Introduction
Interleukin-11 (IL-11) is a cytokine that plays
significant roles in various biological processes. It specifically
interacts with its receptor, the IL-11 receptor α-chain (IL-11R),
with a high affinity (1,2). As a glycoprotein on the cell membrane,
IL-11R, together with gp130 (the corresponding transmembrane signal
transduction glycoprotein), forms a complex closely associated with
the incidence and development of tumors (3–5).
IL-11R is expressed at a high level and functions in the osseous
metastasis process of various types of cancer, including prostate
cancers (6–8). The IL-11 analog
c(Cys-Gly-Arg-Arg-Ala-Gly-Gly-Ser-Cys)NH2, written as
c(CGRRAGGSC), is an artificially synthesized nonapeptide which has
been demonstrated to contain a locus that specifically binds to the
IL-11R α-chain by using phage display technology (9). The present study evaluates the
possibility of using c(CGRRAGGSC) as the exogenous ligand of LNCaP
cells and provides experimental evidence for the visualization of
tissues and organs of experimental animals. This research uses a
near-infrared dye LSS670 and technetium 99m as probes to
investigate the binding characteristics of c(CGRRAGGSC) in human
prostate cancer LNCaP cells in vitro. Our results provide a
basis for the research of the specific molecular targeting used for
the diagnosis of prostate cancer bone metastasis and further
treatment of prostate carcinoma in the future.
Materials and methods
Reagents and cell lines
c(CGRRAGGSC) was purchased from Shanghai Target
Biological (Shanghai, China). LSS670 fluorescent dye (excitation,
670 nm; emission, 755 nm) was provided by Dr Si Xiaoning from the
Molecular Imaging Department of Carestream Health (Shanghai,
China). 99mTc washing buffer was purchased from Nanjing
Senke Technology (Nanjing, China). Human prostate cancer LNCaP
cells were purchased from Nanjing Kaiji Biological (Nanjing,
China). The γ calculator was purchased from Hewlett-Packard (Palo
Alto, CA, USA). The CP224S micro-dose weighing instrument was
purchased from Sartorius (Goettingen, Germany). The FACS Calibur
cell flow instrument was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). The LS-55 fluorescence spectroscopic indicator was
purchased from Perkin-Elmer (Waltham, MA, USA).
Counterstaining of
LSS670-c(CGRRAGGSC)
LSS670 is a near-infrared dye with an excitation
maximum of 670 nm and a long Stoke’s shift (LSS) of 85 nm.
LSS670-c(CGRRAGGSC) was obtained as described below. LSS670 dye (1
mg) was dissolved in 100 μl of DMSO, followed by agitation
for 10 sec and incubation at room temperature for 15 min.
Immediately, 15 μl of the dye solution was added to 30
μl of the c(CGRRAGGSC) solution. The pH was adjusted to 7.6
with 0.1 M PBS to obtain a reaction volume of 500 μl. The
reaction solution was gently mixed for 1 h. The solution was
purified using a Bio-Gel P-30 gel column, with a 10 μl
injection volume and a 0.5 ml/min flow rate. The eluent was then
collected using a tube. Following incubation of the LNCaP cells in
10 μl of LSS670-c(CGRRAGGSC) (200 nM) at 37°C for 4 h, 10
μl of 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI)
dye was added. DAPI was removed following incubation at room
temperature for 5–10 min. The cells were rinsed 2–3 times with PBS
to remove free DAPI. The solution was agitated for 1 h and mounted
onto a Fisherbrand cover glass. The distribution of
LSS670-c(CGRRAGGSC) in LNCaP cells was then observed using a
fluorescence microscope (Olympus-X36, Olympus Corporation, Tokyo,
Japan). The same procedure was performed with normal prostate
epithelium cells that were deficient in the IL-11 receptor and
served as the controls.
Fluorescence labeling of LNCaP cells by
c(CGRRAGGSC)
LNCaP cells were placed into 6-well plates
(1×106 cells/ml) and LSS670-c(CGRRAGGSC) was synthesized
as described above. In the concentration/effect experiment, LNCaP
cells were incubated at 4°C for 4 h and then 37°C for 4 h with
c(CGRRAGGSC) of varying concentrations (0, 20, 50, 100, 200, 500
and 1,000 nM). In the time/effect experiment, LNCaP cells were
incubated with 200 nM c(CGRRAGGSC) at 4°C for 4 h, followed by
incubation at 37°C for 0, 0.125, 0.25, 0.5, 1, 2, 4, 6 or 8 h. In
the competition inhibition experiment, LNCaP cells were incubated
with unlabeled c(CGRRAGGSC) of varying concentrations (20, 50, 100,
200, 500 and 1,000 nM) at 37°C for 4 h. LSS670-c(CGRRAGGSC) (200
nM) was added to the reaction system for another 4-h incubation at
37°C. In the experiments described above, a nonapeptide
c(CGSPGWVRC) served as the control. A fluorescence microscope was
used to observe the distribution of LSS670-c(CGRRAGGSC) in LNCaP
cells.
Radiolabeling
DTPA-c(CGRRAGGSC) was synthesized using the method
described previously (10). The
radiolabeling of DTPA-c(CGRRAGGSC) was performed as follows.
Briefly, 20 μg DTPA-c(CGRRAGGSC), 20 μl of 1 mg/ml
SnCl2 and 280 μl of 0.075 M phosphate-buffered
saline (PBS) were mixed and agitated at room temperature for 2 min.
