Introduction
Hepatocellular carcinoma (HCC) is the 5th most
common type of malignancy worldwide (1,2). Each
year, there are 500,000 to 1 million new cases worldwide,
particularly in Asia and Africa (3). Although the prognosis of HCC has
improved due to new advances in treatment, the overall survival of
patients with HCC remains unsatisfactory (4,5).
Following surgery, the 5-year survival rate is limited to 25–39%
(6), and is much lower elsewhere
(7,8). The aggressive nature of HCC is
associated with mutations of various oncogenes and tumor-suppressor
genes; therefore, novel therapeutic targets for HCC are required
(9). Advances in HCC treatment are
likely to require a more detailed understanding of its biology and
behavior; thus, the mechanisms underlying the rapid metastatic
capacity of HCC require further study (10,11).
The 14-kDa phosphohistidine phosphatase (PHP14),
also known as PHPT1, was the first histidine phosphatase protein
identified in vertebrates and is similar to the janus proteins of
Drosophila (12,13). PHPT1 demonstrates specific
phosphatase activity against peptides and proteins containing
phosphohistidine (14). Studies
investigating the physiological function of PHP14 have revealed
that PHP14 is able to dephosphorylate ATP-citrate lyase (15) and the β-subunit of G proteins in
vitro (16). Among the total
number of phosphorylations in eukaryotes, only 7% are histidine
phosphorylations and they may be involved in the signal
transduction of cancer (17,18).
Klumpp et al revealed that PHP14 may play a role in neuronal
signal transduction (17). It has
also been suggested that PHP14 plays a role in human lung cancer
cell migration and invasion (19);
however, the role of PHP14 in HCC remains unknown.
In the present study, we confirmed that PHP14 is
highly expressed in HCC tissues and cell lines. Additionally, we
used the newly developed lentivirus-mediated delivery of small
interfering RNA (siRNA) technique (20) to observe the effects of PHP14
knockdown on human HCC cell growth and apoptosis in
vitro.
Materials and methods
Cell lines and culture
The HCC cell lines HepG2 and SMMC7721 were preserved
at the Department of Oncology at The First Affiliated Hospital,
Xi’an Jiaotong University, China. Human liver HL-7702 cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and l00 μg/ml
streptomycin, in a humidified incubator with 5% CO2 at
37°C. All cells were passaged twice each week and routinely
examined for mycoplasma contamination. Cells in the logarithmic
growth phase were used for further experiments.
Tissue collection and
immunohistochemistry
Primary site HCC tissues, adjacent benign liver
tissues and normal hepatic tissues were obtained from 38, 37 and 31
patients, respectively. All cases of HCC were clinically and
pathologically confirmed and all patients signed informed consent
forms prior to surgery, which was conducted at the Department of
Pathology, The First Affiliated Hospital of Xi’an Jiaotong
University, China. The protocols used in this study were approved
by the Hospital’s Protection of Human Subjects Committee.
Sections (5 μm) of formalin-fixed
paraffin-embedded specimens were collected. Slides were dewaxed,
rehydrated, incubated in 10% normal goat serum and 0.3% Triton
X-100 in PBS for 1 h, then incubated with monoclonal rabbit
anti-PHP14 polyclonal antibody (1:150; Sigma, Swampscott, MA, USA).
The slides were washed 3 times in PBS for 5 min. The tissues were
then incubated in biotin-labeled rabbit anti-mouse serum (1:200)
for 30 min, rinsed with PBS and incubated with avidin-biotin
peroxidase complex for 1 h. The signal was detected using
3,3′-diaminobenzidine as the chromogen. Negative control slides
omitting the primary antibody were included in all assays and all
sections were examined independently by 2 investigators.
Expression of PHP14 was evaluated according to the
ratio of positive cells per specimen and staining intensity, as
previously described (21). The
ratio of positive cells per specimen was evaluated quantitatively
and scored as follows: 0, staining of <1%; 1, staining of 2–25%;
2, staining of 26–50%; 3, staining of 51–75%; 4, staining of
>75%. Intensity was graded as follows: 0, no signal; 1, weak
signal; 2, moderate signal; 3, strong signal. A total score of 0–12
was finally calculated and graded as: negative (−), score of 0–1;
weak (+), score of 2–4; moderate (++), score of 5–8; strong (+++),
score of 9–12.
Recombinant lentivirus generation
The complementary DNA sequence of PHP14 was designed
from the full-length ZEB1 sequence by Shanghai Gene Chem Company
(Shanghai, China). The potential target sequences for RNA
interference (RNAi) were scanned using the siRNA Target Finder and
Design Tool available on the Ambion website. The
PHP14-siRNA-targeting sequence was CGCCATTTCAACTGAGAAA (19). After testing for knockdown
efficiencies, the stem-loop oligonucleotides were synthesized and
cloned into the lentivirus-based PsicoR vector (Addgene, Boston,
MA, USA). A non-targeting (scrambled) stem-loop DNA PsicoR vector
was generated as a negative control. HCC cells were infected with a
ZEB1-siRNA lentivirus or a negative control virus at 7 days and
examined at 10 days.
