Introduction
Glioblastoma multiforme (GBM) is the most common
malignant glioma in adults (1,2).
Despite recent advancements in treatment modalities that currently
consist of maximally safe surgical resection followed by radiation
therapy plus concomitant and adjuvant temozolomide, GBM remains an
incurable and devastating disease with the median survival time
rarely exceeding 14 months following diagnosis and with less than
5% of GBM patients surviving more than 3 years following diagnosis
(3–5). Gliosarcoma is a rare variant of
malignant glioma that is very similar to GBM in terms of genetic
changes, clinical presentation and poor prognosis (6,7).
Precise and timely determination of individual
prognosis of malignant glioma patients is critical. To date, a
number of clinical, therapeutic and genetic prognostic markers of
malignant glioma patients have been identified. For example, in GBM
patients, O6-methylguanine methyltransferase (MGMT)
promoter methylation, good initial functional status, more radical
resection and treatment with radiation therapy and chemotherapy
have been demonstrated to be associated with better prognosis;
while more advanced age and worse initial neurological status have
been revealed to be associated with poor prognosis (8,9). In
addition, a new generation of genetic and biological prognostic
markers is emerging, although these are not routinely used in
clinical practice (3,8,10).
Therefore, there remains a requirement for new, reliable and widely
available prognostic markers of malignant glioma.
Functional imaging methods, including positron
emission tomography (PET) and single photon emission computed
tomography (SPECT) play an important role in the initial diagnosis
of gliomas and in the follow-up of malignant glioma patients
following surgical treatment or radiotherapy when surrounding edema
and gliosis make it difficult to identify remaining glioma tissue
using only magnetic resonance imaging (MRI). In contrast to PET,
which remains a highly expensive diagnostic modality, SPECT is
widely available and routinely used, even in less developed
countries. In previous studies, higher tracer uptake by glioma on
SPECT was shown to be associated with higher grade and decreased
response to chemotherapy of gliomas (11–14).
In addition, higher technetium-99m-methoxyisobutylisonitrile
(99mTc-MIBI) uptake was demonstrated to correlate with
higher proliferative potential of gliomas and with worse survival
(15–19). However, to the best of our
knowledge, previous studies employed SPECT scans only once during
the treatment period and currently there are no studies evaluating
the association of repeated SPECT scans during perioperative
periods with the prognosis of malignant glioma patients. We have
recently demonstrated that changes in the bulk of malignant gliomas
following surgery and radiation therapy can be reliably evaluated
using SPECT (20). Changes in the
bulk of malignant glioma tissue following surgery evaluated by
SPECT may possibly serve as a novel prognostic marker of malignant
glioma.
Therefore, in this study we aimed to evaluate the
association of overall survival in malignant glioma patients with
uptake of 99mTc-MIBI before and after surgery along with
changes in 99mTc-MIBI uptake after surgery.
Patients and methods
Patients and protocol
Seventeen patients (11 males and 6 females; aged
62.2±8.4 years; range, 39 to 75 years) who underwent resection or
biopsy for histologically confirmed grade IV glioma according to
WHO classification the at the Department of Neurosurgery, Clinic of
Lithuanian University of Health Sciences, Kaunas, Lithuania, were
prospectively included in this study. GBM was diagnosed in 16 (94%)
patients and gliosarcoma was diagnosed in 1 (6%) patient.
All patients underwent preoperative
99mTc-MIBI SPECT scans 2.8±1.9 days prior to surgery or
biopsy (range, 1 to 7 days). Clinical severity of the disease was
evaluated by assessing the number of neurological symptoms prior to
surgery or biopsy. Resection was described by a neurosurgeon as
gross total (more than 90% of microscopically viable glioma
removed) in 11 (65%) patients or as subtotal (less than 90% of
microscopically viable glioma removed) in 5 (29%) patients. Biopsy
was performed in one (9%) patient. A postoperative
99mTc-MIBI SPECT scan was performed 9.8±1.5 days after
surgery or biopsy (range, 7 to 12 days). Following surgery, all
patients received standard external beam radiation treatment that
consisted of a total dose of 60 Gy that was administered in 30
fractions of 2 Gy. None of the patients received chemotherapy with
temozolomide since at the time of the study temozolomide was not
available in Lithuania. Survival data were obtained from the
Residents’ Register Service of Lithuania. Overall survival (OS)
following surgery was defined as the period between the date of
surgery and the date of mortality.
