Introduction
Lung cancer is one of the most common types of
malignant tumor, with a morbidity rate that has increased during
recent years (1). Non-small cell
lung cancer (NSCLC) accounts for approximately 80–85% of all lung
cancer cases (2–4). To date, the most effective therapeutic
measure for treating this type of lung cancer has been surgical
excision of the tumor; however, its efficacy is not always
satisfactory, particularly in patients with advanced lung cancer
for whom the five-year survival rate is relatively low (15–20%)
(3–5). Early diagnosis of lung cancer is not
easy, and chances of overall survival greatly diminish as the
cancer becomes more advanced. Therapeutic measures for detecting
and treating lung cancer have developed rapidly. Methods such as
radiotherapy and chemotherapy are constantly improving and new
chemo-therapeutic drugs are continuously emerging. Overall, the
therapeutic efficacy of existing methods for the treatment of lung
cancer remains unsatisfactory. Therefore, it is necessary to
explore new diagnostic and therapeutic methods to further improve
the outcomes for lung cancer patients.
Currently, great attention has been paid to the
expression of tumor-specific antigen genes and their association
with tumorigenesis. The majority of these studies have evaluated
melanoma-associated antigens (MAGEs). A member of this family,
MAGED4, has been shown to be highly expressed in NSCLC. Therefore,
we consider that the MAGED4 gene may be used as a specific antigen
for NSCLC, and aid in the early diagnosis and successful treatment
of patients with NSCLC.
Patients and methods
Patient data
Among the 54 patients with NSCLC, 33 (61.11%) were
male and 21 (38.89%) were female. Patient ages ranged from 31–78
years, with an average age of 57.33 years (±10.91). A total of 21
(38.89%) patients had a smoking history of ≥20 years (and ≥1
pack/day), and 33 (61.11%) patients had a smoking history of <20
years (or <1 pack/day).
This study was approved by the Institutional Review
Board of Zhongshan Hospital of Fudan University and all patients
signed informed consent forms.
Tissue samples
All tissue samples were obtained by surgical
excision from patients with pulmonary malignant tumors during their
hospitalization at the Department of Thoracic Surgery of the
Huashan Hospital (Shanghai, China). A total of 54 cases were
pathologically diagnosed as primary malignant lung tumors (NSCLC),
of which 34 (62.96%) had a tumor size of >3 cm and 20 (37.04%)
had a tumor size of ≤3 cm. A total of 19 (35.18%) cases were
classified as squamous cell carcinoma, 31 (57.41%) were classified
as adenocarcinoma, three (5.56%) were borderline undifferentiated
large cell carcinoma and one (1.85%) was pulmonary malignant
melanoma. The tumor cells were well-differentiated in 17 (31.48%)
cases, moderately differentiated in 25 (59.25%) cases and poorly
differentiated in 12 (9.00%) cases. Lymph node metastasis occurred
in 26 (48.15%) cases, the remaining 28 (51.85%) cases had no lymph
node metastasis. All patients underwent macroscopical radical
resection and lymph node clearance. Once the tumors were excised,
two sections of tissue of approximately 1 cm3 were
removed; one from the tumor area and the other from an area 5 cm
away from the tumor boundary, which was used as the non-cancerous
tissue sample. The tissues were immediately preserved in liquid
nitrogen at −196°C. Non-cancerous tissues were collected from 20
cases. None of the patients had received any antitumor treatments,
including radiotherapy or chemotherapy, prior to the surgery.
The patients were grouped according to their age,
gender, smoking history, tumor size, pathological classification,
degree of lung cancer cell differentiation and presence of lymph
node metastasis. The patient data for the tissue samples are shown
in Table I.
 | Table I.Data from 54 NSCLC patients. |
Table I.
