Introduction
With regard to incidence and mortality, gastric
cancer is one of the most common types of cancer worldwide. In
Korea, gastric cancer is the most commonly occurring malignancy,
accounting for approximately 15% of newly diagnosed cancer cases
(1). Although its incidence in
individuals younger than 50 years of age has recently increased,
gastric cancer develops more frequently among patients in their
seventh or eighth decade of life. To date, the only potentially
curative treatment is surgery; however, a considerable number of
patients present with advanced stages of the disease at the time of
diagnosis. Moreover, more than half of the patients treated by
complete resection eventually develop recurrent disease (2,3).
Palliative chemotherapy in the first- and second-line setting
improves survival in advanced gastric cancer (AGC) compared to the
best supportive care (4,5); however, the prognosis of the advanced
and recurrent disease remains poor.
Irinotecan with infusional leucovorin (LV) and
5-fluorouracil (5-FU) (FOLFIRI regimen) is widely used as a
first-line treatment for patients with advanced colorectal cancer
(CRC) (6). Certain studies have
also identified the activity and tolerability of FOLFIRI regimens
as first-line chemotherapy in patients with AGC (7,8). The
reported objective response rate (RR) was approximately 40%, with a
median overall survival (OS) of 10–12 months. Phase II studies
using FOLFIRI regimens as salvage therapy for patients with AGC
have demonstrated an RR of approximately 10%, with a median OS of
approximately 6 months (9–11). In terms of toxicity, FOLFIRI
regimens have been well-tolerated.
Since FOLFIRI regimens have demonstrated an
acceptable safety profile with a beneficial activity profile for
patients with AGC (7–11), we used the modified FOLFIRI regimen
(biweekly irinotecan 150 mg/m2 with LV 100
mg/m2 and 5-FU 2000 mg/m2) as salvage
chemotherapy for elderly or frail patients with AGC.
We retrospectively reviewed the efficacy and safety
of the modified FOLFIRI regimen as second-line chemotherapy in AGC
patients in old age or with a poor performance status (PS).
Patients and methods
Patients
Between January 2005 and August 2011, 24 patients
who received systemic chemotherapy with the FOLFIRI regimen as
second-line chemotherapy for AGC were retrospectively analyzed. To
be eligible for this study, patients had to have histologically
confirmed adenocarcinoma of the stomach and unresectable or
metastatic disease with at least 1 measurable lesion. Patients with
a European Clinical Oncology Group performance status (ECOG PS) of
2 were eligible regardless of their age. Among the patients with an
ECOG PS of 1, only those aged 65 years or over were included in the
study. Additionally, patients had to fulfill the following
criteria: no central nervous system metastases, no active
infection, no history of other malignancies and sufficient hepatic,
renal and bone marrow functions. Patients previously treated with
adjuvant chemotherapy were also included. The study was approved by
the Institutional Review Board (IRB) of Hallym Medical Center,
Kangnam Sacred Heart Hospital, Hallym University College of
Medicine, Seoul, South Korea, and as this retrospective study
involved no risk to the patients, the waiver of informed consent
was allowed by the IRB.
Treatment schedule
All 24 patients received irinotecan 150
mg/m2 and LV 100 mg/m2 as a 2 h intravenous
infusion, followed by 5-FU 2,000 mg/m2 as a 46 h
continuous infusion. Antiemetic prophylaxis with 5-HT3
antagonists were administered prior to irinotecan infusion, but
corticosteroids were not routinely administered. Unless there was
no evidence of disease progression or the patient experienced
unacceptable toxicity, this regimen was repeated every 14 days to
the maximum of 12 cycles.
Treatment was delayed for up to 2 weeks until
patients recovered from adverse effects, the absolute granulocyte
count exceeded 1,000/μl and/or the platelet count exceeded
100,000/μl. Dose reductions were made according to the highest
grade of toxicity that had occurred during the previous cycle. The
dose of irinotecan and 5-FU was reduced by 25% for hematological
(neutropenia and thrombocytopenia) or non-hematological toxicities
(mucositis and diarrhea) of grade 3–4.