The study and its consent procedures were approved
by the Ethics Committee of the Lithuanian University of Health
Sciences, Kaunas, Lithuania, and are in agreement with Helsinki
Declaration standards as well as the International Conference on
Harmonization - Good Clinical Practice. All patients provided
signed written informed consent.
99mTc-MIBI SPECT data
acquisition
Radiopharmaceutical 99mTc-MIBI was used
in all cases. 99mTc-MIBI is routinely used at our clinic
for the evaluation of glioma patients since it is intensively
uptaken by highly mitotic tumors, including high-grade, but not
low-grade gliomas, normal brain tissue, necrotic tissue and
fibrotic tissue (21,22). During image acquisition, patients
were placed in a supine position with an appropriate headpiece to
avoid head movement and detectors placed as close as possible to
the patients’ heads. Images were captured 30 to 45 min after
intravenous (i.v.) injection of 500 MBq of 99mTc-MIBI
using a Siemens, E. Cam dual-headed gamma camera (Siemens, Malvern,
PA, USA). The matrix was set at 64×64 pixels due to the relatively
small doses of 99mTc-MIBI used in the study as patients
were exposed to repeated SPECT scans. The tomographic imaging
parameters consisted of a 360° rotation angle and an acquisition
time of 30 sec per frame with a zoom factor of 1.78. For image
reconstruction, Filtered Back Projection was used and the
Butterworth filter was applied with a cut-off of 0.6 and an order
of 7.0. Chang’s attenuation correction was applied with an
attenuation coefficient of 0.12/cm. Raw image data axial plane
reconstructions of SPECT were analyzed.
All SPECT scan results were evaluated by means of
the semi-quantitative total intensity index (TII). We have recently
demonstrated that TII was markedly correlated with the grade of
gliomas and was a reliable index in discriminating high-grade
versus low-grade gliomas (20). In
grade IV glioma patients, TII was used for the semi-quantitative
evaluation of changes in viable glioma tissue following surgery and
radiation treatment (20). In order
to calculate TII, SPECT and diagnostic CT images were matched
according to anatomical features observed on SPECT images including
the scalp, upper parts of orbits, upper parts of frontal sinuses
and the choroid plexus. Four axial slices of each CT and SPECT scan
were included in the final protocol: a) at the level of the most
cranial part of the choroid plexus; b) at the level of the third
ventricle; c) at the level of the body of the lateral ventricles;
d) at the level of the most caudal part of the choroid plexus.
First, two axial slices (a and b) of the CT and SPECT images were
divided into four segments each and the last two axial slices (c
and d) of CT and SPECT images were divided into six segments each.
Thus, each CT and SPECT scan was divided into a total of 20
segments.
The TII was calculated by evaluating the intensity
of pathological 99mTc-MIBI uptake by malignant glioma
tissue, separately in all 20 segments of SPECT scans, using a
four-point scale according to the intensity of the tracer uptake,
ranging from three (the highest intensity of visible tracer uptake)
to zero (no visible tracer uptake). Specifically, 3, 2 and 1 points
were assigned to the segment when the intensity of pathological
99mTc-MIBI uptake in that segment was higher, equal or
lower (but more than background) than the intensity of
99mTc-MIBI uptake in the choroid plexus, respectively.
Zero points were assigned to the segment when 99mTc-MIBI
uptake was not evident. TII was calculated by adding the scores of
all 20 segments with a possible range from 0 to 60 points.
Preoperative TII, postoperative TII and percentage
change in postoperative TII when compared with preoperative TII (Δ
TII) were calculated for all patients.
