Introduction
The L1 cell adhesion molecule (L1CAM) is a
transmembrane adhesion molecule of the immunoglobulin (Ig)
super-family (1), which plays a
pivotal role in neural cell migration and survival, as well as
neurite outgrowth, myelination and synaptic plasticity (2–5).
Several studies have indicated that L1 also plays a significant
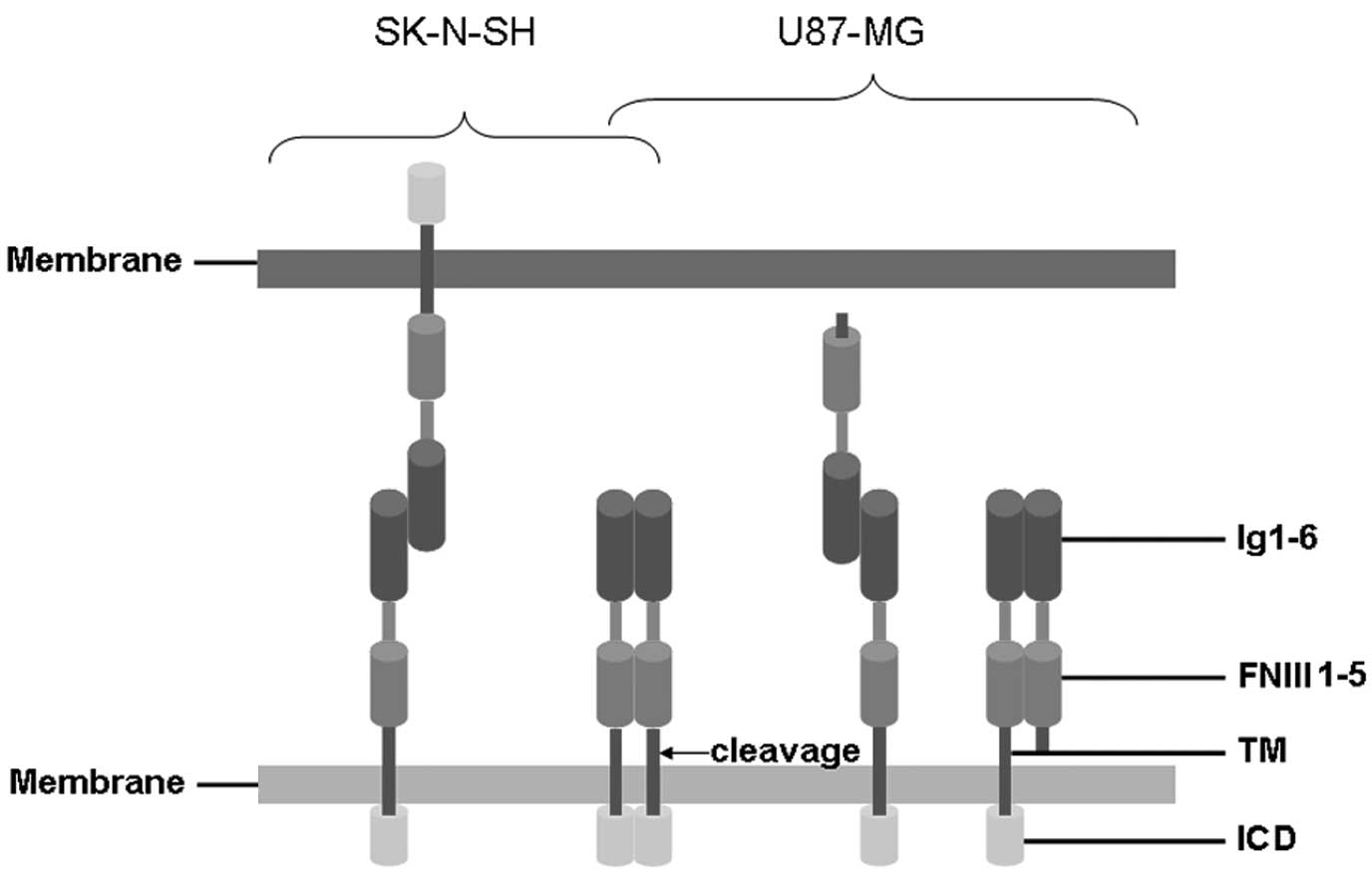
role in tumor progression and metastatic behavior (6). The extracellular portion of L1 is
composed of six Ig-like domains followed by fibronectin type III
(FNIII) domains. The extracellular domain is joined by a short
intracellular cytoplasmic domain (ICD) via a single transmembrane
sequence (Fig. 1) (7). Various functions of L1 are largely
dependent on the complex homophilic and heterophilic interactions
of L1 extracellular domains and other CAMs on the cell surface
(8).
