Furthermore, oligodendrogliomas show a higher sensitivity
to chemotherapy. In particular, pure oligodendrogliomas with a loss
of heterozygosity on chromosomes 1p/19q have been identified as
tumors with a much more favorable natural history, irrespective of
treatment (29). In the present
study, patients who qualified for salvage chemotherapy were young,
without neurological deficits, and with good general condition.
These are commonly known positive prognostic factors that are
associated with better treatment outcomes. The small number of
patients, heterogeneity and retrospective character of the study
does not allow for the generalization of these results to the whole
population.
Additional prospective studies are required for a
more robust description of the association between the response to
postoperative radiotherapy and treatment outcomes. If the evidence
for the existence of this association was demonstrated, it would be
possible to identify high-risk patients with indications for
adjuvant chemotherapy, or escalation of radiation dose. Thus, these
issued should be investigated in future studies.
References
|
1
|
Berger MS, Deliganis AV, Dobbins J and
Keles GE: The effect of extent of resection on recurrence in
patients with low grade cerebral hemisphere gliomas. Cancer.
74:1784–1791. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claus EB, Horlacher A, Hsu L, et al:
Survival rates in patients with low-grade glioma after
intraoperative magnetic resonance image guidance. Cancer.
103:1227–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leighton C, Fisher B, Bauman G, et al:
Supratentorial low-grade in adults: an analysis of prognostic
factors and timing of radiation. J Clin Oncol. 15:1294–1301.
1997.PubMed/NCBI
|
|
4
|
Van den Bent MJ, Afra D, de Witte O, et
al: Long-term efficacy of early versus delayed radiotherapy for
low-grade astrocytoma and oligodendroglioma in adults: the EORTC
22845 randomized trial. Lancet. 366:985–990. 2005.PubMed/NCBI
|
|
5
|
Karim AB, Maat B, Hatlevoll R, et al: A
randomized trial on dose-response in radiation therapy of low-grade
cerebral glioma: European Organization for Research and Treatment
of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys.
36:549–556. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaw E, Arusell R, Scheithauer B, et al:
Prospective randomized trial of low versus high-dose radiation
therapy in adults with supratentorial low-grade glioma: initial
report of a North Central Cancer Treatment Group/Radiation Therapy
Oncology Group/Eastern Cooperative Oncology Group study. J Clin
Oncol. 20:2267–2276. 2002. View Article : Google Scholar
|
|
7
|
Lote K, Egeland T, Hager B, et al:
Survival, prognostic factors, and therapeutic efficacy in low-grade
glioma: a retrospective study in 379 patients. J Clin Oncol.
15:3129–3140. 1997.PubMed/NCBI
|
|
8
|
Janny P, Cure H, Mohr M, et al: Low grade
supratentorial astrocytomas: management and prognostic factors.
Cancer. 73:1937–1945. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pignatti F, van den Bent M, Curran D, et
al: Prognostic factors for survival in adult patients with cerebral
low-grade glioma. J Clin Oncol. 20:2076–2084. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicolato A, Gerosa MA, Fina P, Iuzzolino
P, Giorgiutti F and Bricolo A: Prognostic factors in low-grade
supratentorial astrocytomas: a uni-multivariate statistical
analysis in 76 surgically treated patients. Neurosurgery.
44:208–223. 1995.
|
|
11
|
Soffietti R, Chiò A, Giordana MT, Vasario
E and Schiffer D: Prognostic factors in well-differentiated
cerebral astrocytomas in the adult. Neurosurgery. 24:686–692. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibamoto Y, Kitakabu Y, Takahashi M, et
al: Supratentorial low-grade astrocytoma. Correlation of computed
tomography findings with effect of radiation therapy and prognostic
variables. Cancer. 72:190–195. 1993. View Article : Google Scholar
|
|
13
|
Vecht CJ: Effect of age on treatment
decisions in low-grade glioma. J Neurol Neurosurg Psychiatry.