Statistical analysis
PASW Statistics for Windows 18.0 (IBM Corporation,
Chicago, IL, USA) was used for data analyses. All continuous data
are presented as the mean ± standard deviation, and all categorical
data as a number and percentage. P<0.05 was considered to
indicate a statistically significant result.
First, univariate differences in OS were tested for
statistical significance using a log-rank test with respect to
gender (male versus female), age (≥60 years versus <60 years),
extent of surgery (gross total versus subtotal or biopsy), number
of preoperative symptoms (≥4 symptoms versus <4 symptoms),
preoperative TII (≥12 versus <12), postoperative TII (≥6 versus
<6) and Δ TII (≥50% versus <50%). Survival curves were
calculated using the Kaplan-Meier method in patients with
preoperative TII of <12 versus preoperative TII of ≥12 and in
patients with Δ TII of <50% versus Δ TII of ≥50%.
Next, a multivariate Cox regression analysis was
used to analyze possible independent prognostic factors of OS. The
forward-stepwise model selection procedure was used (P-value of
likelihood-ratio test <0.05 as inclusion criterion and >0.10
as exclusion criterion) to define the final model. The following
variables were entered to the model: gender, age at the time of
surgery, number of symptoms prior to surgery, extent of resection,
preoperative TII, postoperative TII and Δ TII.
Finally, patients who survived 12 months or more
following surgery were compared to patients who survived less than