Glioblastomas represent a critical medical challenge
due to their highly invasive and metastatic characteristics
(9–12). Neuroblastomas are a type of
neuroendocrine tumor derived from any neural crest element of the
sympathetic nervous system (SNS), including the adrenal glands.
They may also originate from nerve tissues in the neck, chest,
abdomen or pelvis (13).
Neuroblastomas are genetic and morphological heterogeneous tumors
with a variable clinical course, manifesting as the most common
type of solid tumor in childhood, with unconventional clinical
behavior (14). Although L1 has
been detected in glioblastomas and neuroblastomas, accumulated
evidence suggests that its roles in these tumors are markedly
different. L1 levels in glioblastomas are higher in those with
greater malignancy and L1 is associated with aggressive clinical
behavior. Additionally, L1 may also be subjected to protease
cleavage to release soluble L1 of different molecular weights
ranging from 140–180 kD. Cleaved L1-mediated homophilic
interactions may facilitate glioma cell adhesion, migration and
metastasis to sites far from the tumor-originating sites. In
contrast to that in human glioblastomas, an association of L1
positivity with greater event-free and overall survival has been
observed in patients with neuroblastoma. L1 negativity is
independently prognostic of event-free and overall survival, and
predicts a good outcome in children with neuroblastoma (15,16).
Thus, L1 may function differently in the two tumor types.
Based on these hypotheses, we examined L1 expression
in human glioblastoma and neuroblastoma samples. We also studied L1
expression and secretion in glioblastoma and neuroblastoma cell
lines. We identified that although a high level of L1 was detected
in all human glioblastoma and neuroblastoma samples, their release
level was markedly different. Neuroblastoma SK-N-SH cells contain
more full-length membrane-tethered L1, while glioblastoma U87-MG
cells contain more soluble L1 within the conditioned culture medium
(CCM).
Materials and methods
Reagents and microarrays
Goat anti-human L1 antibody specifically targeting
the extracellular domain of human L1 was purchased from R&D
Systems (Minneapolis, MN, USA). Donkey anti-goat secondary antibody
conjugated to Daylight™ 488 was obtained from The Jackson
Laboratory (Bar Harbor, ME, USA). A human glioblastoma-containing
tissue microarray and a neuroblastoma-containing tissue microarray
were purchased from Chao Ying Biotechnology Co., Ltd. (GL 2083 and
MC 803; Xi’an, Shaanxi, China) (17). The study was approved by the ethics
committee of Shantou University Medical College, Guangdong,
China.
Cell culture
Human glioblastoma U87-MG cells and neuroblastoma
SK-N-SH cells were purchased from the Chinese Type Culture
Collection (CTCC; Shanghai, China) and maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Thermo Scientific Hyclone, Beijing,
China) supplemented with 50 U/ml of penicillin/streptomycin mixture
(Solarbio Science and Technology Co., Ltd., Beijing, China) and 10%
fetal bovine serum (Sijiqing Biotechnology Co., Hangzhou, China).
Cells were routinely grown in 75 cm2 cell culture plates
(Corning Inc., Corning, NY, USA) at 37°C in a humidified 5%
CO2 atmosphere.