Data from 54 NSCLC patients.
| Patient data | No. of cases | % |
|---|
| Age (years) | | | |
| <60 | 31 | 57.41 |
| ≥60 | 23 | 42.59 |
| Gender | | | |
| Male | 33 | 61.11 |
| Female | 21 | 38.89 |
| Smoking history
(years) | | | |
| ≥20 (and ≥1
pack/day) | 21 | 38.89 |
| <20 (or <1
pack/day) | 33 | 61.11 |
| Tumor size
(diameter, cm) | | | |
| ≤3 | 20 | 37.04 |
| >3 | 34 | 62.96 |
| Pathological
classification | | | |
| Squamous cell
carcinoma | 19 | 35.18 |
|
Adenocarcinoma | 31 | 57.41 |
| Other | 4 | 7.41 |
| Cell
differentiation (group) | | | |
| Well (a) | 17 | 31.48 |
| Moderate (b) | 25 | 59.52 |
| Poor (c) | 12 | 9.00 |
| Lymph node
metastasis | | | |
|
N0 | 28 | 51.85 |
|
N1–2 | 26 | 48.15 |
Real-time fluorescence quantitative polymerase chain
reaction (qPCR) was used to detect MAGED4 expression in the tumor
and normal tissues according to the data collected (6,7), and
to analyze the correlation between the various groups.
Reagents and instruments
TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) was used for RNA extraction, and RNase-Free
DNase M610A (Promega Corporation, Madison WI, USA) was used for DNA
treatment. The Reverse Transcription System A3500 (Promega
Corporation) was used for reverse transcription (RT) reactions, and
SYBR-Green PCR Master mix (Cat No. 4309155; Applied Biosystems,
Carlsbad, CA, USA) was used for qPCR. An ABI7900 fluorescence
quantitative PCR instrument (Applied Biosystems) was used for PCR
analysis.
Methods
RT-PCR was conducted to detect the expression level
of MAGED4 mRNA. The primer sequences for MAGED4 were as follows:
positive-sense strand, 5′-CCAGAATCAGAA CCGAGA-3′ and antisense
strand, 5′-CCAAAATCTCCG TCCTCA-3′; internal reference
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) positive-sense
strand, 5′-TGAACG GGAAGCTCACTGG-3′ and antisense strand, 5′-TCCACC
ACCCTGTTGCTGTA-3′. Primers were synthesized by Shanghai Shenggong
Biotechnology Co. (Shanghai, China). Total RNA extraction using an
RNA extraction kit (Invitrogen Life Technologies) was conducted
according to the manufacturer’s instructions. The RT kit was from
Promega Corporation, and total RNAs were reverse transcribed into
cDNA according to the manufacturer’s instructions. Real-time PCR
was conducted using the Applied Biosystem high-throughput
fluorescent quantitative PCR system (7900HT). The conditions for
PCR reactions were as follows: 50°C for 2 min, 95°C for 10 min,
95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec, 60°C for 15 sec
and 95°C for 15 sec. A total of 40 cycles were conducted and the
values of MAGED4 and GAPDH were determined.
Correction of real-time PCR analysis
For real-time qPCR, all 74 sample volumes were 1
μl. Since the cDNA concentrations in each sample were not
identical, possibly due to errors in concentration quantitation or
the RT efficiency of RNA, results were normalized to GAPDH
expression. ΔCt was obtained by subtracting the measured value of
GAPDH from the measured value of MAGED4 and a ΔCt value was set as
the standard value. Thus, ΔΔCt was obtained by comparing the ΔCt
values of other samples, and 2−ΔΔCt was obtained after
comparison and conversion using formulas. Finally, the relative
expression level of MAGED4 mRNA in the sample was obtained
(7).
Statistical analysis
All statistical analyses were conducted using SPSS
software version 11.5 (SPSS Inc., Chicago, IL, USA). Analysis of
variance (ANOVA) and the Student-Newman-Keuls test (q-test) were
used for statistical analysis of data between multiple groups, and
a t-test was used to evaluate the difference in means between the
two groups. P<0.05 was used to indicate a statistically
significant difference.