12 months following surgery in terms of age at the time of surgery,
gender, number of symptoms prior to surgery, preoperative TII,
postoperative TII and Δ TII using an independent samples t-test for
continuous data and two-sided Fisher’s exact test for categorical
data.
Results
The mean preoperative TII was 8.8±2.6, ranging from
4 to 12. Following surgery, TII decreased in 16 patients and
increased in 1 patient. The mean postoperative TII was 4.0±3.1,
ranging from 0 to 13, and the mean Δ TII was 55.9±27.9%, ranging
from a TII increase of 8.3% to a TII decrease of 100%. The mean OS
time was 18.9±23.7 months and the median OS time was 12.4 months,
ranging from 1.4 to 88 months. There were nine (45%) 1-year
survivors and three (15%) 2-year survivors.
In univariate analyses using the log-rank test, we
identifed that a greater number of preoperative neurological
symptoms, higher preoperative TII, higher postoperative TII and
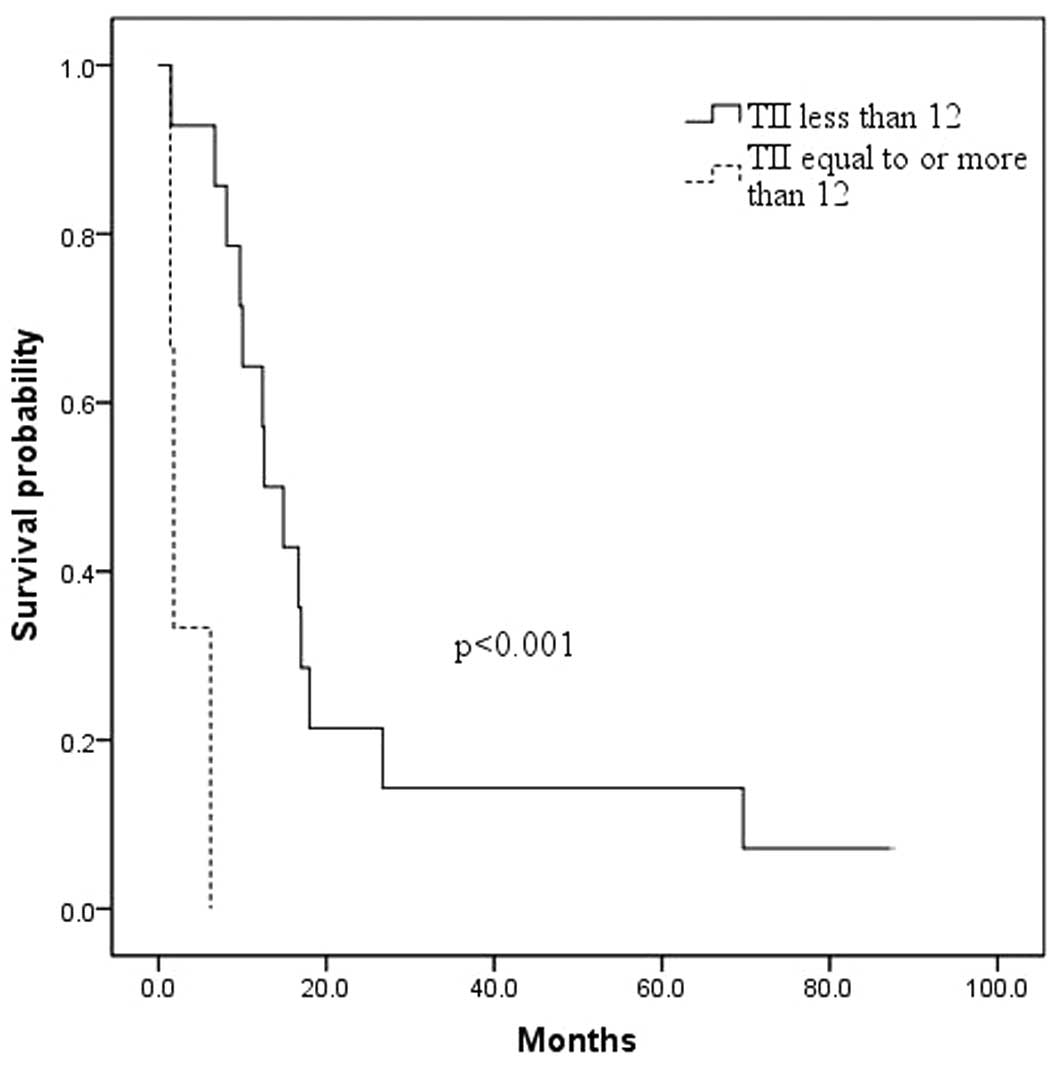
lower Δ TII were associated with OS (Table I). Specifically, worse OS survival
was associated with a preoperative TII of ≥12 when compared to a
preoperative TII of <12 (3.1±1.5 months versus 22.3±6.4 months,
respectively; p<0.001; Fig. 1),
with a postoperative TII of ≥6 when compared to a postoperative TII
of <6 (3.7±2.2 months versus 22.2±6.4 months, respectively;
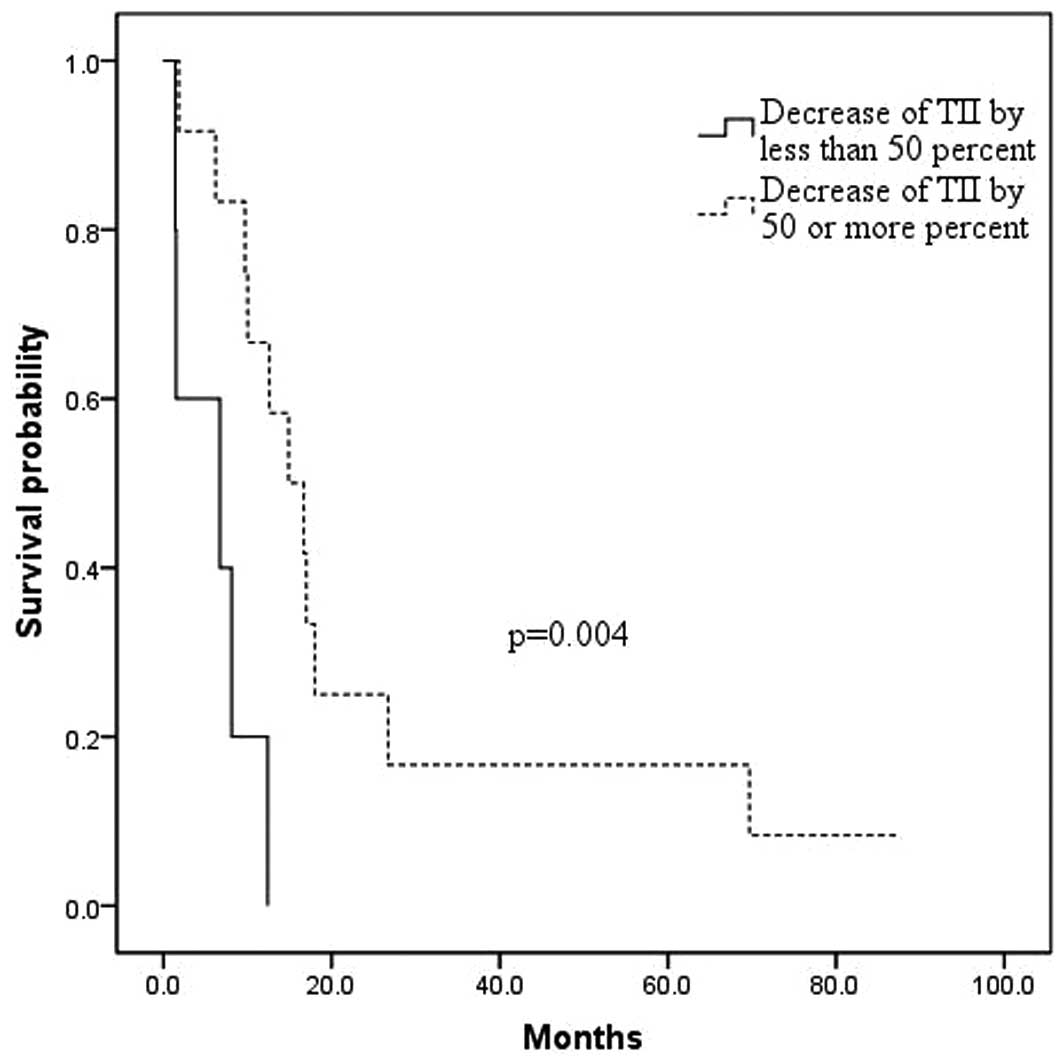
p=0.001) and with a Δ TII of <50% when compared to a Δ TII of