Response and toxicity evaluation
Baseline evaluation included physical examination,
complete blood counts (CBC), blood chemistries and radiology
examinations. Physical examination, CBC and blood chemistry were
performed prior to each cycle. Tumor assessments by CT scans were
repeated every 4 cycles. The response to the modified FOLFIRI
regimen was assessed according to the guidelines of the Response
Evaluation Criteria in Solid Tumors (RECIST) committee. Complete
response (CR) was defined as the disappearance of all evidence of
the disease, and partial response (PR) was defined as a reduction
in the unidimensional tumor measurements by at least 30%, without
the formation of a new lesion or the progression of an existing
lesion. Progressive disease (PD) was defined as an increase in the
sum of the products of all measurable lesions by at least 20% or
the appearance of a new lesion, including the reappearance of any
lesion that previously disappeared. Stable disease (SD) was defined
as a tumor response that did not fulfill the criteria for CR, PR or
PD.
Toxicities were recorded according to the National
Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0.
For toxicity analysis, the highest grade of toxicity in all cycles
of chemotherapy was used for each patient.
Statistical analysis
Response and toxicity data were analyzed using
simple descriptive statistics. Time to progression (TTP) and OS
were determined from the first day of chemotherapy using the
modified FOLFIRI regimen. TTP was calculated until tumor
progression or mortality, and OS was calculated until the date of
mortality or the last follow-up. Survival curves were established
using the Kaplan-Meier method.
Results
Patient characteristics
A total of 24 patients were included in the study.
Their baseline characteristics are summarized in Table I. The patients consisted of 17 males
(70.8%) and 7 females with a median age of 60.5 years (range, 30–83
years). A total of 11 patients (45.8%) were aged 65 years or over
and 18 (75%) had an ECOG PS of 2. Amongst the 24 patients, 21
(87.5%) had adenocarcinoma and the remaining 3 had signet ring cell
carcinoma. The most common metastatic site was the peritoneum
(54.2%), followed by the abdominal lymph node (50%), liver (41.6%)
and lung (16.7%). A total of 17 (70.8%) patients had metastatic
diseases and the remaining 7 had recurrent disease following
curative resection. As first-line chemotherapy, 14 patients (58.3%)
had received oxaliplatin, LV and 5-FU (FOLFOX regimen), and 6 (25%)
had received taxane (paclitaxel or docetaxel) with cisplatin.
 | Table I.Characteristics of 24 frail or elderly
patients with advanced gastric cancer. |
Table I.
Characteristics of 24 frail or elderly
patients with advanced gastric cancer.
| Characteristics | No. of patients
(%) |
|---|
| Age (years) |
| ≥65 | 11 (45.8) |
| <65 | 13 (54.2) |
| Median | 60.5 |
| Range | 30–83 |
| Gender |
| Male | 17 (70.8) |
| Female | 7 (29.2) |
| ECOG PS |
| 1 | 6 (25.0) |
| 2 | 18 (75.0) |
| Histology |
| Adenocarcinoma
differentiation | 21 (87.5) |
| Well | 6 (25.0) |
| Moderate | 4 (16.7) |
| Poor | 11 (45.8) |
| Signet ring cell
carcinoma | 3 (12.5) |
| Disease status |
| Recurrent | 7 (29.2) |
| Metastatic | 17 (70.8) |
| Sites of
metastases |
| Liver | 10 (41.6) |
| Abdominal lymph
node | 12 (50.0) |
| Lung | 4 (16.7) |
| Peritoneum | 13 (54.2) |
| Ovary | 2 (8.3) |
| Bone | 3 (12.5) |
| First-line
chemotherapy |
| Oxaliplatin + LV
+ 5-FU | 14 (58.3) |
| Taxane and
cisplatin | 6 (25.0) |
| S-1 +/−
cisplatin | 4 (16.7) |
Treatment and outcomes
A total of 113 cycles were conducted, with a median
of 4 cycles per patient (range, 1–12 cycles). However, 4 patients
who demonstrated early disease progression and 3 patients who
demonstratated clinical impairment or toxicity received only 1 or 2
cycles of chemotherapy.