Results
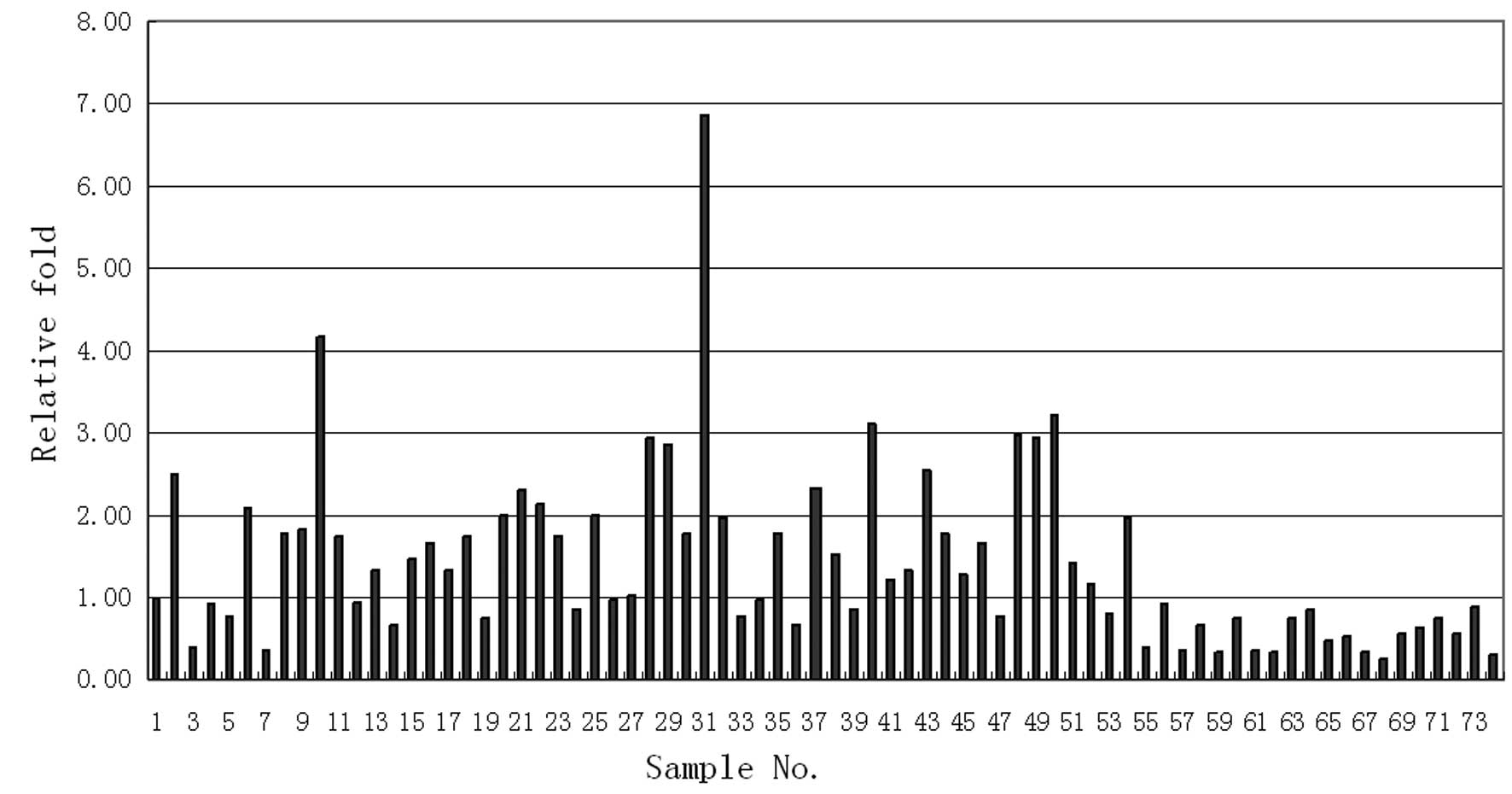
The measured MAGE4 values of the 74 patients are
shown in Fig. 1. The X-axis
represents the number of cases and the Y-axis represents the
corresponding measured value of MAGED4 for each patient. We
identified that the measured values of MAGED4 in the 54 NSCLC
tissues were significantly higher compared to those of the 20
normal lung tissues (Fig. 1).