≥50% (6.0±2.1 months versus 24.3±7.3 months, respectively; p=
0.004; Fig. 2). Also, patients with
≥4 neurological symptoms prior to surgery had a worse OS when
compared to patients with <4 preoperative neurological symptoms
(7.9±2.2 months versus 23.5±7.5 months, respectively, p=0.004).
 | Table I.Univariate analysis of the effect of
prognostic factors on survival in 17 malignant glioma patients. |
Table I.
Univariate analysis of the effect of
prognostic factors on survival in 17 malignant glioma patients.
| Variable | Number of
cases | Mean survival
(months) | Univariate analysis
P-value (log-rank) |
|---|
| Gender |
| Male | 11 | 10.9±2.5 | 0.194 |
| Female | 6 | 33.5±13.3 | |
| Age (years) |
| ≥60 | 10 | 14.2±6.4 | 0.151 |
| <60 | 7 | 25.7±9.8 | |
| Resection |
| Gross total | 11 | 19.4±5.3 | 0.372 |
| Subtotal or
biopsy | 6 | 17.9±12.8 | |
| Number of
symptoms |
| ≥4 | 5 | 7.9±2.2 | 0.044 |
| <4 | 12 | 23.5±7.5 | |
| Preoperative
TII |
| ≥12 | 3 | 3.1±1.5 |
<0.001 |
| <12 | 14 | 22.3±6.4 | |
| Postoperative
TII |
| ≥6 | 3 | 3.7±2.2 | 0.001 |
| <6 | 14 | 22.2±6.4 | |
| Decrease of TII
≥50% after surgery |
| Yes | 12 | 24.3±7.3 | 0.004 |
| No | 5 | 6.0±2.1 | |
In multivariate analyses, postoperative TII
(p=0.008; 95% confidence interval, 1.12–2.13%), number of
preoperative neurological symptoms (p=0.02; 95% confidence
interval, 1.19–5.90%) and gender (p=0.03; 95% confidence interval,
1.21–15.98%) were found to be factors with independent prognostic
value.