Immunofluorescence analysis
Paraffin-embedded 4 μm human glioblastoma and
neuroblastoma tissues containing microarray sections were dewaxed,
and antigen retrieval using 10 mM citrate buffer (pH 6.0) was
conducted. Deparaffinized sections were rehydrated through a graded
series of ethanol to phosphate-buffered saline (PBS; pH 7.4).
Sections were briefly washed in PBS for 5 min each and blocked with
10% normal donkey serum in PBS at room temperature for 60 min. They
were then subjected to incubation with goat anti-human L1 (1:200)
primary antibody mixture. After three washes in PBS for 5 min,
samples were incubated at room temperature with donkey anti-goat
secondary antibody conjugated to Daylight 488 (1:500) for 90 min.
Tissues were co-stained with 4′,6-diamidino-2-phenylindole (DAPI)
and mounted using anti-fade mounting solution (Beyotime Institution
of Biotechnology, Jiangsu, China). Confocal images were obtained
using the Olympus confocal system (FV-1000, Olympus, Japan). DAPI
and Daylight 488 were excited at 405 and 488 nm, respectively.
Evaluation of L1 immunostaining
intensities in the whole field of each microarray set
Staining intensities were scored between 0 and 4
based on their immunofluorescence signal: 0 (−), no detectable
signal; 1 (+), weak; 2 (++), intermediate; 3 (+++), strong; and 4
(++++), very strong.
Western blot analysis
Equivalent quantities of cell lysates (CLs) from
U87-MG and SKN-SH cells or 60 μl of serum free CCM from the cells
were heated to 95°C in 20% sample loading buffer containing 0.125 M
Tris-HCl (pH 6.8), 20% glycerol, 10% sodium dodecyl sulfate (SDS),
0.1% bromophenol blue and 5% β-mercaptoethanol. They were then
resolved by 8% SDS-polyacrylamide gel electrophoresis (PAGE) and
electroblotted onto polyvinylidene difluoride membranes (PVDFs;
Millipore, Billerica, MA, USA). Non-specific protein binding sites
were blocked with 5% non-fat dry milk diluted in Tris-buffered
saline (TBS; pH 7.4) buffer containing 0.05% Tween-20 (TBST).
Membranes were incubated with the primary antibody for human L1
(1:500; R&D Systems) and mouse glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (1:1000; Beyotime Institution of
Biotechnology) overnight at 4°C. After three washes for 5 min each,
horseradish peroxidase (HRP)-conjugated goat anti-mouse and rabbit
anti-goat secondary antibodies (1:1000; Boster, Wuhan, China)
diluted in TBST were applied. After three washes in TBST for 5 min
each at room temperature, antigens were visualized using enhanced
chemiluminescence (Beyotime Institution of Biotechnology). Signal
intensity was quantified using Image Tool II software (Dental
Diagnosis Science, San Antonio, TX, USA) by multiplying the average
densitometry by the area indexed by the number of pixels (18,19).
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Statistical analyses were performed using SPSS version 10.0
software (SPSS, Chicago, IL, USA). Data were analyzed using the
independent samples Student’s t-test and P<0.05 was used to
indicate a statistically significant difference.