This was followed by the addition of 0.2 ml of 99mTc
eluent (370–740 MBq). Following incubation at room temperature for
30 min, the labeled product 99mTc-DTPA-c(CGRRAGGSC) was
obtained. Analytical HPLC was performed using an SCL-10AVP HPLC
system equipped with an EKA Chemicals reverse phase C-18 analytical
column (KR100-5, 250×4.6 mm). The flow rate was 1 ml/min. The
samples were eluted with H2O/acetonitrile containing
0.15% trifluoroacetic acid of three linear gradients (A, 0–40%; B,
10–80%; and C, 30–100%, each for 30 min). The resultant eluent was
collected in a tube. The fluorescence properties (ultraviolet
wavelength, 210 nm) were measured with a PDA100 ultraviolet
detector (Shimadzu Co., Kyoto, Japan). The stability of
99mTc-DTPA-c(CGRRAGGSC) in vitro was measured at
different time points.
Saturation binding analysis in vitro
Aliquots (75 μl) of the sample extracts were
incubated for 30 min at 4°C with 50 μl of 0.5% Tris buffer
solution containing varying concentrations (0.1–10 nM) of
99mTc-DTPA-c(CGRRAGGSC). Parallel incubations were
conducted in the presence of 1 μM unlabeled
DTPA-c(CGRRAGGSC) to determine the non-specific binding (NSB) at
each 99mTc-DTPA-c(CGRRAGGSC) concentration. When the
samples were mixed, the radioactivity of each tube in the sample
group was measured and the mean dpm (disintegrations per min) of
the tubes was used as total radioactivity. The samples were
incubated at 25°C for 90 min with gentle agitation. The reactions
were terminated by the addition of 1 ml cold Tris buffer. The
samples were centrifuged at 10,000 rpm for 15 min at 4°C and the
precipitates were washed with cold buffer. Following two more
washes, specific binding (SB) was calculated as the difference
between total binding [measured in the absence of
DTPA-c(CGRRAGGSC)] and NSB [measured in the presence of
DTPA-c(CGRRAGGSC)]. A saturation curve was created with dpm as the
ordinate and the quantity of added
99mTc-DTPA-c(CGRRAGGSC) as the abscissa. The equilibrium
dissociation constant (Kd) and the maximum binding
capacity (Bmax) of the receptor were obtained by
Scatchard analysis.
Competitive inhibition experiment in
vitro
Aliquots (75 μl) of the sample extracts were
incubated for 30 min at 4°C with 50 μl of 0.5% Tris buffer
containing 1 nmol of 99mTc-DTPA-c(CGRRAGGSC). In
addition, parallel experiments were conducted in the presence of
unlabeled DTPA-c(CGRRAGGSC) of varying concentrations (0.1–10
μM) to determine NSB. The procedures conducted were the same
as those described in saturation binding analysis. A competitive