Lentivirus infection
The HCC cell lines, HepG2 and SMMC7721, were
incubated with lentivirus in a small volume of serum-free DMEM at
37°C for 4 h. Following this, 10% DMEM was added to the cells and
they were placed in an incubator for an additional indicated time
for the following experiments. Subsequently, the following
experiments were conducted using viruses at a multiplicity of
infection (MOI) of 20, unless indicated otherwise. HepG2 and
SMMC7721 cells transfected with the PHP14-siRNA or scramble-siRNA
were designated lenti-siRNA/PHP14 and src-siRNA, respectively.
Cell viability and proliferation
Cell viability was examined using routine 3-
(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT)
assay 3 days following virus infection at the indicated MOI. A cell
proliferation assay was conducted by counting the number of cells.
Cells were plated in triplicate at a density of 5.0×104
cells/ml in 24-well plates and infected with the virus at the
indicated MOIs. They were harvested daily and counted using a
hemocytometer.
Colony formation assay
A soft-agar colony formation assay was conducted to
assess the anchorage-independent growth ability of cells as a
characteristic of in vitro tumorigenicity using a Cellomics
Arrayscan (Genechem, Shanghai, China). Briefly, HepG2 and SMMC-7721
cells were infected with the virus for 24 h. The cells were then
detached and plated in 6-well plates (1.0×104
cells/well) containing 0.3% agarose and a 0.5% agarose underlay.
The number of foci (>100 μm) was counted after 17 days.
Each experiment was conducted in triplicate.
Flow cytometry analysis
Flow cytometry analysis was used to determine the
distribution of the cells in the cell cycle and the cells
undergoing apoptosis. Briefly, HepG2 and SMMC7721 cells were seeded
and infected with the virus for 96 h in complete medium and placed
in a serum-free medium for 48 h. Once the adherent cells were
collected by trypsinization, the cells were suspended in ∼0.5 ml of
70% alcohol and maintained at 4°C for 30 min. The suspension was
filtered through a 50-μm nylon mesh and the DNA content of
the stained nuclei was analyzed using a flow cytometer (EPICS XL;
Beckman Coulter, Inc., Brea, CA, USA). Cell cycle analysis was
conducted using MultiCycle DNA cell cycle analysis software.
Apoptotic cells were identified as a hypodiploid DNA peak, which
represented the cells in the sub-G1 phase. Results from
at least 20,000 cells were collected and analyzed using CellQuest
software (BD Biosciences, Franklin Lakes, NJ, USA).
Reverse-transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. A total of 1 μg of RNA was subjected to RT.
The PCR primers used for the PHP14 internal control were: sense,
5′-GCCACAGAGCCACCCCCA-3′; antisense, 5′-ATCCAGACAAATCCTTCCAGCA-3′.
The PCR primers used for glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were: forward, 5′-TGGTATCGTGGAAGGACTCA-3′; reverse,
5′-CCAGTAGAGGCAGGGATGAT-3′ (19).
PCR products were separated on a 1% agarose gel, then visualized
and photographed under ultraviolet light.
Western blot analysis
Following protein quantization using a Coomassie
Brilliant Blue assay, 50 μg of protein was boiled in loading
buffer, resolved on 10% SDS polyacrylamide gels, electrotransferred
to nitrocellulose membranes and incubated overnight with mouse
monoclonal antibodies against PHP14, Bcl-2, Bax (1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and β-actin (1:5000;
Sigma, St. Louis, MO, USA). The secondary antibody (1:1000;
peroxidase-conjugated anti-mouse IgG) was applied, and the relative
content of the target proteins was detected by
chemiluminescence.