Results
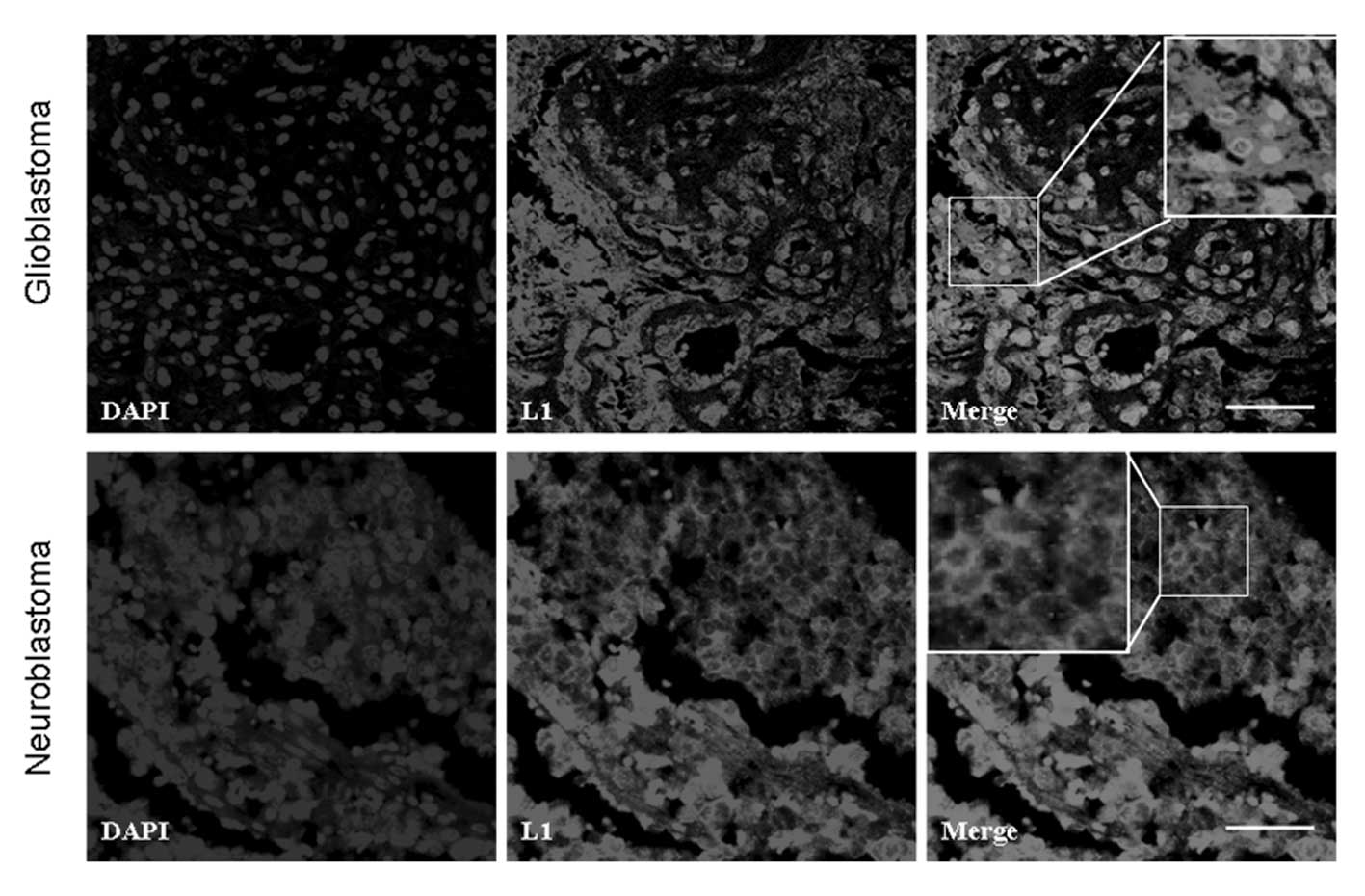
Immunofluorescence staining of L1
Human glioblastoma and neuroblastoma tissues
demonstrated extensive positive staining for L1. In one
representative glioblastoma sample, extensive L1 staining was
localized to glioblastoma cells and the extra-cellular matrix
(Fig. 2). However, in another
representative neuroblastoma sample, L1 was mainly localized at or
around the cell membrane (Fig. 2).
These observations suggested the differential localization pattern
of L1 in glioblastomas and neuroblastomas.