inhibition curve was created with SB as the ordinate and the amount
of added unlabeled DTPA-c(CGRRAGGSC) as the abscissa. The 50%
inhibiting concentration (IC50) and the equilibrium
inhibition constant (Ki) were calculated.
Statistical analyses
The measurements were expressed as means ± standard
deviation (± sd) and analyzed using one-way ANOVA. P<0.05 was
considered to indicate a statistically significant result. Each
experiment was repeated at least three times.
Results
Binding characteristics of
LSS670-c(CGRRAGGSC) in LNCaP cells
To investigate the binding characteristics of
c(CGRRAGGSC) in LNCaP cells, c(CGRRAGGSC) was labeled with a
fluorescent dye, LSS670, generating the probe LSS670-c(CGRRAGGSC).
In the concentration/effect experiments, LNCaP cells were incubated
with varying concentrations (0, 20, 50, 100, 200, 500 and 1,000 nM)
of LSS670-c(CGRRAGGSC). This was followed by detection of the
fluorescence intensity within cells with a fluorescence microscope.
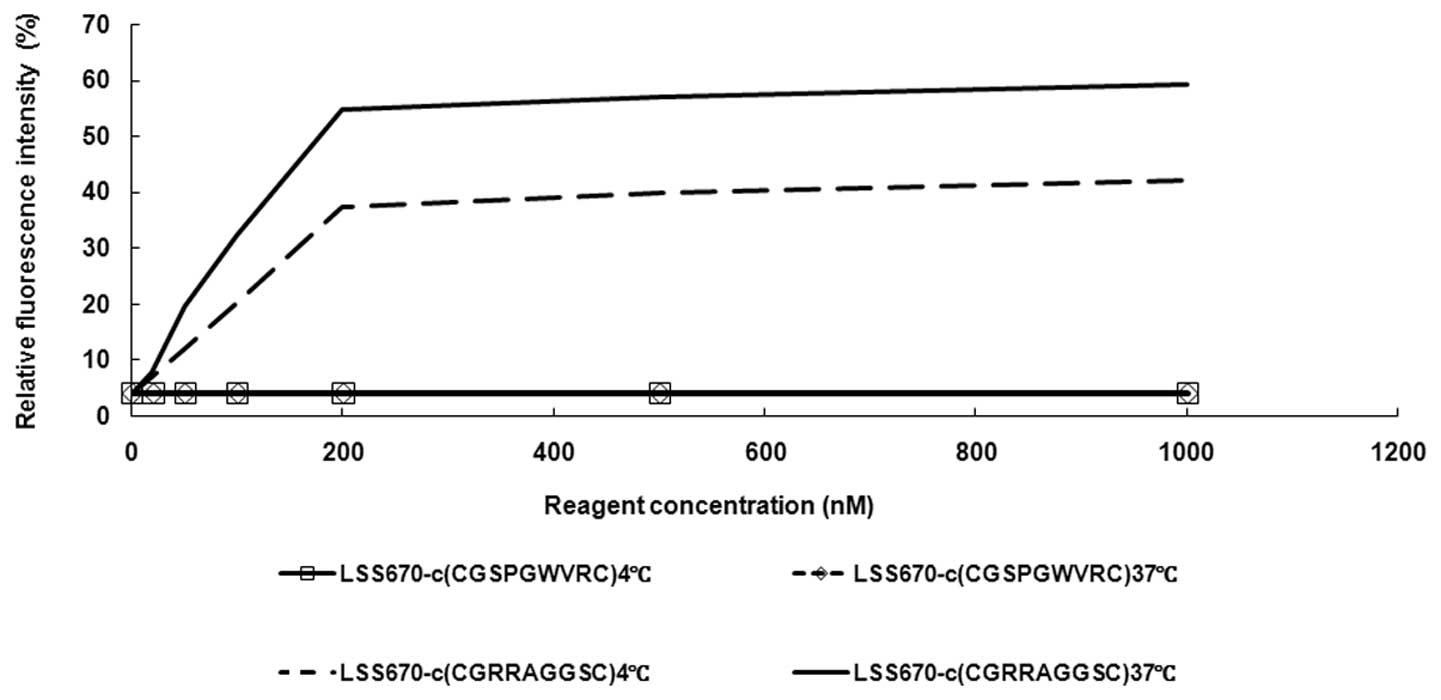
As shown in Fig. 1, the
fluorescence intensity of the cells incubated with 200 nM
LSS670-c(CGRRAGGSC) was 6.58-fold higher than that of cells
incubated with 20 nM LSS670-c(CGRRAGGSC), while the fluorescence
intensity of the cells incubated with 500 nM LSS670-c(CGRRAGGSC)
was only 1.2-fold higher than cells incubated with 200 nM
LSS670-c(CGRRAGGSC). In cells incubated with a nonapeptide
c(CGSPGWVRC), which served as a control probe, no fluorescence was
detected.
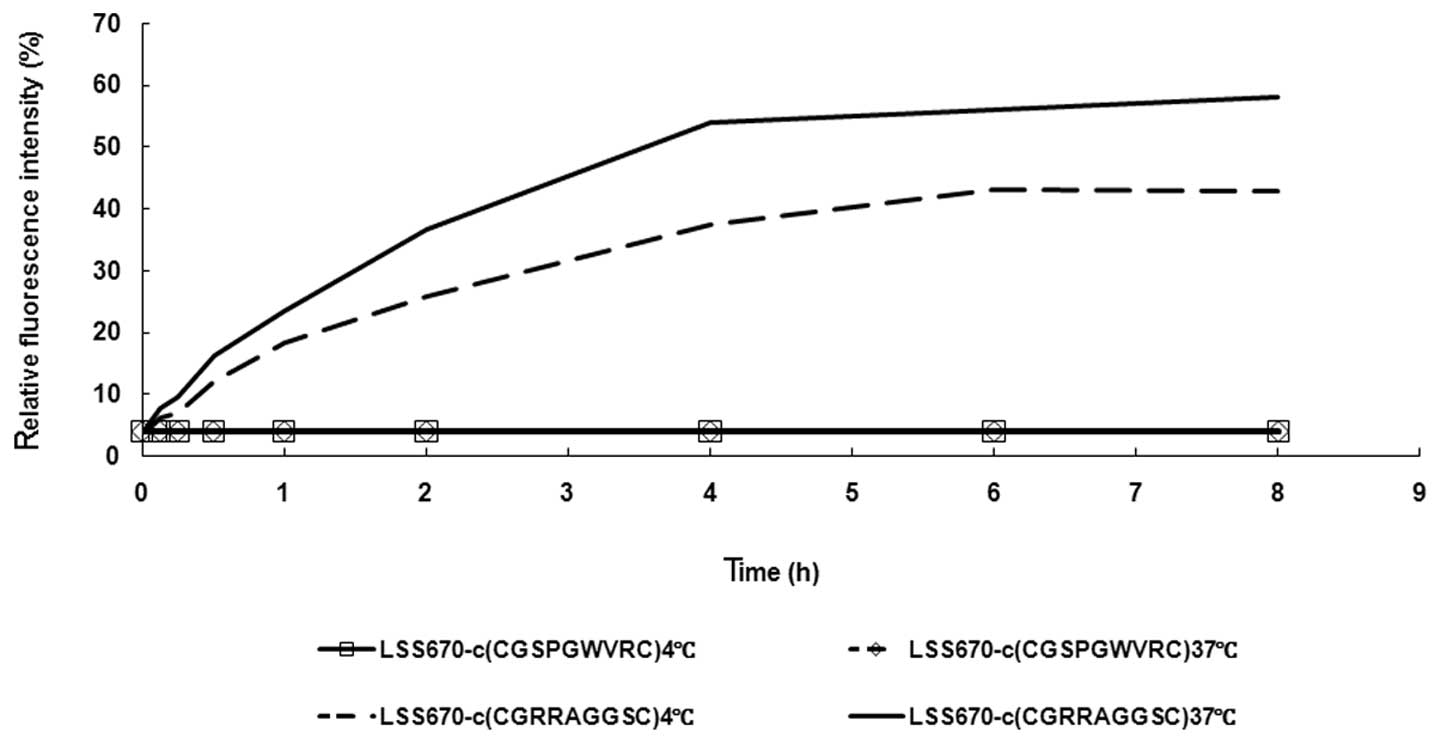
In the time/effect experiment, LNCaP cells were
incubated with 200 nM LSS670-c(CGRRAGGSC) at 4°C for 4 h, followed
by continued incubation at 37°C for various time courses (0, 0.125,
0.25, 0.5, 1, 2, 4, 6 or 8 h). As shown in Fig. 2, the fluorescence intensity within
cells incubated with the control probe LSS670-c(CGSPGWVRC) was
always extremely low at different incubation times (from 0 to 8 h)
at either 4°C or 37°C. However, the fluorescence was detected in
LNCaP cells incubated with LSS670-c(CGRRAGGSC) for 0.125 h or
longer at 37°C. The fluorescence intensity within cells incubated
with LSS670-c(CGRRAGGSC) at 37°C increased to a relatively high
level at 4 h and then the intensity continued to increase gradually
to the highest level at 8 h. When compared with the cells incubated
with the control probe LSS670-c(CGSPGWVRC), the fluorescence
intensity in cells incubated with LSS670-c(CGRRAGGSC) was
105.68-fold higher at 4 h (37°C). It was noted that when incubated
with LSS670-c(CGRRAGGSC) at 4°C, the fluorescence intensity in
LNCaP cells reached its peak following 6-h incubation.