Of the 24 patients, 3 achieved PR and 8 demonstrated
SD. On an intent-to-treat basis, the overall RR was 12.5% and the
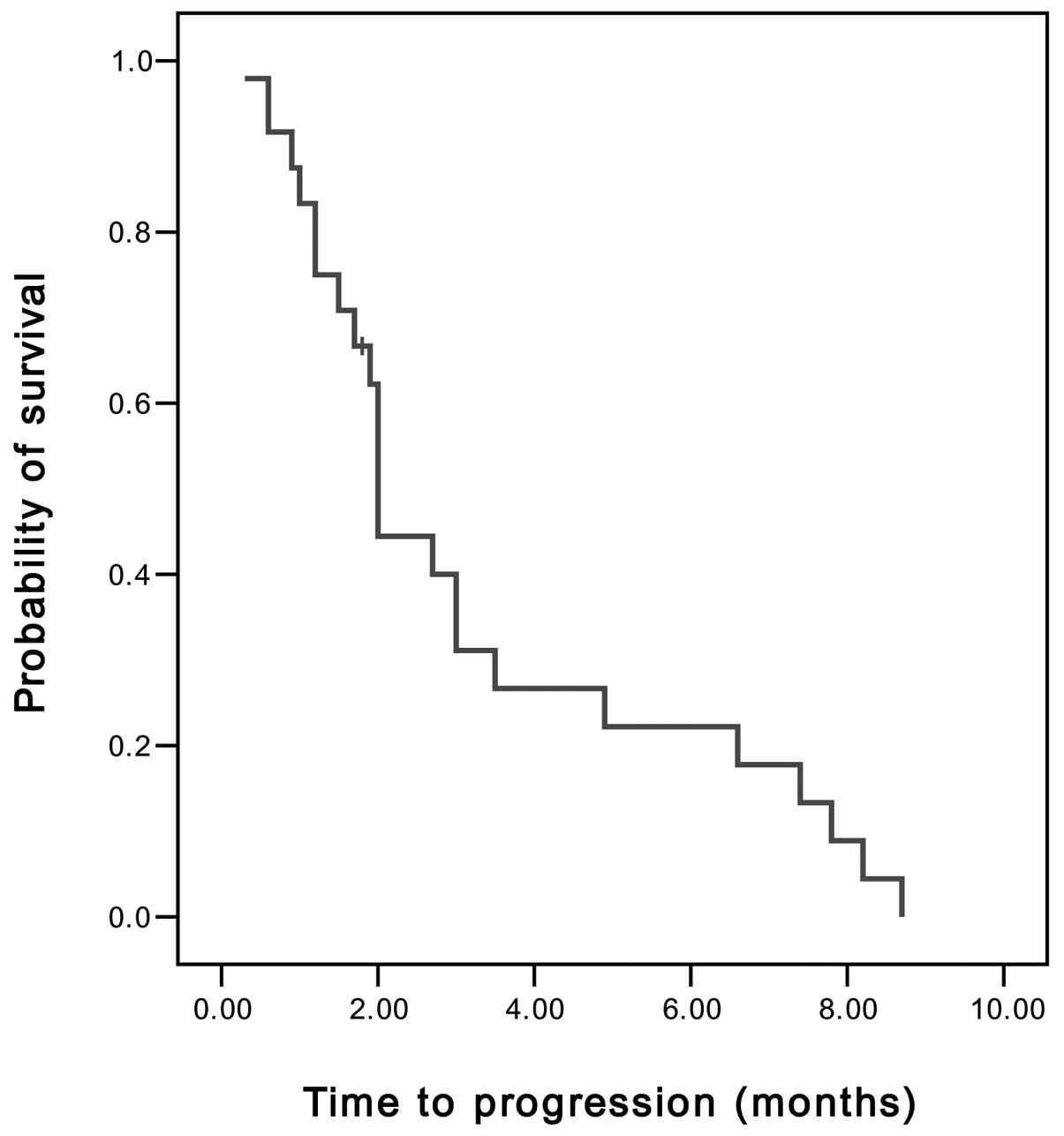
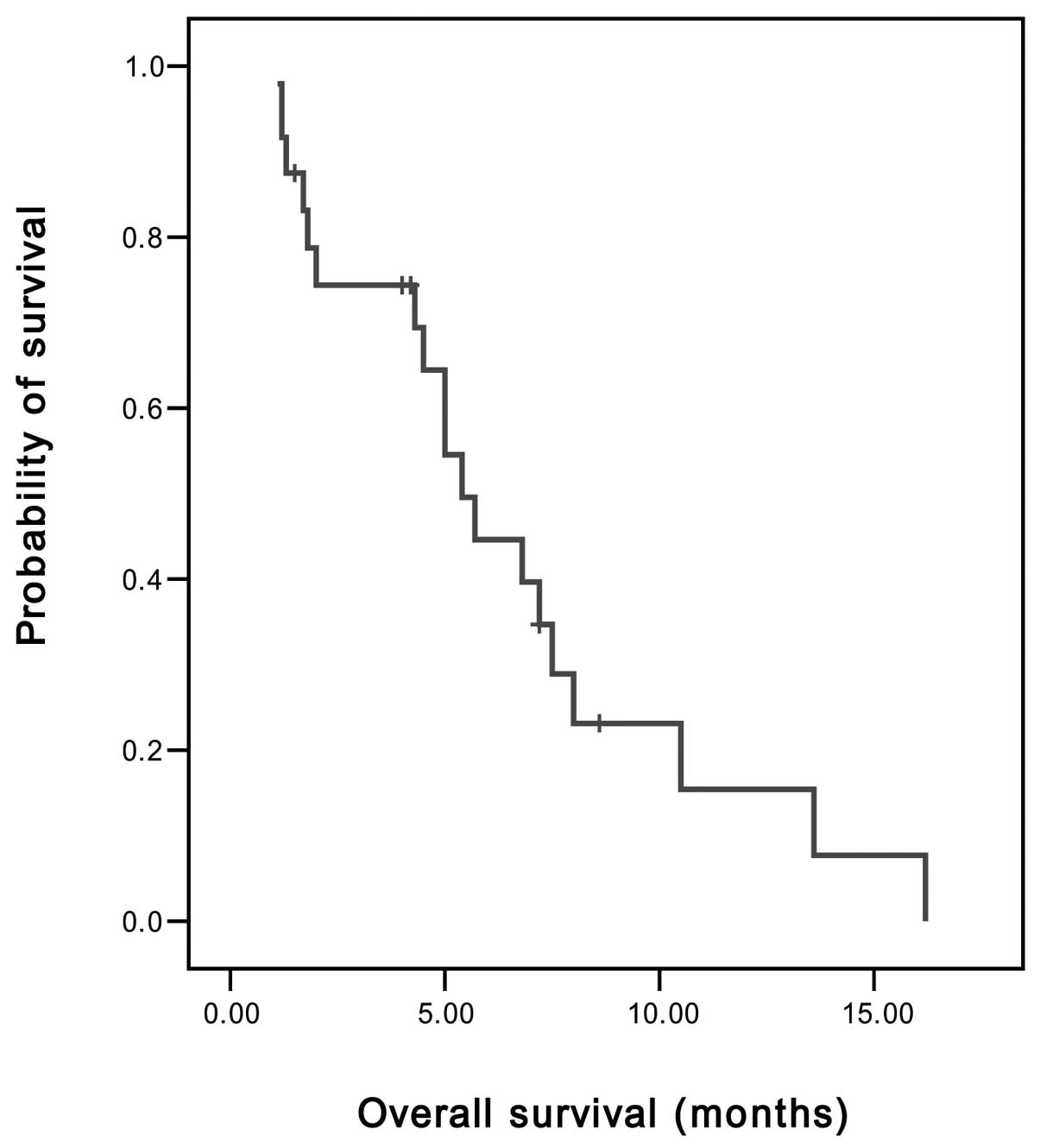
disease control rate (PR and SD) was 45.8%. The median TTP was 2
months [95% confidence interval (CI), 1.89–2.11 months] (Fig. 1), the median OS was 5.4 months (95%
CI, 4.11–6.69 months) and the 1-year survival rate was 8.3%
(Fig. 2).
Toxicities
All 24 patients were evaluable for toxicity. The
toxicity profiles are shown in Table
II. A total of 6 (25%) patients delayed the next cycle of
chemotherapy, primarily due to myelosuppression, and 5 (20.8%)
patients required dose reduction due to grade 3–4 toxicities. Grade
3–4 hematological toxicities, including neutropenia, anemia and
thrombocytopenia, were observed in 6 (25%), 4 (16.7%) and 1 (4.2%)
patients, respectively. Additionally, 3 (12.5%) patients developed
febrile neutropenia, of which 1 succumbed to pneumonia.
 | Table II.Toxicity profile by grade of 24 frail
or elderly patients with advanced gastric cancer. |
Table II.