56:1259–1264. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Veelen ML, Avezaat CJ, Kros JM, van
Putten W and Vecht C: Supratentorial low grade astrocytoma:
prognostic factors, dedifferentiation, and the issue of early
versus late surgery. J Neurol Neurosurg Psych. 64:581–587.
1998.PubMed/NCBI
|
|
15
|
Bauman G, Fischer B, Walting C, Cairncross
JG and Macdonald D: Adult Supratentorial low-grade gliomas: long
experience at a single institution. J Radiat Oncol Biol Phys.
75:1401–1407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauman G, Pahapill P, Macdonald D, Fisher
B, Leighton C and Cairncross G: Low grade glioma: a measuring
radiographic response to radiotherapy. Can J Neurol Sci. 26:18–22.
1999.PubMed/NCBI
|
|
17
|
Eyre HJ, Crowley JJ, Townsend JJ, et al: A
randomized trial of radiotherapy versus radiotherapy plus CCNU for
incompletely resected low-grade gliomas: a Southwest Oncology Group
study. J Neurosurg. 78:909–914. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lunsford LD, Somaza S, Kondziolka D and
Flickenger JC: Survival after stereotactic biopsy and radiation of
cerebral non pilocytic astrocytoma. J Neurosurg. 82:523–529. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaw EG, Berkey B, Coons SW, et al:
Initial report of Radiation Therapy Oncology Group (RTOG) 9802:
Prospective studies in adult low-grade glioma (LGG). ASCO 2006
Annual Meeting Proceedings. J Clin Oncol. 24:Abstr 1500. 2006.
|
|
20
|
Quinn JA, Reardon DA, Friedman AH, et al:
Phase II trial of temozolomide in patients with progressive
low-grade glioma. J Clin Oncol. 21:646–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van den Bent MJ, Taphoorn MJ, Brandes AA,
et al: Phase II study of first-line chemotherapy with temozolomide
in recurrent oligodendroglial tumors: the European Organization for
Research and Treatment of Cancer Brain Tumor Group study 26971. J
Clin Oncol. 21:2525–2528. 2003.PubMed/NCBI
|
|
22
|
van den Bent MJ, Chinot O, Boogerd W, et
al: Second-line chemotherapy with temozolomide in recurrent
oligodendroglioma after PCV (procarbazine, lomustine and
vincristine) chemotherapy: EORTC Brain Tumor Group phase II study
26972. Ann Oncol. 14:599–602. 2003.
|
|
23
|
Hoang-Xuan K, Capelle L, Kujas M, et al:
Temozolomide as initial treatment for adults with low-grade
oligodendrogliomas or oligoastrocytomas and correlation with
chromosome 1p deletions. J Clin Oncol. 22:3133–3138. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buckner JC, Gesme D Jr, O’Fallon JR, et
al: Phase II trial of procarbazine, lomustine, and vincristine as
initial therapy for patients with low-grade oligodendroglioma or
oligoastrocytoma: efficacy and associations with chromosomal
abnormalities. J Clin Oncol. 21:251–255. 2003. View Article : Google Scholar
|
|
25
|
Brada M, Viviers L, Abson C, et al: Phase
II study of primary temozolomide chemotherapy in patients with WHO
grade II gliomas. Ann Oncol. 14:1715–1721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pace A, Vidiri A, Galiè E, Carosi M,
Telera S, Cianciulli AM, et al: Temozolomide chemotherapy for
progressive low-grade glioma: clinical benefits and radiological
response. Ann Oncol. 14:1722–1726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stege EM, Kros JM, de Bruin HG, et al:
Successful treatment of low-grade oligodendroglial tumors with a
chemotherapy regimen of procarbazine, lomustine, and vincristine.
Cancer. 103:802–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chahlavi A, Kanner A, Peereboom D,
Staugaitis SM, Elson P and Barnett G: Impact of chromosome 1p
status in response of oligodendroglioma to temozolomide:
preliminary results. J Neurooncol. 61:267–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jenkins RB, Blair H, Ballman KV, et al: A
t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and
predicts a better prognosis of patients with oligodendroglioma.
Cancer Res. 66:9852–9861. 2006. View Article : Google Scholar : PubMed/NCBI
|