Statistical analysis revealed that the expression
level of MAGED4 in tumor tissues (mean, 1.74±1.07) was
significantly higher than that of normal lung tissues,
non-cancerous tissues excised more than 5 cm away from the focus,
(mean, 0.55±0.22; P<0.01; Table
II).
 | Table II.MAGED4 expression levels in NSCLC and
normal lung tissues (n=74). |
Table II.
MAGED4 expression levels in NSCLC and
normal lung tissues (n=74).
| Tissue | No. of cases | Mean MAGED4
expression | P-value |
|---|
| NSCLC | 54 | 1.74±1.07 | <0.01 |
| Normal lung | 20 | 0.55±0.22 | |
MAGED4 expression in NSCLC tumor tissues was then
compared between the various groups. The results revealed that
MAGED4 expression was not significantly correlated to patient age,
gender, smoking history or tumor size (P>0.05). Notably, MAGED4
expression in squamous cell carcinoma was significantly higher than
that in adenocarcinoma (P<0.01). In the various cell
differentiation types, expression levels of MAGED4 were relatively
higher when the cells were poorly differentiated compared to when
the cells were well differentiated. The expression levels of MAGED4
increased from poorly differentiated cells to well-differentiated
cells, with moderately differentiated cells having an intermediate
level of expression (P<0.01). MAGED4 expression levels in
patients with lymph node metastasis were significantly higher
compared to patients without lymph node metastasis (P<0.05;
Table III).
 | Table III.MAGED4 gene expression in NSCLC tumor
tissues compared between various patient groups (n=54). |
Table III.
MAGED4 gene expression in NSCLC tumor
tissues compared between various patient groups (n=54).
| Patient data | No. of cases
(%) | Mean MAGED4
expression | P-value |
|---|
| Age (years) | | | |
| <60 | 31 (57.41) | 1.96±1.29 | 0.08 |
| ≥60 | 23 (42.59) | 1.44±0.60 | |
| Gender | | | |
| Male | 33 (61.11) | 1.75±0.85 | 0.92 |
| Female | 21 (38.89) | 1.72±1.39 | |
| Smoking history
(years) | | | |
| ≥20 (and ≥1
pack/day) | 21 (38.89) | 1.73±0.82 | 0.95 |
| <20 (or <1
pack/day) | 33 (61.11) | 1. 75±1.22 | |
| Tumor size
(cm) | | | |
| ≤3 | 20 (37.04) | 1.52±0.63 | 0.27 |
| >3 | 34 (62.96) | 1.87±1.26 | |
| Pathological
classification | | | |
| Squamous cell
carcinoma | 19 (34.55) | 1.99±0.74 | <0.01 |
|
Adenocarcinoma | 32 (58.18) | 1.27±0.53 | Not included in
statistical analysis |
| Other | 4 (7.27) | 4.23±1.85 |
| Cell
differentiation (group) | | | |
| Well (a) | 17 (31.48) | 1.01±0.45 | <0.01 |
| Moderate (b) | 25 (46.29) | 1.65±0.59 |
(c>b>a)a |
| Poor (c) | 12 (22.22) | 2.96±1.44 | |
| Lymph node
metastasis | | | |
|
N0 | 28 (51.85) | 1.42±0.60 | 0.031 |
|
N1–2 | 26 (48.15) | 2.07±1.36 | |
Discussion
Lung cancer is one of the leading causes of
mortality. With continually rising rates of morbidity and
mortality, lung cancer has become the leading cause of mortality
among carcinomas in numerous countries worldwide (1). Although a vast amount of research has
been conducted regarding early prevention, diagnosis and clinical
treatments of lung cancer, the five-year survival rate remains
unsatisfactory (approximately 15%) (2–4).