Immunostaining intensities of L1 in
glioblastoma and neuroblastoma samples
Positive immunostaining for L1 was detected in all
46 glioblastoma samples. Three, five and 38 samples demonstrated
moderate, strong and very strong immunostaining, respectively. L1
was also detected in five neuroblastoma samples. Two samples
demonstrated strong and three samples demonstrated very strong
staining intensity (Table I).
 | Table I.Immunostaining intensity analysis of
the cell adhesion molecule L1 in human glioblastoma and
neuroblastoma tissues. |
Table I.
Immunostaining intensity analysis of
the cell adhesion molecule L1 in human glioblastoma and
neuroblastoma tissues.
| Tumor type | n | L1 cell adhesion
molecule
|
|---|
| − | + | ++ | +++ | ++++ |
|---|
| Glioblastoma | 46 | 0 | 0 | 3 | 5 | 38 |
| Neuroblastoma | 5 | 0 | 0 | 0 | 2 | 3 |
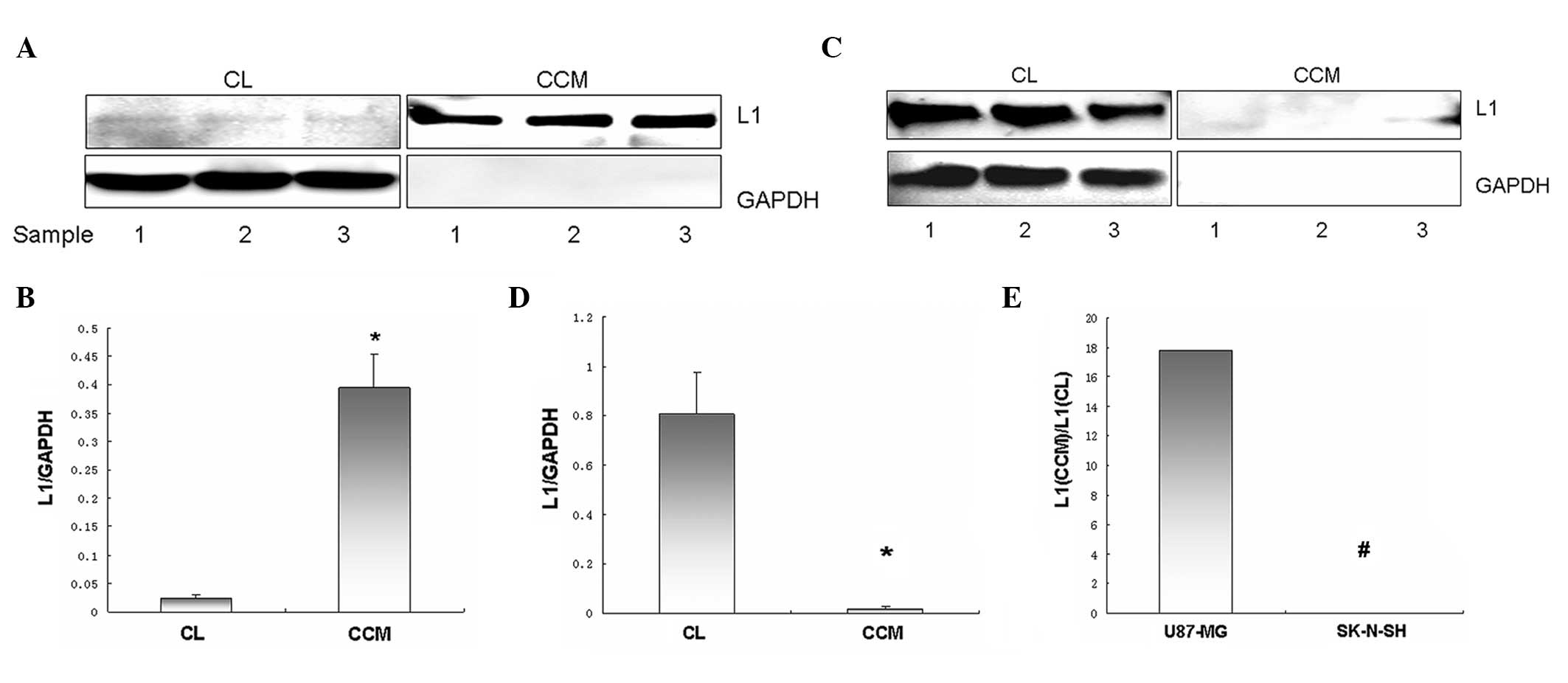
Western blot analysis of full-length L1
and released soluble L1 expression in U87-MG and SK-N-SH cells
In U87-MG cells, full-length L1 in CLs were weakly
detected, while the level of soluble L1 in the CCM was notably high
(Fig. 3A). Full-length L1 and
soluble L1 levels derived from the same amount of U87-MG cells, as
indexed by L1/GAPDH, were 0.023±0.006 and 0.394±0.06, respectively.