In the competition/inhibition experiment, LNCaP
cells were incubated with varying concentrations (20, 50, 100, 200,
500 and 1,000 nM) of unlabeled c(CGRRAGGSC) at 37°C for 4 h.
LSS670-c(CGRRAGGSC) (200 nM) was then added into the reaction
system for an additional 4-h incubation at 37°C. In the experiments
described above, a nonapeptide c(CGSPGWVRC) served as the
control.
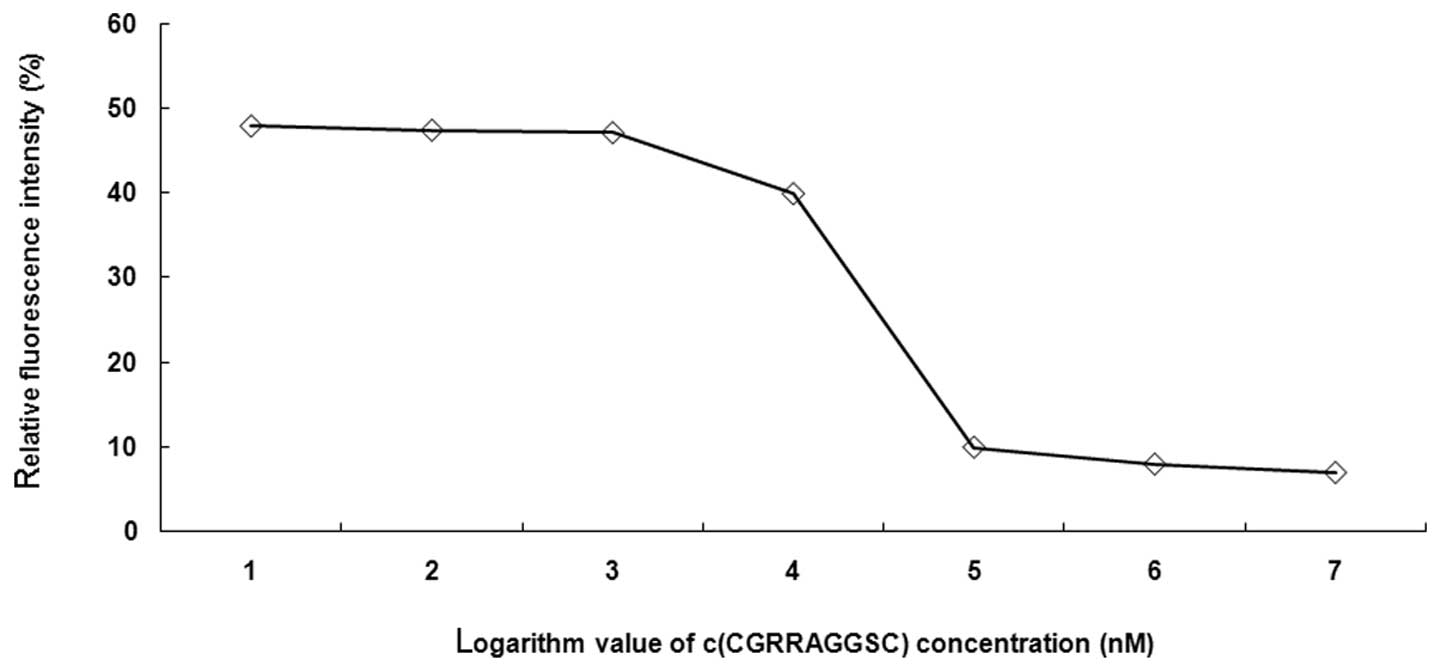
Competition inhibition curve
As shown in Fig. 3,
the competition inhibition curve, which was drawn using the
logarithm value of the fluorescence intensity in LNCaP cells to the
concentration of unlabeled c(CGRRAGGSC), indicated that the
LSS670-c(CGRRAGGSC) fluorescence intensity decreases with the
increase in unlabeled c(CGRRAGGSC) concentration (Fig. 3). These results suggest that LNCaP
cells are able to be specifically labeled by the
LSS670-c(CGRRAGGSC) probes and that the probe binding efficacy is
affected by the probe concentration and incubation time.