Toxicity profile by grade of 24 frail
or elderly patients with advanced gastric cancer.
| Toxicity | Grade 1–2 (%) | Grade 3–4 (%) |
|---|
| Hematological |
| Neutropenia | 9 (37.5) | 6 (25.0) |
| Febrile
neutropenia | - | 3a (12.5) |
| Anemia | 18 (75.0) | 4 (16.7) |
|
Thrombocytopenia | 6 (25.0) | 1 (4.2) |
|
Non-hematological |
| Nausea | 10 (41.7) | 3 (12.5) |
| Vomiting | 7 (29.2) | 2 (8.3) |
| Diarrhea | 4 (16.7) | 1 (4.2) |
| Mucositis | 4 (16.7) | 1 (4.2) |
| Hepatotoxity | 5 (20.8) | 0 |
Non-hematological toxicities were generally mild.
Grade 1–2 nausea, vomiting and diarrhea were observed in 10
(41.7%), 7 (29.2%) and 4 (16.7%) patients, respectively. Grade 3–4
gastrointestinal toxicities, including nausea, vomiting, diarrhea
and mucositis, were observed in 3 (12.5%), 2 (8.3%), 1 (4.2%) and 1
(4.2%) patients, respectively.
Discussion
Over the past decade, new chemotherapeutic agents,
including docetaxel, paclitaxel, capecitabine, S-1, irinotecan and
oxaliplatin, have been extensively studied for use in gastric
cancer therapy. Although AGC is considered to be chemosensitive,
its prognosis has remained poor, with a median OS of approximately
10 months. Gastric cancer develops more frequently among elderly
patients (1). Additionally, a
considerable number of patients with AGC have poor PS in
association with a previous radical gastrectomy or comorbidity;
therefore, attempts to develop optimal regimens for frail or
elderly patients are required.
The combination of irinotecan with 5-FU/LV (FOLFIRI)
as first-line chemotherapy has demonstrated an acceptable safety
profile with beneficial activity for AGC patients (7,8). A
variety of FOLFIRI regimens have been examined in the clinical
setting (9–11); however, in this study we used the
modified FOLFIRI regimen (biweekly irinotecan 150 mg/m2
with LV 100 mg/m2 and 5-FU 2,000 mg/m2) for
patients with AGC. We adopted an infusional 5-FU regimen due to low
hematological toxicity levels observed compared to bolus 5-FU
(12).
Several phase II studies have demonstrated the
activity and tolerability of FOLFIRI regimens as salvage
chemotherapy (9–11); however, information regarding frail
or elderly patients with AGC remains limited. In this study, we
reviewed the efficacy and safety of the modified FOLFIRI regimen as
salvage chemotherapy in AGC patients with old age or poor PS. On an
intent-to-treat basis, the overall RR was 12.5%, with a median TTP
of 2 months and a median OS of 5.4 months, which are comparable
results to those in other studies using FOLFIRI regimens as salvage
treatment (10,11). Jeon et al studied another
FOLFIRI regimen (biweekly irinotecan 150 mg/m2 with LV
20 mg/m2 and bolus 5-FU 400 mg/m2 followed by
5-FU 600 g/m2 as a 22 h infusion on day 1 and 2) as
second-line chemotherapy in AGC patients who were previously
unsuccessfully treated with the modified FOLFOX-4 regimen (10). In this study, approximately 40% of
patients demonstrated a poor PS, with an RR of 9.4% and a median
TTP and OS of 2 months and 5.84 months, respectively (10). Sym et al have also reviewed
various FOLFIRI regimens in AGC patients previously treated with
fluoropyrimidine, platinum and taxane (11). Among 131 patients, 12 (12.3%)
demonstrated a tumor response, with a median TTP of 2.2 months and
a median OS of 6.2 months (11).