Patients who survived more than 12 months following
surgery had significantly higher rates of gross total resection
(n=8/9 (89%) versus n=3/8 (38%), respectively; p=0.05), lower
postoperative TII (2.3±1.7 versus 5.9±3.4, respectively; p=0.01)
and greater Δ TII (70.0±21.7 versus 40.1±26.4, respectively;
p=0.02) when compared to patients who survived less than 12 months
following surgery (Table III).
Discussion
The main finding of the present study was that a
higher tracer uptake on SPECT scans was associated with a worse
survival in malignant glioma patients. Specifically, we identified
that higher postoperative 99mTc-MIBI uptake was an
independent predictor of worse survival in malignant glioma
patients. In univariate analyses, worse OS was associated with
higher preoperative and postoperative 99mTc-MIBI uptake
as well as with a smaller decrease in 99mTc-MIBI uptake
following surgery. Finally, patients who survived one year or more
following surgery had a significantly lower postoperative
99mTc-MIBI uptake and a higher decrease in
99mTc-MIBI uptake following surgery.
To the best of our knowledge, this is the first
study evaluating the association of preoperative and postoperative
99mTc-MIBI uptake as well as changes in 99mTc-MIBI
uptake following surgery with survival in the same cohort of
malignant glioma patients. It is also the first study evaluating
the association between 99mTc-MIBI uptake assessed using
the semi-quantitative TII method and survival of malignant glioma
patients. The main findings were that higher 99mTc-MIBI
uptake following surgery was independently associated with worse OS
of malignant glioma patients after adjusting for age, gender,
extent of surgery and clinical disease severity prior to surgery
(number of neurological symptoms). In addition, in univariate
analyses, higher 99mTc-MIBI uptake before surgery
(TII≥12), after surgery (TII<6) and a smaller decrease in
99mTc-MIBI uptake after surgery were all found to be
associated with worse survival. Finally, patients who survived more
than a year following surgery had significantly lower postoperative
TII and a more pronounced decrease in 99mTc-MIBI uptake
following surgery. Together, our findings suggest that tracer
uptake by malignant glioma on SPECT scans evaluated using a
semi-quantitative TII method may be valuable predictors of survival
in malignant glioma patients. However, our findings remain to be
replicated in larger samples of malignant glioma patients.
Currently, there are a few studies evaluating the
association between uptake of different tracers on SPECT scans and
survival of glioma patients. In a recent study, Alexiou et
al (2010) evaluated the prognostic value of preoperative
99mTc-tetrofosmin uptake in predicting the OS of 18 GBM
patients and found that higher 99mTc-tetrofosmin uptake
was associated with significantly worse survival (16). Another study reported that higher
preoperative thalium-201 uptake was associated with significantly
worse survival in high grade glioma patients (23). However, in a subgroup of patients
diagnosed with WHO grade IV gliomas there were no statistically
significant differences in survival between the low lesion to
normal tissue ratio (L/N) ratio group and the high L/N ratio group.
Beauchesne et al (2004) reported that larger metabolic tumor
volume assessed using the 99mTc-MIBI at the end of
radiation therapy was associated with worse prognosis of malignant
glioma patients (17). Another
study by the same group demonstrated that a larger tumor volume and
a higher L/N ratio were associated with worse prognosis of
malignant glioma patients following treatment failure (18). To the best of our knowledge, we were
the first to report on the association of a change in tracer uptake
following surgery with survival of malignant glioma patients. Also,
we were the first to report that higher preoperative
99mTc-MIBI uptake was correlated with worse survival.
Higher uptake of fluorine-18 (18F)-2-deoxyglucose (FDG) on PET
scans was reported to be a reliable predictor of worse survival in
glioma patients (24,25). However, the use of PET remains
limited due to its high cost, in contrast to SPECT, which is a
cheaper and more widely available diagnostic modality even in less
developed countries.