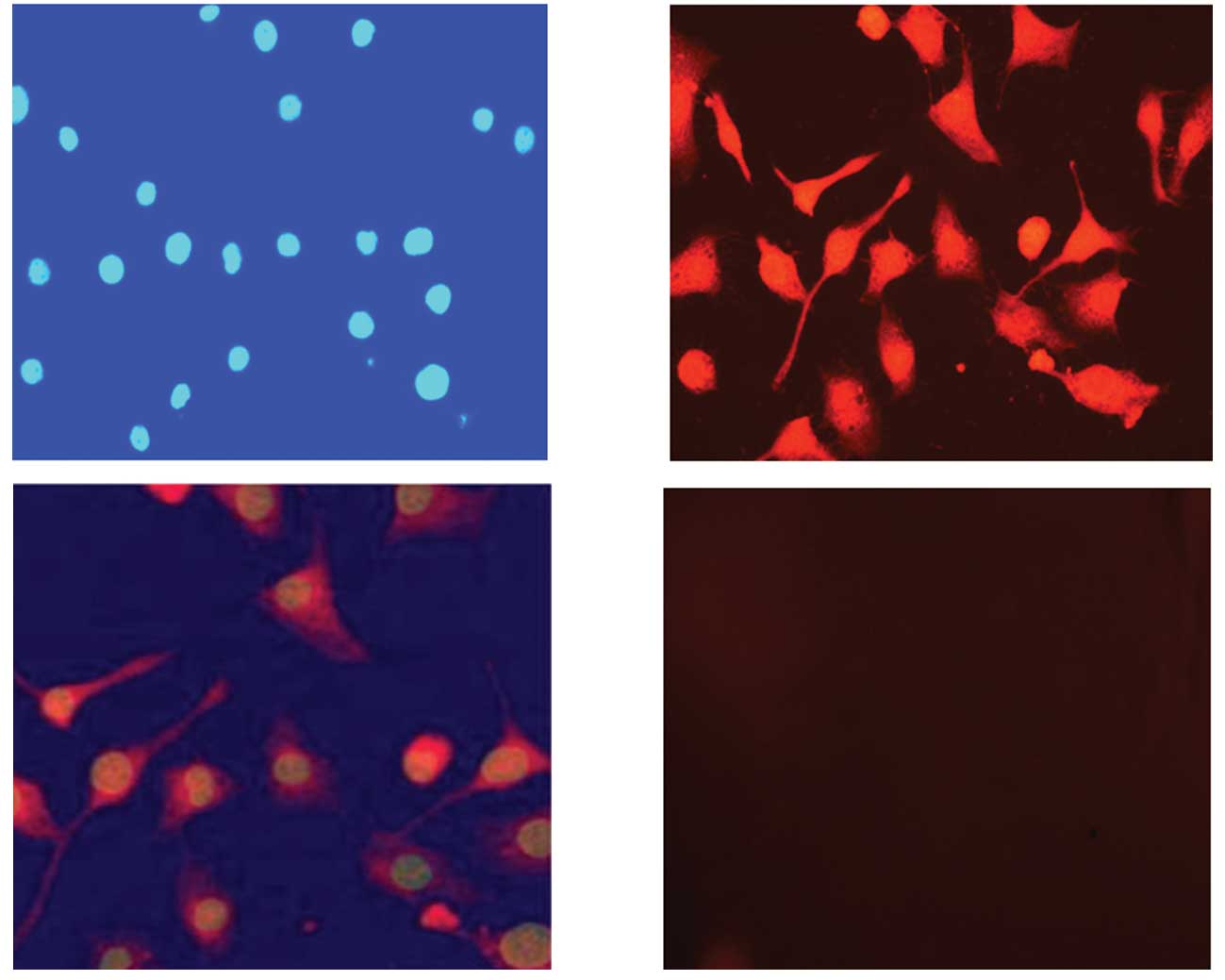
LSS670-c(CGRRAGGSC) localization
The majority of LSS670-c(CGRRAGGSC) was concentrated
in the LNCaP cell membranes and cytoplasm. To investigate the
localization of LSS670-c(CGRRAGGSC) probes in LNCaP cells, the
LSS670-c(CGRRAGGSC)-labeled cells were stained with DAPI
(excitation wavelength, 350 nm; emission wavelength, 425 nm) and
observed through a fluorescence microscope. As shown in Fig. 4, the majority of LSS670-c(CGRRAGGSC)
was concentrated in LNCaP cell membranes and cytoplasm (Fig. 4A, B and C). No LSS670 fluorescence
was demonstrated in the cell nucleus. No obvious LSS670
fluorescence was observed in the control cells labeled with
LSS670-c(CGSPGWVRC) (Fig. 4D).
These results suggest that the binding between c(CGRRAGGSC) and
LNCaP cells is specific.