Therefore, there is an urgent requirement to identify new
diagnostic and therapeutic methods for the treatment of lung
cancer.
Genetic diagnosis and treatments for lung cancer may
be one direction for the development of diagnosis and treatments
for lung cancer in the future. MAGEs are a group of
tumor-associated antigens (TAA), which were first discovered in
melanomas (8). The MAGE gene family
is almost completely silent in normal tissues and is mainly
expressed in malignant tumor tissues, particularly in melanomas.
MAGE expression correlates with tumor specificity to a relatively
high degree (9), and its expression
is closely associated with the development, progression and
prognosis of tumors. Thus, its products may be used in molecular
diagnosis and immunotherapy of tumors. Van der Bruggen et al
discovered the first MAGE, MAGE1, during a study of malignant
melanomas in 1991 (8). Following
this study, attempts were made to further characterize other
members of the MAGE gene family.
Previous studies revealed that MAGE antigens were
expressed in various malignant tumors (10–15),
while there was low or no expression of MAGE in normal tissues.
Certain MAGE family members also demonstrated relatively high
levels of positive expression in lung cancer tissues. Certain
studies indicate that the positive expression rate of MAGE-A3 in
lung cancer is as high as 30–50% (16,17).
However, findings obtained by other studies vary widely. One study
revealed that MAGE-A3 expression levels in lung cancer were only
13% (18). Identification of a
consistent lung cancer-specific antigen remains an aim for
researchers in the field.
MAGED4 is a member of the MAGE gene family that has
been recently discovered (19). In
contrast to other MAGE members, MAGED4 demonstrates low levels of
expression in many malignant tumors outside of the lung, while its
expression level in NSCLC is relatively high (19).
Our study compared the expression levels of MAGED4
between 54 cases of surgically-resected NSCLC and non-cancerous
lung tissues proximal to the cancer using real-time fluorescence
qPCR. Expression of MAGED4 in normal tissues was significantly
lower compared to that in tumor tissues (0.55 vs. 1.74;
P<0.01).
Previous studies have reported that genes in the
MAGED4 family may be detected at a significantly increased level in
the majority of malignant tumor tissues. This is due to the
characterization of malignant tumors by melanoma, and due to the
fact that low levels that are typically found in normal tissues,
with the exception of the testicles and placenta (20). However, Jang et al discovered
that MAGE mRNA was detected in corresponding normal tissues
(21), and in 2006 Ito et al
also revealed that MAGED4 expression was detected at low levels in
normal tissues (19). These results
correspond with our findings in the present study which indicate
that MAGED4 expression can be detected at low levels in normal
tissues. We suggest that the reason for this discrepancy may be due
to the slightly different expression features of various members of
the MAGE family in tissues. It may also be related to the
processing of the tissue samples, methods for experimental
detection or other factors. The real-time fluorescence qPCR
detection in this study had a higher sensitivity and accuracy in
comparison to previous staining, immunohistological methods and
traditional PCR detection. Its high sensitivity is able to detect
less than 10 tumor cells from 1×105–1×107
nucleated cells; thus, it is a well-received accurate RNA detection
technique.
We conducted a more detailed analysis for the high
expression levels of MAGED4 in NSCLC and revealed that expression
in NSCLC tissues was not significantly correlated with patient age
or gender (P>0.05). These results were in accordance with the
properties of other members of the MAGE gene family discovered in
previous studies (15,17,18).
Since the development of lung cancer has previously
been shown to be correlated with smoking (22), we divided patients into two groups;
those with a smoking history of ≥20 years and those with a smoking
history of <20 years. Notably, the expression levels of MAGED4
in these two groups did not demonstrate a significant difference
(P>0.05). Ito et al similarly revealed that MAGED4
expression level did not correlate with patient smoking history
(19); while a number of earlier
studies on other members of the MAGE gene family, including
MAGE-A1, -A3 and -B2, indicated that the activation and expression
of these genes had a certain degree of correlation with smoking
(21). We suggest that the
discrepancy in these findings is due to different biological
behaviors of the various members of the MAGE gene family. We
presume that the reason for this result may be attributed to the
difference in biological behaviors in various genes in the MAGE
family.