Thus, released soluble L1 levels were significantly higher compared
to membrane-tethered full-length L1 levels in U87-MG cells
(P<0.01; Fig. 3B). In contrast,
full-length L1 in SK-N-SH CLs was strongly detected, while soluble
L1 in the CCM was almost undetectable (Fig. 3C). Full-length L1 and soluble L1
levels derived from the same amount of SK-N-SH cells, as indexed by
L1/GAPDH, were 0.807±0.17 and 0.02±0.01, respectively. Thus,
full-length L1 levels were significantly higher compared to
released soluble L1 levels in SK-N-SH cells (P<0.05; Fig. 3D). The ratio of soluble L1 to
full-length L1 in U87-MG cells (17.833:0.024) was significantly
higher compared to that observed in SK-N-SH cells (0.024:0.008)
(P<0.01; Fig. 3E).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to
indicate equal loading volume and ensure no significant leaking of
cell content into the CCM.
Discussion
Although it has been demonstrated that glioblastomas
and neuroblastomas contain high levels of L1, their biological
behaviors are distinct. In this study, we identified the different
expression patterns of L1 between U87-MG and SK-N-SH cells,
suggesting that this pattern may partially explain the
heterogeneity of two tumor types in cell migration, metastasis and
proliferation.
L1 stimulates glioblastoma cell motility (20), and promotes growth and survival of
glioma stem cells in an autocrine/paracrine manner, which can be
inhibited by siRNA and L1 ectodomain-binding antibodies (20,21).
It has been identified that L1 is poorly expressed in gemistocytic
astrocytomas, which are less prone to invading the brain parenchyma
(22). Unlike previous results of
neuroblastoma cells, cells in the invasive fronts of glioblastoma
express more L1 and display greater invasive potential (23). The interaction of another important
CAM member, NCAM, and integrin has been studied in neuroblastoma
SK-N-5Y cells, where the NCAM and β1-integrin interaction
contributes to cell differentiation and decreases malignancy
(24). Thus, more full-length L1
may interact with molecules including integrin. However, in more
malignant glioblastoma cells, native interaction of L1 with
integrin was impaired, and the soluble L1-based signal responsible
for cell proliferation and migration increased. Soluble L1 derived
from the full-length cleavage in the third FNIII domain may disrupt
its arginine-glycine-aspartic acid (RGD)-independent interaction
with integrin through a sequence within this domain on the cell
membrane. As a consequence, cell differentiation signaling was
impaired. In contrast, dispersed soluble L1 may significantly
increase the heterophilic interaction of L1 with integrin through
the RGD sequence in the sixth Ig-like domain, and signaling
responsible for metastasis and migration may be augmented.
Transformation from the full length to the protease cleavage form
of L1 may lead to the transition of cell differentiation to
malignancy-related cell behaviours. L1 was identified to be
preferentially and markedly expressed in glioma-related gliomatosis
cerebri characterized by glial fibrillary acidic protein expression
and cell invasion into preferentially the white matter (22). Thus, it was proposed that the
cleaved L1 ectodomain and/or exosomal vesicles containing L1
support the extensive and diffuse migratory behavior of
glioblastoma cells within the brain tissue, forming the basis of an
autocrine/paracrine model for glioblastoma invasion (20). In vivo existence of soluble
L1 allows the interaction of L1 with more diverse molecules on the
surface of cells from the cleaving site (Fig. 1). By contrast, cell-cell adhesion
through full-length L1-based homophilic interaction or L1/integrin
heterophilic interaction should be the predominant form in SK-N-SH
or other neuroblastoma cells, which are more prone to differentiate
with less malignancy.