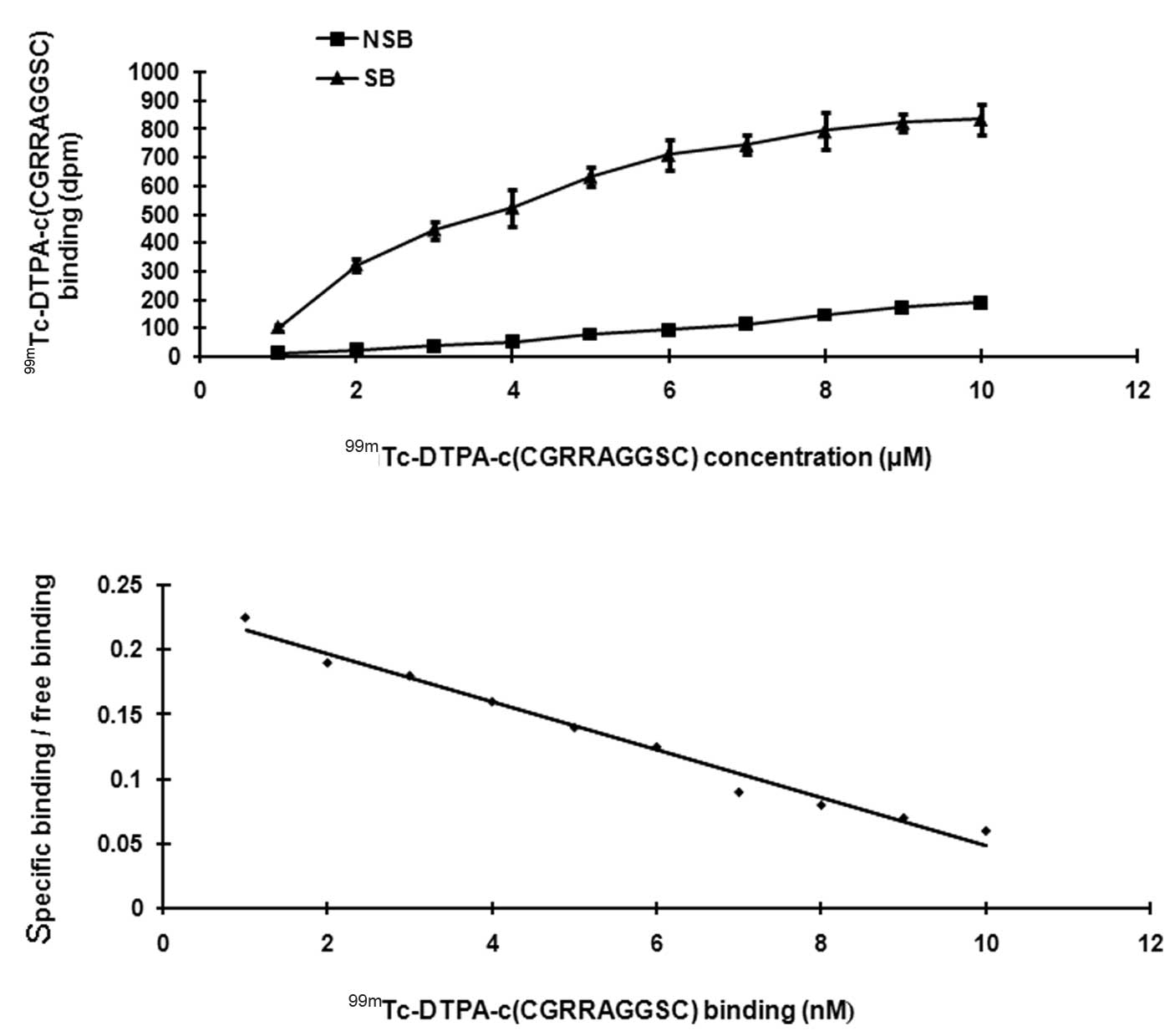
Saturation binding analysis
The SB increased as the radioligand concentration
increased, but gradually decreased as radioligand concentration
approached saturation (Fig. 5A).
NSB increased consistently as the radioligand concentration
increased, without showing a tendency of saturation. The Scatchard
plot is a straight line (Fig. 5B).
Linear regression was used to determine the Kd value and
Bmax value. The Kd value was 3.2±0.02 nM. The
Bmax value was 754±34 fmol/mg.pro. These results suggest
that the binding between c(CGRRAGGSC) and LNCaP cells has
sufficient affinity and provides further evidence for the existence
of a receptor.
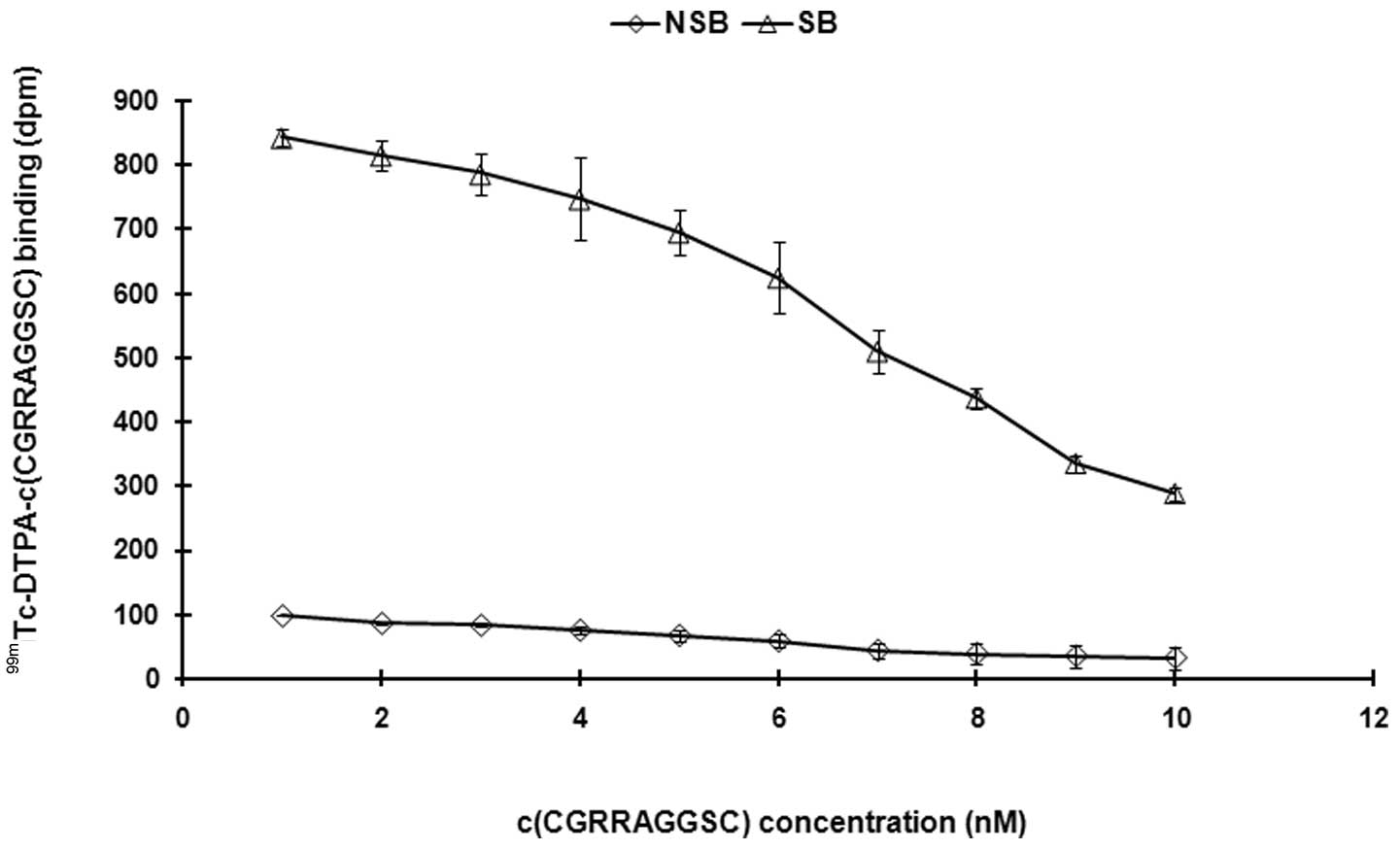
Competitive inhibition analysis
Unlabeled peptides were used to perform the
competitive inhibition analysis. With c(CGRRAGGSC) concentrations
in the reaction system gradually increasing (0.1–10 μM), the
binding of 99mTc-DTPA-c(CGRRAGGSC) to the receptor
gradually decreased. The IC50 and the equilibrium
inhibition constant (Ki) were calculated
(IC50=6.31±0.12 nM, Ki=2.11±0.14 nM; Fig. 6). These results suggest that the
labeled peptides and unlabeled peptides have the same biological
activity.
Discussion
The bone metastasis of human prostate cancer is a
relatively long process, which can be difficult to diagnose. Early
clinical diagnosis is particularly difficult since current
medicines and biological agents lack targeted specificity to the
cancer tissue due to their low deposition on the tumor and normal
body structures. Due to the inaccuracy of drug effect, doses
reaching the tumor are far from ideal (11,12). A
number of studies proved that IL-11, as a cytokine with
multi-biological effects, when specifically bound with the α-chain
of its receptor, becomes a complex with high affinity for the
inter-membrane signal transduction glycoprotein 130. The complex
activates the Jak-STAT signal pathway and facilitates the
phosphorylation of p-hydroxyphenylalanine kinase, thus exerting
related biological effects (6,13,14).
Using phage display peptide library screening technology, it was
revealed that the nonapeptide c(CGRRAGGSC), as an analog of IL-11,
is able to specificically target to the prostate tumor. Previous
studies have reported that when breast cancer cells undergo bone
metastasis, they also generate IL-11, which accelerates the spread
of tumor cells into the surrounding bones (15). Therefore, bone metastasis can be
inhibited through the inhibition of the expression of IL-11. Kang