With regards to toxicity, the modified FOLFIRI
regimen used in the current study was acceptable. A total of 6
patients delayed their next cycle of chemotherapy due to
myelosuppression and 5 required dose reduction due to grade 3–4
toxicities. Although there was 1 treatment-related mortality,
hematological toxicities were manageable. The incidence of grade
3–4 myelotoxicities in our study was similar to those reported in
other studies that employed the same drugs (10,11).
The most common non-hematological toxicities, nausea and vomiting,
were observed in 54.2 and 37.5% of patients, respectively. Our
results achieved in frail or elderly patients are encouraging in
terms of the efficacy and safety. However, considering that 7
patients terminated chemotherapy of FOLFIRI after only 1 or 2
cycles due to early disease progression, clinical impairment or
toxicity, further trials are required to identify the patients most
likely to benefit from salvage chemotherapy.
In conclusion, in our study, the modified FOLFIRI
regimen used as salvage chemotherapy was effective and acceptable
for frail or elderly patients with AGC. Our results suggest that
this regimen may be an effective option for these patients.
References
|
1.
|
KW JungS ParkHJ KongCancer statistics in
Korea: incidence, mortality, survival, and prevalence in 2008Cancer
Res Treat43111201110.4143/crt.2011.43.1.121509157
|
|
2.
|
RT GreenleeT MurrayS BoldenPA WingoCancer
statistics, 2000CA Cancer J
Clin50733200010.3322/canjclin.50.1.7
|
|
3.
|
LL GundersonH SosinAdenocarcinoma of the
stomach: areas of failure in a re-operation series (second or
symptomatic look) clinicopathologic correlation and implications
for adjuvant therapyInt J Radiat Oncol Biol
Phys8111198210.1016/0360-3016(82)90377-77061243
|
|
4.
|
V CatalanoR LabiancaGD BerettaG GattaF de
BraudE Van CutsemGastric cancerCrit Rev Oncol
Hematol54209241200510.1016/j.critrevonc.2005.01.002
|
|
5.
|
PC Thuss-PatienceA KretzschmarD
BichevSurvival advantage for irinotecan versus best supportive care
as second-line therapy in gastric cancer: a randomized phase III
study of the Arbeitsgemeinschaft Internistiche Onkolgie (AIO)Eur J
Cancer4723062314201110.1016/j.ejca.2011.06.002
|
|
6.
|
LB SaltzJV CoxC BlankeIrinotecan plus
fluorouracil and leucovorin for metastatic colorectal cancerN Engl
J Med343905914200410.1056/NEJM20000928343130211006366
|
|
7.
|
BG KimSY OhHC KwonA phase II study of
irinotecan with biweekly, low dose leucovorin and bolus and
continuous infusion 5-fluorouracil (modified FOLFIRI) as first line
therapy for patients with recurrent or metastatic gastric cancerAm
J Clin Oncol33246250201019770628
|
|
8.
|
O BoucheJL RaoulF BonnetainRandomized
multi-center phase II trial of a biweekly regimen of fluorouracil
and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus
irinotecan in patients with previously untreated metastatic gastric
cancer: a Federation Francophone de Cancerologie Digestive Group
Study - FFCD 9803J Clin Oncol22431943282004
|
|
9.
|
SG KimSY OhHC KwonA phase II study of
irinotecan with bi-weekly, low-dose leucovorin and bolus and
continuous infusion 5-fluorouracil (modified FOLFIRI) as salvage
therapy for patients with advanced or metastatic gastric cancerJpn
J Clin Oncol37744749200710.1093/jjco/hym10317923456
|
|
10.
|
EK JeonSH HongTH KimModified FOLFIRI as
second-line chemotherapy after failure of modified FOLFOX-4 in
advanced gastric cancerCancer Res
Treat43148153201110.4143/crt.2011.43.3.14822022291
|
|
11.
|
SJ SymMH RyuJL LeeSalvage chemotherapy
with biweekly irinotecan, plus 5-fluorouracil and leucovorin in
patients with advanced gastric cancer previously treated with
fluoropyrimidine, platinum, and taxaneAm J Clin
Oncol31151156200810.1097/COC.0b013e31815878a2
|
|
12.
|
E Diaz-RubioNew chemotherapeutic advances
in pancreatic, colorectal, and gastric
cancersOncologist9282294200410.1634/theoncologist.9-3-28215169983
|