This study demonstrated that expression of MAGED4 in
the younger age group was higher than that of the older age group
(1.96 vs. 1.44; P>0.05). This difference in expression may be
attributed to the slightly more rapid tumor cell proliferation in
young patients due to an increased cellular activity (19). Studies have revealed that the
proliferation speed of tumor cells has a certain degree of
correlation with expression of the MAGE gene family (19). However, we identified that there was
no significant difference between the two age groups (P>0.05),
which may be due to the relatively small sample size used in this
study.
Members of the MAGE gene family have been identified
to have higher expression levels in tumors with relatively larger
diameters (23). In this study, we
discovered that MAGED4 expression was not significantly correlated
with tumor size (P>0.05). This may result from the different
expression characteristics of the various subtypes of genes in the
MAGE family. Research data for MAGED4 regarding this topic requires
further investigation.
Currently, there is no agreement with regards to the
correlation between MAGE expression and pathological types of
tumors. Yoshimatsu et al revealed that the expression levels
of MAGE-1 and -3 were higher in squamous cell carcinomas compared
to adenocarcinomas (15), while
Shen et al revealed that there was no significant difference
in MAGE-1 expression between these two types of carcinomas
(24). There have been few studies
focusing on the correlation between MAGED4 expression and
pathological types of tumors. In accordance with the results of
this study, Ito et al identified that expression levels of
MAGED4 in squamous cell carcinomas were higher than those in
adenocarcinomas (19). The
difference in expression levels may be due to the different
proliferation profiles and molecular genetic characteristics
between squamous cell carcinoma and adenocarcinoma cells.
In the present study, there was a certain degree of
correlation between the expression level of MAGED4 and the
differentiation degree of tumor cells. The expression level of
MAGED4 was higher when the tumor cells were poorly differentiated
and lower when the tumor cells were well differentiated. We
categorized the pathological sections of the samples into three
groups according to the tumor cell differentiation degree: a,
well-differentiated carcinoma; b, moderately differentiated
carcinoma; c, poorly differentiated carcinoma. Multiple comparisons
demonstrated that expression levels of MAGED4 were highest in c,
followed by b, then a (P<0.01). This result provides a general
guideline that may be helpful in selecting therapies and providing
prognostic information for NSCLC patients.
It is well-known that the metastatic status of lymph
nodes is an important prognostic factor in cancer; therefore, we
categorized the samples into two groups according to whether lymph
node metastasis had occurred. Our results demonstrated that the
expression level of MAGED4 in the lymph node metastasis group was
higher than that in the group without lymph node metastasis
(P<0.05). In the literature, there has been no agreement in
terms of the correlation between MAGE gene family expression and
metastatic status of lymph nodes. Dango et al reported that
the expression of MAGE-A was correlated with lymph node metastasis
(25), while Chang et al
proposed that they were not correlated (26). The results of this study indicate
that there is a certain degree of correlation between MAGED4
expression and the metastatic status of lymph nodes. These results
will aid in providing prognostic information with regards to
NSCLC.
In the present study, we discovered that MAGED4
expression in NSCLC tissues was significantly higher than that in
normal lung tissues. This is in accordance with results from other
members of the MAGE family. MAGE-A1, -A2 and -A3 demonstrated
higher expression levels in malignant tumor tissues compared to
normal tissues; however, the expression of MAGED4 in NSCLC tissues
revealed its particularity as it did not follow the consistent
expression of the other genes in the MAGE family.