Statistical analysis
Statistical analysis was conducted using the
Kruskal-Wallis rank test and the Mann-Whitney U test to calculate
P-values and to compare the differences between the
immunohistochemistry groups. Assays for characterizing cell
phenotypes were analyzed using the Student’s t-test. The
statistical SPSS software package (SPSS, Chicago, IL, USA) was used
to analyze the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
PHP14 expression is elevated in the
majority of HCC patient samples and cell lines
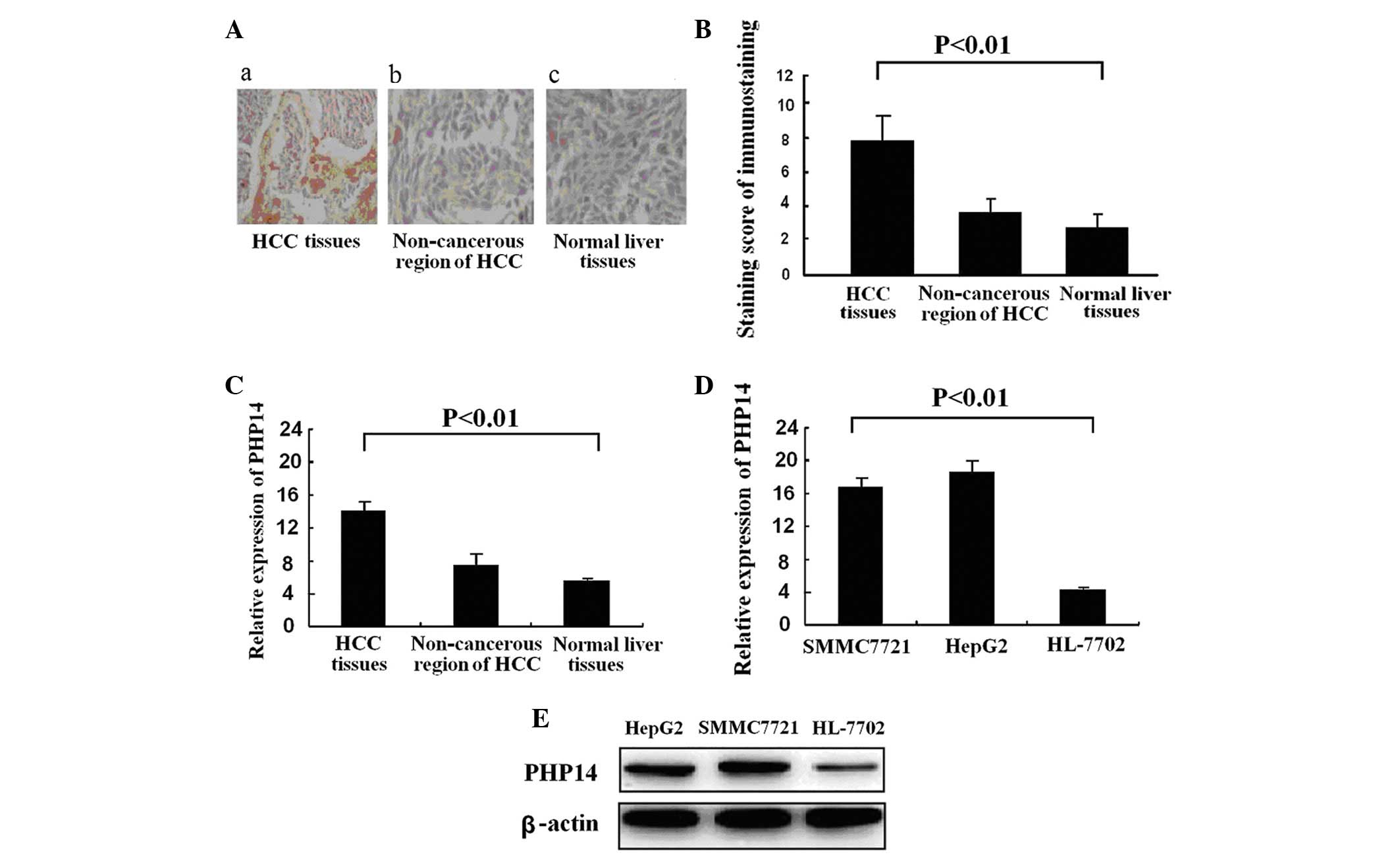
We examined the expression of PHP14 in 58 tissue
samples collected from HCC patients. Immunohistochemical images
revealed that the expression of PHP14 in HCC tissues was
significantly higher compared with that in the adjacent
non-cancerous HCC and normal liver tissues (Fig. 1A). A summary of PHP14 expression
using immunohistochemical analysis in HCC tissues, adjacent benign
liver tissues and normal liver tissues is presented in Table I. The average staining score of
PHP14 in the HCC tissues was significantly higher compared with
that of the adjacent non-cancerous HCC tissues and normal liver
tissues (Fig. 1B) (7.53±0.78 vs.
3.67±0.36 vs. 3.21±0.42, respectively; P<0.01). Expression of
PHP14 mRNA in the 3 tissue groups confirmed the same result
(Fig. 1C). PHP14 expression was
clearly elevated in HCC tumor tissues compared with adjacent
non-cancerous liver tissues. PHP14 mRNA expression was markedly
increased in the 2 cancer cell lines, HepG2 and SMMC7721, compared
with that of the human liver cell line, HL-7702 (Fig. 1D). Similarly, differential
expression of the PHP14 protein was confirmed by western blot
analysis (Fig. 1E). These results
indicate that PHP14 expression is closely correlated with HCC.
 | Table I.Immunohistochemical analysis of PHP14
in HCC tissues, adjacent benign liver tissues and normal liver
tissues. |
Table I.