Malignant gliomas have increased metabolic needs,
increased cellular mitochondrial content and maintain the negative
mitochondrial transmembrane potential that is associated with the
active diffusion of 99mTc-MIBI into the mitochondria
(7,10). Higher 99mTc-MIBI uptake
was demonstrated to correlate with biological markers of malignancy
of malignant gliomas, including aneuploidy level and percentage of
cells in the S-phase fraction, and with proliferative activities of
glioma as assessed by the means of Ki-67 (26,27).
In the majority of previous studies, tracer uptake was evaluated
using the L/N ratio; the most widely used index for
semi-quantitative evaluation of brain SPECT results. However, the
L/N ratio corresponds to tracer uptake at the point of the brain
tumor where tracer uptake is the highest, but does not include
quantitative evaluation of the total tracer uptake area. TII is a
semi-quantitative index that corresponds to tracer uptake in 20
segments of brain SPECT and is an index of malignancy and size of
glioma. TII is a highly sensitive and specific method for
identifying high-grade gliomas and may be used for the follow-up of
grade IV glioma patients following surgery and radiation treatment
when surrounding edema prevents accurate interpretation of CT and
MRI scans (20). We have recently
revealed that higher 99mTc-MIBI uptake prior to surgery
evaluated using TII was positively correlated with histological WHO
glioma grade (r=0.64) (20).
Therefore, TII may be used to supplement the L/N ratio for initial
diagnosis and at follow-up.
We found that more radical resection of high-grade
gliomas as evaluated by the means of SPECT was associated with
improved OS. Specifically, a decrease in TII of more than 50%
following surgery and a lower postoperative TII were associated
with longer survival. Also, patients who survived more than a year
following surgery had higher rates of total resection, lower
postoperative tracer uptake and a higher decrease in TII following
surgery when compared to patients who survived less than a year
following surgery. Currently used multimodal treatment algorithms
for malignant gliomas include maximally safe microsurgical
resection followed by chemotherapy and radiotherapy. However,
despite recent advances in chemotherapy and radiotherapy, maximally
safe microsurgical resection remains the pivotal treatment modality
of malignant glioma patients and more radical resection is
associated with increased survival (28,29).
We have also identifed that more preoperative
neurologic symptoms were associated with worse OS, suggesting that
preoperative neurological status is an important marker of disease
severity and possesses an important prognostic value in malignant
glioma patients. A recent study by Chaichana et al has also
reported that preoperative clinical symptoms, including motor and
language deficit, were independent predictors of poor survival in
GBM patients undergoing resection (9).
Limitations of this study should be acknowledged.
Firstly, standard chemotherapy with temozolomide was not available
at the time of the study in Lithuania; therefore, the value of TII
in predicting the survival of patients receiving combined
radiotherapy and chemotherapy should be addressed in the future.
Also, other clinical (e.g., functional status) and genetic (e.g.,
MGMT promoter methylation) prognostic markers were not
systematically evaluated and therefore were not included in the
analyses. Finally, due to the small sample size, our findings need
to be considered with caution until replicated in a larger sample
of malignant glioma patients. Furthermore, more advanced nuclear
medicine imaging modalities, including PET and SPECT/CT were not
used at our institution at the time of the study.
It should be noted that GBM patients were considered
together with one gliosarcoma patient, as it was previously shown
that both types of tumors share substantial clinical and genetic
similarities, have similar survival rates, and should therefore be
considered together in terms of treatment and enrolment to research
protocols (6,7).
In conclusion, the results of this study suggest
that higher 99mTc-MIBI uptake before and after surgery
and less pronounced decrease of 99mTc-MIBI uptake after
surgery are associated with worse survival of malignant glioma
patients. Evaluation of tracer uptake by means of TII during
perioperative periods may be used as an additional prognostic
factor of malignant glioma patients. However, further studies in
larger samples are required.