The mechanism of MAGED4 activity in tumor cells also
remains unclear. Studies have been conducted on more
well-characterized family members, including MAGE-A1, -A2 and -A3,
but further study is required in order to compare MAGED4 to these
family members. Studies on these genes have entered the stage of
immunotherapy investigation. Studies on active specific
immunotherapy with MAGE peptide-based tumor vaccines and adoptive
therapy with in vitro-induced MAGE-specific cytotoxic T
lymphocyte (CTL) of certain MAGE-positive cancers have been
conducted, and a degree of efficacy has been demonstrated (27,28).
In conclusion, MAGED4 is a newly discovered member
of the MAGE gene family. Its expression level in NSCLC is higher
than that of other members of the MAGE family, but studies on the
MAGED4 gene remain insufficient (29). In our study, the expression of
MAGED4 in NSCLC tissues revealed a certain degree of specificity
and sensitivity. The results may be beneficial for the diagnosis
and treatments of NSCLC in the future.
Acknowledgements
This study was supported by the Youth
Foundation of Fudan University and the Startup Foundation of
Huashan Hospital, Fudan University.
References
|
1.
|
HL HowePA WingoMJ ThunAnnual report to the
nation on the status of cancer (1973 through 1998), featuring
cancers with recent increasing trendsJ Natl Cancer
Inst93824842200110.1093/jnci/93.11.82411390532
|
|
2.
|
L CrinòW WederJ van MeerbeeckE FelipESMO
Guidelines Working GroupEarly stage and locally advanced
(non-metastatic) non-small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-upAnn
Oncol21v103v1152010
|
|
3.
|
DS EttingerW AkerleyG BeplerMG BlumA
ChangRT CheneyLR ChirieacTA D’AmicoTL DemmyAK GantiNCCN Non-Small
Cell Lung Cancer Panel MembersNon-small cell lung cancerJ Natl
Compr Canc Netw8740801201020679538
|
|
4.
|
A JemalR SiegelE WardCancer statistics,
2008CA Cancer J Clin587196200810.3322/CA.2007.0010
|
|
5.
|
KM PistersE VallièresJJ CrowleySurgery
with or without preoperative paclitaxel and carboplatin in
early-stage non-small-cell lung cancer: Southwest Oncology Group
Trial S9900, an intergroup, randomized, phase III trialJ Clin
Oncol2818431849201010.1200/JCO.2009.26.1685
|
|
6.
|
VJ JohnsonB YucesoyMI LusterGenotyping of
single nucleotide polymorphisms in cytokine genes using real-time
PCR allelic discrimination
technologyCytokine27135141200410.1016/j.cyto.2004.05.00215304242
|
|
7.
|
KJ LivakTD SchmittgenAnalysis of relative
gene expression data using real-time quantitative PCR and the
2(-Delta Delta C(T))
methodMethods25402408200110.1006/meth.2001.126211846609
|
|
8.
|
P van der BruggenC TraversariP ChomezA
gene encoding an antigen recognized by cytolytic T lymphocytes on a
human melanomaScience254164316471991
|
|
9.
|
E De PlaenK ArdenC TraversariStructure,
chromosomal localization, and expression of 12 genes of the MAGE
familyImmunogenetics4036036919947927540
|
|
10.
|
K KariyamaT HigashiY KobayashiExpression
of MAGE-1 and -3 genes and gene products in human hepatocellular
carcinomaBr J
Cancer8110801087199910.1038/sj.bjc.669081010576668
|
|
11.
|
S NoguchiT AiharaK MotomuraH InajiS
ImaokaH KoyamaDetection of breast cancer micrometastases in
axillary lymph nodes by means of reverse transcriptase-polymerase
chain reaction. Comparison between MUC1 mRNA and keratin 19 mRNA
amplificationAm J Pathol1486496561996
|
|
12.
|
S OfujiM IkedaS TsujitaniExpression of
MAGE-1, MAGE-2 and MAGE-3 genes in human gastric carcinomas; lack
of evidence for cytotoxic effects in cases with simultaneous
expression of MAGE-3 and HLA-A2Anticancer
Res183639364419989854470
|
|
13.