Immunohistochemical analysis of PHP14
in HCC tissues, adjacent benign liver tissues and normal liver
tissues.
| Immunostaining | Liver tissue, n (%)
(n=68)
| HCC
differentiation, n (%) (n=38)
|
|---|
| Normal (n=31) | Adjacent benign
(n=37) | Well (n=12) | Moderate
(n=14) | Poor (n=12) |
|---|
| − | 16 (51.61) | 17 (45.95) | 2 (16.78) | 2 (14.29) | 1 (8.33) |
| + | 10 (32.26) | 14 (37.84) | 3 (25) | 2 (14.29) | 2 (16.78) |
| ++ | 3 (9.68) | 5 (13.51) | 3 (25) | 3 (21.43) | 3 (25) |
| +++ | 2 (6.45) | 1 (2.70) | 4 (33.33) | 7 (50) | 6 (50) |
Lentivirus-delivered siRNA knockdown of
PHP14 expression and inhibition of HCC cell proliferation
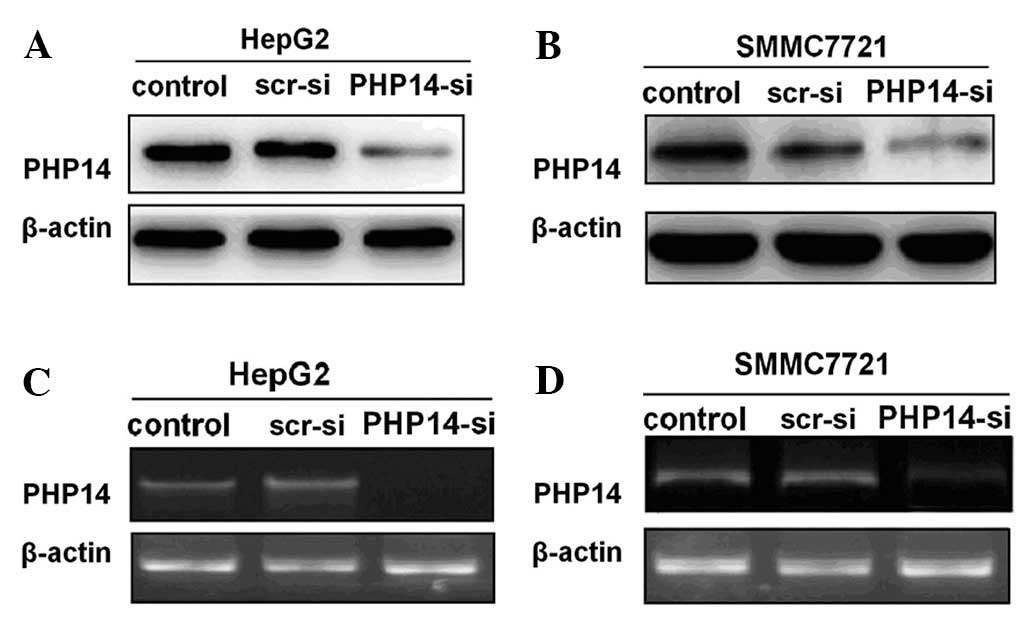
To further explore the correlation between PHP14 and
HCC, we constructed lentivirus-delivered vectors, a PHP14-specific
siRNA vector (lenti-siRNA/PHP14) and a scramble siRNA vector
(src-siRNA). The vectors were then transfected into HepG2 and
SMMC7721 cells. A suppression rate of up to 80% was observed at 96
h in the cells infected with lenti-siRNA/PHP14. The inhibitory
effect of the lenti-siRNA/PHP14 was revealed to be specific as the
control and src-siRNA group had little effect on PHP14 expression
levels (Fig. 2A and B).
To examine the reduction level of target mRNA
induced by the lentivirus-delivered siRNAs, RT-PCR was conducted
using the prepared mRNA from the infected HepG2 and SMMC-7721
cells. As shown in Fig. 2C and D,
lenti-siRNA/PHP14-transfection caused a marked reduction in the
level of PHP14 mRNA, while the src-siRNA-transfected and control
cells did not. Altogether, these data indicated that the
PHP14-siRNA suppressed the PHP14 overexpression in HCC cells
effectively.