et al revealed that the signaling pathway of IL-11 has a
significant role in the bone metastasis of cancer, therefore bone
metastasis may be specifically accelerated by raising the
expression level of IL-11 (16).
Other relevant studies have also demonstrated that IL-11 is closely
associated with the occurrence and development of bone metastasis
(17). Therefore, by employing an
artificially synthesized nonapeptide as the ligand and determining
its binding characteristics with human prostate cancer LNCaP cells,
this study aimed to investigate the possibility of a nonapeptide as
the specific external ligand of LNCaP cells in order to provide
evidence for further studies.
Our team labeled c(CGRRAGGSC) with LSS670 and used
fluorescence cytochemistry to investigate the binding
characteristics of c(CGRRAGGSC) and human prostate cancer LNCaP
cells. This method has the following advantages: i)
LSS670-c(CGRRAGGSC) and LNCaP cells bind in a
concentration-dependent manner and demonstrate a saturation
tendency with increasing concentration. ii) LSS670-c(CGRRAGGSC) and
LNCaP cells bind in a time-dependent manner and demonstrate a
saturation tendency with time. iii) The binding of
LSS670-c(CGRRAGGSC) and LNCaP is challenged by the unlabeled
nonapeptide c(CGRRAGGSC) and shows a clear competitive inhibition,
which suggests that c(CGRRAGGSC) has the ability to bind with LNCaP
cells but that the binding quantity is limited. Fluorescence
microscopy revealed the concentration bound to the cell membranes.
LSS670 labeling and 99mTc did not affect the binding
characteristics of c(CGRRAGGSC) and demonstrated a saturation
tendency as well as competitive inhibition. In the binding
experiments, internalization and releasing LSS670-c(CGRRAGGSC), it
was revealed that c(CGRRAGGSC) acts with LNCaP cells via IL-11 and
its receptor and may be released as its prototype, while the
negative peptide in the control group has no such ability. It is
that the specificity is resulted from binding of the receptor and
the ligand; Kd and Bmax are calculated as
3.2±0.02 nmol/l and 754±34 fmol/mg pro, respectively, which proves
that their binding has sufficient affinity and provides further
evidence for the existence of the receptor. The nonapeptide has the
ability to bind specifically with the surface receptor of LNCaP and
become a complete membrane receptor, which meets the requirement of
being an aglucone. This also suggests that c(CGRRAGGSC) and
LSS670-c(CGRRAGGSC) compete in order to bind with sites on the cell
membranes of LNCAP cells.