Based on our observations regarding the differential
expression patterns of L1 between glioblastoma and neuroblastoma
cells, we suggest that soluble L1 may contribute to
malignancy-related cell migration and metastasis. It also suggests
methods which may be able to interfere with glioma cell migration
by targeting L1 and related molecules clinically.
Acknowledgements
The author is grateful for the support
of the General Program of the National Natural Science Foundation
of China (grant no. 81171138).
References
|
1.
|
PF ManessM SchachnerNeural recognition
molecules of the immunoglobulin superfamily: signaling transducers
of axon guidance and neuronal migrationNat
Neurosci101926200710.1038/nn1827
|
|
2.
|
AH ZischWB StallcupLD ChongK Dahlin-HuppeJ
VosholM SchachnerEB PasqualeTyrosine phosphorylation of L1 family
adhesion molecules: implication of the Eph kinase Cek5J Neurosci
Res47655665200710.1002/(SICI)1097-4547(19970315)47:6%3C655::AID-JNR12%3E3.0.CO;2-U9089215
|
|
3.
|
CG BeckerA ArtolaR Gerardy-SchahnT BeckerH
WelzlM SchachnerThe polysialic acid modification of the neural cell
adhesion molecule is involved in spatial learning and hippo-campal
long-term potentiationJ Neurosci
Res45143152199610.1002/(SICI)1097-4547(19960715)45:2%3C143::AID-JNR6%3E3.0.CO;2-A8843031
|
|
4.
|
JW LawAY LeeM SunAG NikonenkoSK ChungA
DityatevM SchachnerF MorelliniDecreased anxiety, altered place
learning, and increased CA1 basal excitatory synaptic transmission
in mice with conditional ablation of the neural cell adhesion
molecule L1J Neurosci2310419104322003
|
|
5.
|
ZG ZhongS YokoyamaM NodaH
HigashidaOverexpression of adhesion molecule L1 in NG108-15
neuroblastoma X glioma hybrid cells enhances dibutyryl cyclic
AMP-induced neurite outgrowth and functional synapse formation with
myotubesJ
Neurochem6822912299199710.1046/j.1471-4159.1997.68062291.x
|
|
6.
|
S RavehN GavertA Ben-Ze’evL1 cell adhesion
molecule (L1CAM) in invasive tumorsCancer
Lett282137145200910.1016/j.canlet.2008.12.02119144458
|
|
7.
|
MK SchäferP AltevogtL1CAM malfunction in
the nervous system and human carcinomasCell Mol Life
Sci6724252437201020237819
|
|
8.
|
Y HeGJ JensenPJ BjorkmanCryo-electron
tomography of homophilic adhesion mediated by the neural cell
adhesion molecule
L1Structure17460471200910.1016/j.str.2009.01.00919278660
|
|
9.
|
C FiggeG LoersM SchachnerT TillingNeurite
outgrowth triggered by the cell adhesion molecule L1 requires
activation and inactivation of the cytoskeletal protein cofilinMol
Cell Neurosci49196204201210.1016/j.mcn.2011.10.00222019611
|
|
10.
|
N GavertM Conacci-SorrellD GastA
SchneiderP AltevogtT BrabletzA Ben-Ze’evL1, a novel target of
beta-catenin signaling, transforms cells and is expressed at the
invasive front of colon cancersJ Cell
Biol168633642200510.1083/jcb.20040805115716380
|
|
11.