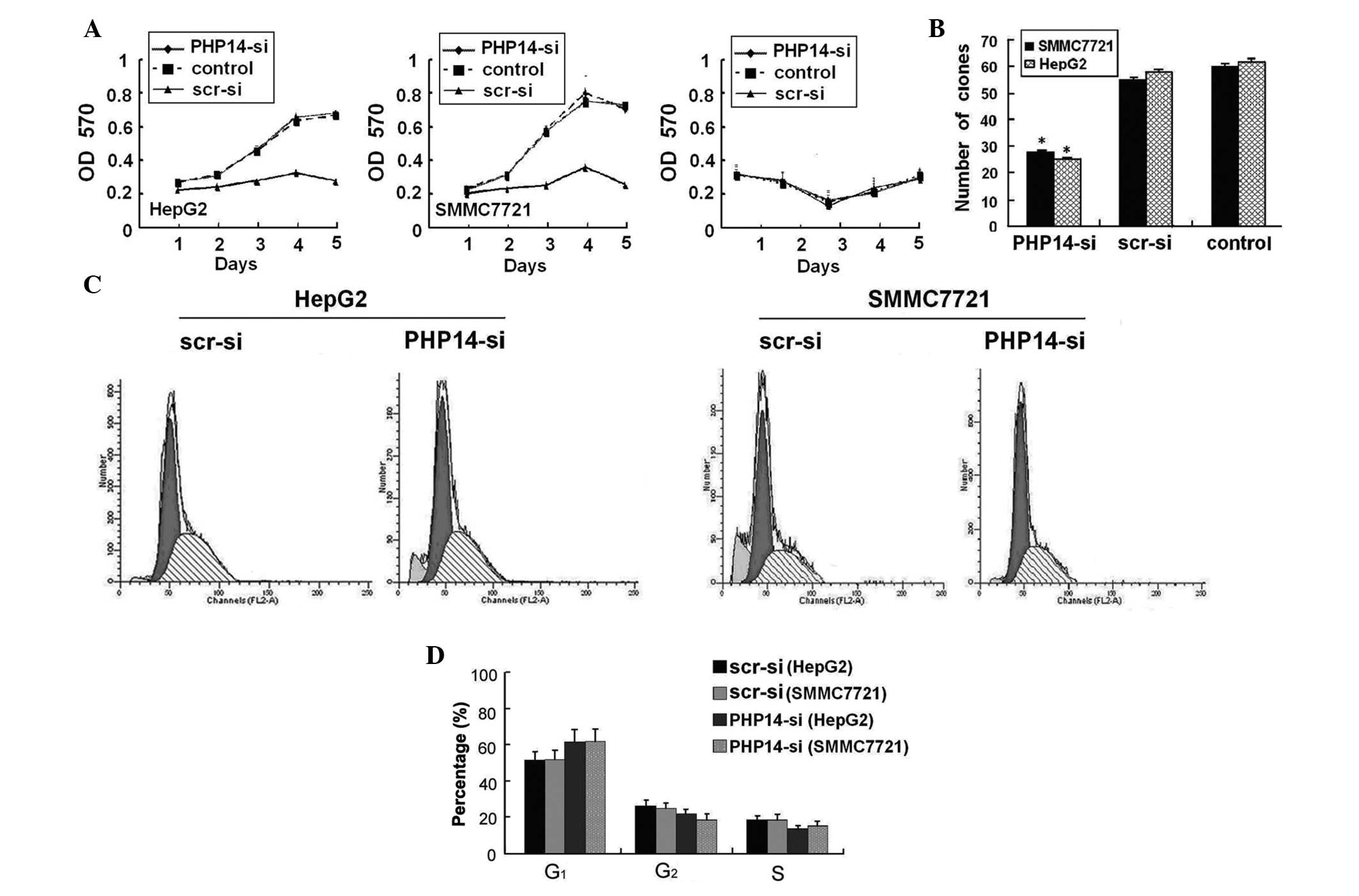
These results confirmed that lenti-siRNA/PHP14
efficiently repressed PHP14 expression. We then tested the effect
of lenti-siRNA/PHP14 on the cell viability of HepG2 and SMMC7721
cells in culture. As shown in Fig.
3A, lenti-siRNA/PHP14 significantly inhibited cell growth of
HepG2 and SMMC7721 cells compared with scr-siRNA-transfected and
control cells. The inhibitory effect on HepG2 and SMMC7721 cell
growth was also was confirmed by colony formation assays, which
demonstrated that the colony-forming ability of HCC cells was
significantly inhibited by transfection with lenti-siRNA/PHP14
(Fig. 3B). These results suggest
that knockdown of PHP14 by lenti-siRNA/PHP14 inhibited the
expression of PHP14 in HepG2 and SMMC7721 cells compared with the
scr-siRNA-transfected and control group.
Lenti-siRNA/PHP14 inhibits cell cycle S
phase entry of HCC cells
We examined the effect of lenti-siRNA/PHP14 on cell
viability of HepG2 and SMMC7721 cells in cell culture. The human
liver cell strain HL-7702, infected with the virus at a MOI of 20,
was taken as a negative control. As shown in Fig. 3C, following virus infection,
lenti-siRNA/PHP14 significantly inhibited cell growth of both HCC
cell lines compared with the control group. The difference was more
pronounced after day 3, a time in which essentially all the cells
established cell-cell contacts. The inhibitory effects on HCC cell
growth were also confirmed by colony formation assays, which
demonstrated that the colony-forming ability of HCC cells was
significantly inhibited by transfection with lenti-siRNA/PHP14.
Flow cytometry was conducted 96 h after infection.