Near-infrared ray fluorescence imaging is a new
optical visualization technology that observes structures and blood
at greater depth due to its greater penetration ability and lower
scattering activation disturbance (18–20). The present study used
LSS670 to label c(CGRRAGGSC) as it has wider scope of activation
and emission and quickly and specifically binds with the external
amino groups of the nonapeptide as a new near-infrared ray
fluorescent dye. This fluorescence labeling is observed using an
appropriate light source, such as camera control (CC) and other
optical detectors. This fluorescence labeling has better
sensitivity and spatial resolution for real-time observation on
sites in and outside of the cell membranes. The results of the
study prove that the dye clearly visualizes the binding sites of
c(CGRRAGGSC) and LNCaP cells and outperforms other near-infrared
ray dyes including cy5.5. In further studies, the dye could be used
to visualize the body structures of small animals.
Through the preliminary study of the binding
characteristics of c(CGRRAGGSC) and LNCaP cells, we draw the
conclusion that c(CGRRAGGSC) specifically binds to LNCaP cells
through a receptor-mediated pathway.
Acknowledgements
We thank Dr Xiaoning Si for providing the LSS670
dye. This study was supported by the National Nature Scientific
Research Foundation of China (30470500).
References
|
1
|
Du X and Williams DA: Interleukin-11:
review of molecular, cell biology, and clinical use. Blood.
89:3897–3908. 1997.PubMed/NCBI
|
|
2
|
Schleinkofer K, Dingley A, Tacken I,
Federwisch M and Müller-Newen G: Identification of the domain in
the human interleukin-11 receptor that mediates ligand binding. J
Mol Biol. 306:263–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dahmen H, Horsten U, Küster A, et al:
Activation of the signal transducer gp130 by interleukin-11 and
interleukin-6 is mediated by similar molecular interactions.
Biochem J. 331:695–702. 1998.PubMed/NCBI
|
|
4
|
Kurth I, Horsten U, Pflanz S, et al:
Activation of the signal transducer glycoprotein 130 by both IL-6
and IL-11 requires two distinct binding epitopes. J Immunol.
162:1480–1487. 1999.PubMed/NCBI
|
|
5
|
Zurita AJ, Troncoso P, Cardó-Vila M,
Logothetis CJ, Pasqualini R and Arap W: Combinatorial screenings in
patients: the interleukin-11 receptor alpha as a candidate target
in the progression of human prostate cancer. Cancer Res.
64:435–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakayama T, Yoshizaki A, Izumida S, et al:
Expression of interleukin-11(IL-11) and IL-11 receptor alpha in
human gastric carcinoma and IL-11 upregulates the invasive activity
of human gastric carcinoma cells. Int J Oncol. 30:825–833.
2007.PubMed/NCBI
|
|
7
|
Yoshizaki A, Nakayama T, Yamazumi K,
Yakata Y, Taba M and Sekine I: Expression of interleukin (IL)-11
and IL-11 receptor in human colorectal adenocarcinoma: IL-11
up-regulation of the invasive and proliferative activity of human
colorectal carcinoma cells. Int J Oncol. 29:869–876.
2006.PubMed/NCBI
|
|
8
|
Campbell CL, Jiang Z, Savarese DM and
Savarese TM: Increased expression of the interleukin-11 receptor
and evidence of STAT3 activation in prostate carcinoma. Am J
Pathol. 158:25–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Ke S, Kwon S, et al: A new optical
and nuclear dual-labeled imaging agent targeting interleukin 11
receptor alpha-chain. Bioconjug Chem. 18:397–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cook RJ and Major P: Multistate analysis
of skeletal events in patients with bone metastases. Clin Cancer
Res. 12:6264s–6269s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgentaler A: Testosterone and prostate
cancer: an historical perspective on a modern myth. Eur Urol.
50:935–939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ernst M, Najdovska M, Grail D, et al:
STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated
gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest.
118:1727–1738. 2008.PubMed/NCBI
|
|
13
|
Nandurkar HH, Hilton DJ, Nathan P, Willson
T, Nicola N and Begley CG: The human IL-11 receptor requires gp130
for signalling: demonstration by molecular cloning of the receptor.
Oncogene. 12:585–593. 1996.PubMed/NCBI
|
|
14
|
Morgan H, Tumber A and Hill PA: Breast
cancer cells induce osteoclast formation by stimulating host IL-11
production and downregulating granulocyte/macrophage
colony-stimulating factor. Int J Cancer. 109:653–660. 2004.
View Article : Google Scholar
|
|
15
|
Kang Y, He W, Tulley S, et al: Breast
cancer bone metastasis mediated by the Smad tumor suppressor
pathway. PNAS. 102:13909–13914. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Javelaud D, Mohammad KS, McKenna CR, et
al: Stable overexpression of Smad7 in human melanoma cells impairs
bone metastasis. Cancer Res. 67:2317–2324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shah K and Weissleder R: Molecular optical
imaging: applications leading to the development of present day
therapeutics. NeuroRx. 2:215–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Wang W, Wu Q, et al: Dual optical
and nuclear imaging in human melanoma xenografts using a single
targeted imaging probe. Nucl Med Biol. 33:349–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinbrink J, Liebert A, Wabnitz H, et al:
Towards noninvasive molecular fluorescence imaging of the human
brain. Neurodegener Dis. 5:296–303. 2008. View Article : Google Scholar : PubMed/NCBI
|