|
V QuillienJL RaoulD HeresbachB ColletL
ToujasF BrasseurExpression of MAGE genes in esophageal
squamous-cell carcinomaAnticancer Res1738739119979066682
|
|
14.
|
U SahinM KoslowskiO TureciExpression of
cancer testis genes in human brain tumorsClin Cancer
Res639163922200011051238
|
|
15.
|
T YoshimatsuI YoshinoA OhgamiExpression of
the melanoma antigen-encoding gene in human lung cancerJ Surg
Oncol67126129199810.1002/(SICI)1096-9098(199802)67:2%3C126::AID-JSO10%3E3.0.CO;2-19486785
|
|
16.
|
C GrunwaldM KoslowskiT ArsirayExpression
of multiple epigenetically regulated cancer/germline genes in
nonsmall cell lung cancerInt J
Cancer11825222528200610.1002/ijc.2166916353146
|
|
17.
|
K TajimaY ObataH TamakiExpression of
cancer/testis (CT) antigens in lung cancerLung
Cancer422333200310.1016/S0169-5002(03)00244-714512184
|
|
18.
|
JR TsaiIW ChongYH ChenDifferential
expression profile of MAGE family in non-small-cell lung cancerLung
Cancer56185192200710.1016/j.lungcan.2006.12.00417208331
|
|
19.
|
S ItoY KawanoH KatakuraExpression of
MAGE-D4, a novel MAGE family antigen, is correlated with tumor-cell
proliferation of non-small cell lung cancerLung
Cancer517988200610.1016/j.lungcan.2005.08.01216225959
|
|
20.
|
F MuscatelliAP WalkerE De PlaenAN
StaffordAP MonacoIsolation and characterization of a MAGE gene
family in the Xp21.3 regionProc Natl Acad Sci
USA9249874991199510.1073/pnas.92.11.49877761436
|
|
21.
|
SJ JangJC SoriaL WangActivation of
melanoma antigen tumor antigens occurs early in lung
carcinogenesisCancer Res6179597963200111691819
|
|
22.
|
A ParsonsA DaleyR BeghP AveyardInfluence
of smoking cessation after diagnosis of early stage lung cancer on
prognosis: systematic review of observational studies with
meta-analysisBMJ340b5569201010.1136/bmj.b5569
|
|
23.
|
S ShichijoA HayashiS TakamoriDetection of
MAGE-4 protein in lung cancersInt J
Cancer64158165199510.1002/ijc.29106403037622303
|
|
24.
|
C ShenD WangG JiangJ LiuG ZhangmRNA
expressions of MAGE genes in human lung cancerZhongguo Fei Ai Za
Zhi62682712003(In Chinese)
|
|
25.
|
S DangoB CucuruzO MayerDetection of
disseminated tumour cells in mediastinoscopic lymph node biopsies
and endobronchial ultrasonography-guided transbronchial needle
aspiration in patients with suspected lung cancerLung
Cancer68383388201010.1016/j.lungcan.2009.08.003
|
|
26.
|
HK ChangJ ParkW KimThe expression of MAGE
and GAGE genes in uterine cervical carcinoma of Korea by RT-PCR
with common primersGynecol
Oncol97342347200510.1016/j.ygyno.2004.12.05115863128
|
|
27.
|
R EifukuM TakenoyamaI YoshinoAnalysis of
MAGE-3 derived synthetic peptide as a human lung cancer antigen
recognized by cytotoxic T lymphocytesInt J Clin
Oncol63439200110.1007/PL0001207711706525
|
|
28.
|
M MarchandN van BarenP WeynantsTumor
regressions observed in patients with metastatic melanoma treated
with an antigenic peptide encoded by gene MAGE-3 and presented by
HLA-A1Int J
Cancer80219230199910.1002/(SICI)1097-0215(19990118)80:2%3C219::AID-IJC10%3E3.0.CO;2-S9935203
|
|
29.
|
M SangL WangC DingMelanoma-associated
antigen genes - an updateCancer
Lett3028590201110.1016/j.canlet.2010.10.02121093980
|