The percentage of HepG2 and SMMC77221 cells in the
G0/G1 phase (63.9±2.6 and 64.57±1.9,
respectively) of the lenti-siRNA/PHP14-transfected group was
significantly higher compared with that in the
src-siRNA-transfected group (53.56±2.3 and 54.26±2.5, respectively;
P<0.01). However, the percentage of HepG2 and SMMC7721 cells in
the S phase (13.74±2.5 and 15.89±2.3, respectively) of the
lenti-siRNA/PHP14-transfected group was significantly lower
compared with that in the src-siRNA-transfected group (19.22±1.6
and 19.52±1.4, respectively) (P<0.01; Fig. 3D).
Collectively these data indicate that
lenti-siRNA/PHP14 exhibited a specific inhibitory effect on HepG2
and SMMC7721 cell growth. They also suggest that lenti-siRNA/PHP14
induces G0/G1 phase arrest and inhibits S
phase entry.
Lenti-siRNA/PHP14 induces cell apoptosis
in HCC cells
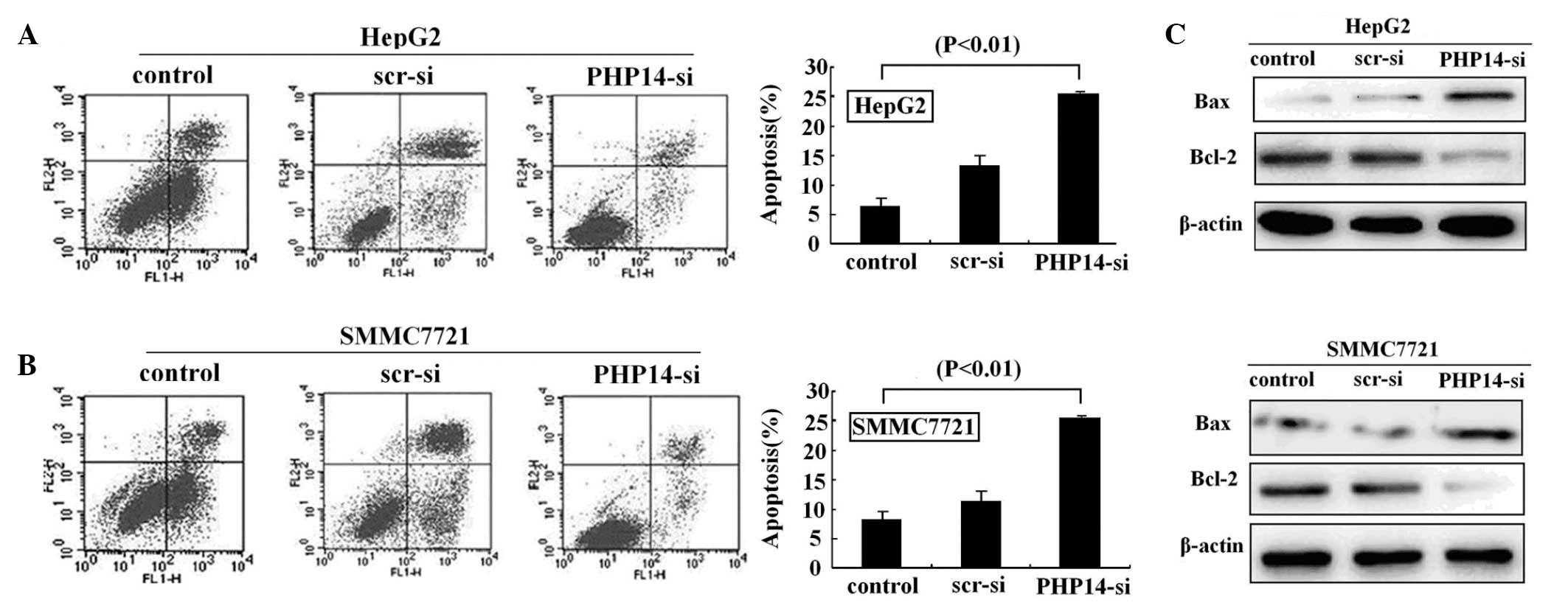
The effect of lenti-siRNA/PHP14 on the apoptosis of
HCC cells was investigated using flow cytometry (Fig. 4A). It was identified that 8.91% of
the HepG2 cells transfected with src-siRNA were apoptotic, while
17.82% of the HepG2 cells transfected with lenti-siRNA/PHP14 were
apoptotic. We also identified that 8.23% of the SMMC7721 cells
transfected with src-siRNA were apoptotic, while 15.98% of the
SMMC7221 cells transfected with lenti-siRNA/PHP14 were apoptotic.
These data suggest that knockdown of PHP14 by lenti-siRNA/PHP14
specifically induces apoptosis of the PHP14-overexpressing HCC cell
lines, HepG2 and SMMC7721 (Fig.
4B).
In order to explore the underlying molecular
mechanism of PHP14 and cell apoptosis, we analyzed the Bcl-2 family
mediators of apoptosis, including Bcl-2 and Bax, using western blot
analysis. The results indicated that knockdown of PHP14
downregulated Bcl-2 and upregulated Bax expression in
lenti-siRNA/PHP14-transfected cells, suggesting that the apoptotic
effect of PHP14 may be partly mediated by these Bcl-2 family
proteins (Fig. 4C).