|
RS SchmidPF ManessL1 and NCAM adhesion
molecules as signaling coreceptors in neuronal migration and
process outgrowthCurr Opin
Neurobiol18245250200810.1016/j.conb.2008.07.01518760361
|
|
12.
|
PF SiesserPF ManessL1 cell adhesion
molecules as regulators of tumor cell invasivenessCell Adh
Migr3275277200910.4161/cam.3.3.868919483471
|
|
13.
|
S KarmakarSR ChoudhuryNL BanikSK
RayCombination of N-(4-hydroxyphenyl) retinamide and genistein
increased apoptosis in neuroblastoma SK-N-BE2 and SH-SY5Y
xenograftsNeuroscience163286295200910.1016/j.neuroscience.2009.06.03719540315
|
|
14.
|
C RechnitzerIncreased survival of children
with solid tumours: how did we get there and how to keep the
success going?Cancer
Imaging11S65S69201110.1102/1470-7330.2011.901022187133
|
|
15.
|
R WachowiakHC FiegelJT KaifiA QuaasA
KrickhahnPG SchurrR ErttmannM SchachnerD KluthG SauterJR IzbickiL1
is associated with favorable outcome in neuroblastomas in contrast
to adult tumorsAnn Surg
Oncol1435753580200710.1245/s10434-007-9608-017917782
|
|
16.
|
R WachowiakT RawnaqR MetzgerA QuaasH
FiegelN KählerU RolleJR IzbickiJ KaifiH TillUniversal expression of
cell adhesion molecule NCAM in neuroblastoma in contrast to L1:
implications for different roles in tumor biology of
neuroblastoma?Pediatr Surg
Int2413611364200810.1007/s00383-008-2264-z18972120
|
|
17.
|
DN LouisH OhgakiOD WiestlerWK CaveneePC
BurgerA JouvetBW ScheithauerP KleihuesThe 2007 WHO classification
of tumours of the central nervous systemActa
Neuropathol11497109200710.1007/s00401-007-0243-417618441
|
|
18.
|
W ZhaoSG RenNeuregulin-1 (Nrg1) is mainly
expressed in rat pituitary gonadotroph cells and possibly regulates
prolactin (PRL) secretion in a juxtacrine mannerJ
Neuroendocrinol2312521262201110.1111/j.1365-2826.2011.02223.x21919974
|
|
19.
|
WJ ZhaoSG RenEndogenous neuregulin-1
expression in the anterior pituitary of female Wistar-Furth rats
during the estrous cycleNan Fang Yi Ke Da Xue Xue
Bao319219272011(In Chinese).
|
|
20.
|
M YangS AdlaMK TemburniVP PatelEL LagowOA
BradyJ TianMI BoulosDS GalileoStimulation of glioma cell motility
by expression, proteolysis, and release of the L1 neural cell
recognition moleculeCancer Cell
Int927200910.1186/1475-2867-9-2719874583
|
|
21.
|
S IzumotoT OhnishiN AritaS HiragaT TakiT
HayakawaGene expression of neural cell adhesion molecule L1 in
malignant gliomas and biological significance of L1 in glioma
invasionCancer Res561440144419968640837
|
|
22.
|
T SuzukiS IzumotoY FujimotoM MarunoY ItoT
YoshimineClinicopathological study of cellular proliferation and
invasion in gliomatosis cerebri: important role of neural cell
adhesion molecule L1 in tumour invasionJ Clin
Pathol58166171200510.1136/jcp.2004.02090915677537
|
|
23.
|
L ChengQ WuOA GuryanovaZ HuangQ HuangJN
RichS BaoElevated invasive potential of glioblastoma stem
cellsBiochem Biophys Res
Commun406643648201110.1016/j.bbrc.2011.02.12321371437
|
|
24.
|
AK PetridisSN NikolopoulosA
El-MaaroufPhysical and functional cooperation of neural cell
adhesion molecule and β1-integrin in neurite outgrowth inductionJ
Clin Neurosci1811091113201121719291
|