Discussion
HCC is an aggressive malignant tumor with high
incidence and poor prognosis, particularly in Asia and Africa
(3). Previous studies have
demonstrated that the incidence of HCC in the USA and the UK has
increased in past 2 decades (22,23).
Although varies therapies for HCC are available, the recovery rate
remains unsatisfactory. Thus, an effective treatment method for HCC
is urgently required.
PHP14 was the first histidine phosphatase protein
identified in vertebrates, and is similar to the janus proteins of
Drosophila (12,13). PHP14 and its role in human lung
cancer cell migration and invasion has been identified (19). PHP14 may play a role in neuronal
signaling transduction; however, the role of PHP14 in other cancers
remains unknown.
In this study, we demonstrated that PHP14 expression
is elevated in the majority of HCC patient samples and HCC cell
lines. Consequently, we hypothesized that the knockdown of PHP14
may inhibit tumorigenic growth of HCC in which PHP14 is
overexpressed.
Using immunohistochemistry, we studied the PHP14
expression of 38 HCC, 37 adjacent non-cancerous and 31 normal
hepatic tissues. Using western blot analysis and RT-PCR, we
identified that PHP14 protein and mRNA are highly expressed in HCC
tissues compared with adjacent non-cancerous tissue. The average
staining score in HCC tissues was significantly higher compared
with that in adjacent non-cancerous and normal hepatic tissue. This
suggested that PHP14 plays a role in HCC carcinogenesis and may
have a facilitative effect on the proliferation of HCC cells. Using
western blot analysis, we demonstrated that PHP14 expression was
upregulated in HCC cell lines compared with the normal liver cell
line. Our study also confirmed that PHP14 was upregulated in
HCC.
To determine whether the ectopic expression of PHP14
is able to modulate the proliferation of HCC cells, lentivirus
vector siRNAs targeting PHP14 were transfected into HepG2 and
SMMC7721 cells. Lentivirus, a type of reversal virus, is able to
integrate into the host genome and demonstrate long-term expression
of integrated genes (24).
Lentivirus has a higher titer than a retroviral virus and does not
only infect dividing phase cells, but also infects non-dividing
cells. Using lentivirus vectors, one pair of siRNAs targeting PHP14
were transfected into HepG2 and SMMC7721 cells. As a result, we
revealed that PHP14-si significantly inhibited HCC cell
proliferation and growth in vitro. The stimulatory effects
of PHP14 on HepG2 and SMMC7721 cells demonstrated that the PHP14
gene may be a growth promoter gene that acts directly or indirectly
to control the proliferation of cells. The products of such genes
regulate cell growth and differentiation in a positive way and thus
promote neoplastic development, which further indicates that PHP14
may act as a tumor promoter in HCC. These result are different from
those of Xu et al who suggested that PHP14 expression had no
effect on lung cancer cell growth and proliferation (19).
We also used flow cytometry to examine how PHP14-si
affects the cell cycle. Once cells were treated with PHP14-si for
96 h, we observed a significant increase of G1 phase population in
all HCC cells. This result indicated that the knockdown of PHP14
expression inhibited HCC cell proliferation, which is necessary for
entering into the S phase.
In this study, we examined the expression level of
two Bcl-2 family members, Bcl-2 and Bax, in this PHP14
depletion-triggered apoptosis in HCC cells. We identified that the
knockdown of PHP14 downregulated Bcl-2 and upregulated Bax in
PHP14-siRNA-transduced tumor cells, suggesting that Bcl-2 family
members may partially contribute to the apoptosis of HCC cells with
PHP14 depletion.
In conclusion, PHP14 may be an effective gene target
for HCC treatment. The results of this study suggested that the
significant downregulation of PHP14 expression by lenti-siRNA/PHP14
in HCC cells results in the inhibition of S phase entry, inhibition
of cell proliferation and increased cell apoptosis. Therefore,
knockdown of PHP14 using lenti-virus-delivered siRNA may be a
valuable approach for the treatment of HCC. The correlation between
PHP14 and HCC cell invasion and metastasis requires further
study.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
PHP14
|
14-kDa phosphohistidine
phosphatase
|
|
mRNA
|
messenger RNA
|
|
MOI
|
multiplicity of infection
|
|
PCR
|
polymerase chain reaction
|
|
PBS
|
phosphate-buffered saline
|
|
RNAi
|
RNA interference
|
|
siRNA
|
small interfering RNA
|
References
|
1.
|
A JemalR SiegelE WardY HaoJ XuT MurrayMJ
ThunCancer statistics, 2008CA Cancer J
Clin587196200810.3322/CA.2007.0010
|
|
2.
|
HB El-SeragJA DavilaNJ PetersenKA
McGlynnThe continuing increase in the incidence of hepatocellular
carcinoma in the United States: an updateAnn Intern
Med139817823200310.7326/0003-4819-139-10-200311180-0000914623619
|
|
3.
|
DM ParkinF BrayJ FerlayP PisaniGlobal
cancer statistics, 2002CA Cancer J
Clin5574108200510.3322/canjclin.55.2.74
|
|
4.
|
A JemalA ThomasT MurrayM ThunCancer
statistics, 2002CA Cancer J
Clin522347200210.3322/canjclin.52.1.23
|
|
5.
|
JM LlovetA BurroughsJ BruixHepatocellular
carcinomaLancet36219071917200310.1016/S0140-6736(03)14964-1
|
|
6.
|
M ColomboHepatocellular carcinomaJ
Hepatol15225236199210.1016/0168-8278(92)90041-M
|
|
7.
|
EC LaiST FanCM LoKM ChuCL LiuJ WongHepatic
resection for hepatocellular carcinoma. An audit of 343 patientsAnn
Surg221291298199510.1097/00000658-199503000-000127717783
|
|
8.
|
K TakenakaN KawaharaK YamamotoResults of
280 liver resections for hepatocellular carcinomaArch
Surg1317176199610.1001/archsurg.1996.014301300730148546582
|
|
9.
|
J BruixJM LlovetPrognostic prediction and
treatment strategy in hepatocellular
carcinomaHepatology35519524200210.1053/jhep.2002.3208911870363
|
|
10.
|
C QianM DrozdzikWH CaselmannJ PrietoThe
potential of gene therapy in the treatment of hepatocellular
carcinomaJ
Hepatol32344351200010.1016/S0168-8278(00)80082-310707877
|
|
11.
|
J RuizC QianM DrozdzikJ PrietoGene therapy
of viral hepatitis and hepatocellular carcinomaJ Viral
Hepat61734199910.1046/j.1365-2893.1999.6120136.x10847127
|
|
12.
|
P EkG PetterssonB EkF GongJP LiO
ZetterqvistIdentification and characterization of a mammalian
14-kDa phosphohistidine phosphataseEur J
Biochem26950165023200210.1046/j.1432-1033.2002.03206.x12383260
|
|
13.
|
S KlumppJ HermesmeierD SelkeR BaumeisterR
KellnerJ KrieglsteinProtein histidine phosphatase: a novel enzyme
with potency for neuronal signalingJ Cereb Blood Flow
Metab2214201424200210.1097/00004647-200212000-0000212468887
|
|
14.
|
S KlumppJ KrieglsteinReversible
phosphorylation of histidine residues in vertebrate proteinsBiochim
Biophys Acta1754291295200510.1016/j.bbapap.2005.07.03516194631
|
|
15.
|
S KlumppG BechmannA MaurerD SelkeJ
KrieglsteinATP-citrate lyase as a substrate of protein histidine
phosphatase in vertebratesBiochem Biophys Res
Commun306110115200310.1016/S0006-291X(03)00920-312788074
|
|
16.
|
A MaurerT WielandF MeisslThe beta-subunit
of G proteins is a substrate of protein histidine
phosphataseBiochem Biophys Res
Commun33411151120200510.1016/j.bbrc.2005.06.20016039992
|
|
17.
|
S KlumppJ KrieglsteinPhosphorylation and
dephosphorylation of histidine residues in proteinsEur J
Biochem26910671071200210.1046/j.1432-1033.2002.02755.x11856347
|
|
18.
|
PS SteegD PalmieriT OuatasM
SalernoHistidine kinases and histidine phosphorylated proteins in
mammalian cell biology, signal transduction and cancerCancer
Lett190112200310.1016/S0304-3835(02)00499-812536071
|
|
19.
|
A XuJ HaoZ Zhang14-kDa phosphohistidine
phosphatase and its role in human lung cancer cell migration and
invasionLung
Cancer674856201010.1016/j.lungcan.2009.03.00519344975
|
|
20.
|
C ShenAK BuckX LiuM WinklerSN ReskeGene
silencing by adenovirus-delivered siRNAFEBS
Lett539111114200310.1016/S0014-5793(03)00209-612650936
|
|
21.
|
K MaaserP DaublerB BarthelOesophageal
squamous cell neoplasia in head and neck cancer patients:
upregulation of COX-2 during carcinogenesisBr J
Cancer8812171222200310.1038/sj.bjc.660086512698187
|
|
22.
|
HB El-SeragAC MasonRising incidence of
hepatocellular carcinoma in the United StatesN Engl J
Med340745750199910.1056/NEJM19990311340100110072408
|
|
23.
|
SD Taylor-RobinsonGR FosterS AroraS
HargreavesHC ThomasIncrease in primary liver cancer in the UK,
1979–94Lancet350114211431997
|
|
24.
|
A PfeiferT LimK ZimmermannLentivirus
transgensisMethods
Enzymol477315201010.1016/S0076-6879(10)77001-420699133
|