References
|
1.
|
LM DeAngelisBrain tumorsN Engl J
Med34411423200110.1056/NEJM200101113440207
|
|
2.
|
JA SchwartzbaumJL FisherKD AldapeM
WrenschEpidemiology and molecular pathology of gliomaNat Clin Pract
Neurol2494503200610.1038/ncpneuro028916932614
|
|
3.
|
Y LiuS SheteCJ EtzelM ScheurerG AlexiouG
ArmstrongPolymorphisms of LIG4, BTBD2, HMGA2, and RTEL1 genes
involved in the double-strand break repair pathway predict
glioblastoma survivalJ Clin
Oncol2824672474201010.1200/JCO.2009.26.621320368557
|
|
4.
|
JN ScottNB RewcastlePM BrasherD FultonJA
MacKinnonM HamiltonWhich glioblastoma multiforme patient will
become a long-term survivor? A population-based studyAnn
Neurol46183188199910.1002/1531-8249(199908)46:2%3C183::AID-ANA7%3E3.0.CO;2-710443883
|
|
5.
|
R StuppWP MasonMJ van den BentM WellerB
FisherMJ TaphoornRadiotherapy plus concomitant and adjuvant
temozolomide for glioblastomaN Engl J
Med352987996200510.1056/NEJMoa04333015758009
|
|
6.
|
E GalanisJC BucknerRP DinapoliBW
ScheithauerRB JenkinsCH WangClinical outcome of gliosarcoma
compared with glioblastoma multiforme: North Central Cancer
Treatment Group resultsJ
Neurosurg89425430199810.3171/jns.1998.89.3.0425
|
|
7.
|
J LutterbachR GuttenbergerA
PagenstecherGliosarcoma: a clinical studyRadiother
Oncol615764200110.1016/S0167-8140(01)00415-711578729
|
|
8.
|
C AdamsonOO KanuAI MehtaC DiN LinAK
MattoxGlioblastoma multiforme: a review of where we have been and
where we are goingExpert Opin Investig
Drugs1810611083200910.1517/1354378090305276419555299
|
|
9.
|
K ChaichanaS ParkerA OliviA
Quinones-HinojosaA proposed classification system that projects
outcomes based on preoperative variables for adult patients with
glioblastoma multiformeJ
Neurosurg1129971004201010.3171/2009.9.JNS09805
|
|
10.
|
N El HindyHS BachmannN LambertzM AdamzikH
NückelK WormAssociation of the CC genotype of the regulatory BCL2
promoter polymorphism (−938C>A) with better 2-year survival in
patients with glioblastoma multiformeJ Neurosurg114163116392011
|
|
11.
|
X ChengY LiZ XuD LiJ WangA meta-analysis
of (99m) Tc-MIBI SPECT for detection of recurrent glioma after
radiation therapyJ Clin
Neurosci18307312201110.1016/j.jocn.2010.07.11321251837
|
|
12.
|
K KallénIM BurtscherS HoltåsE RydingI
Rosén201Thallium SPECT and 1H-MRS compared with MRI in chemotherapy
monitoring of high-grade malignant astrocytomasJ
Neurooncol46173185200010894370
|
|
13.
|
F Prigent-Le JeuneF DuboisS PerezS BlondM
SteinlingTechnetium-99m sestamibi brain SPECT in the follow-up of
glioma for evaluation of response to chemotherapy: first resultsEur
J Nucl Med Mol Imaging31714719200414985865
|
|
14.
|
RB SchwartzBL HolmanJF PolakBM GaradaMS
SchwartzR FolkerthDual-isotope single-photon emission computerized
tomography scanning in patients with glioblastoma multiforme:
association with patient survival and histopatho-logical
characteristics of tumor after high-dose radiotherapyJ
Neurosurg896068199810.3171/jns.1998.89.1.0060
|
|
15.
|
I AkZ GulbasF AltinelE VardareliTc-99m
MIBI uptake and its relation to the proliferative potential of
brain tumorsClin Nucl
Med282933200310.1097/00003072-200301000-0000712493957
|
|
16.
|
GA AlexiouS TsiourisAP KyritsisG
FotakopoulosA GoussiaS VoulgarisThe value of 99mTc-tetrofosmin
brain SPECT in predicting survival in patients with glioblastoma
multiformeJ Nucl
Med5119231926201010.2967/jnumed.110.08092921078797
|
|
17.
|
P BeauchesneR PedeuxM BoniolC
Soler99mTc-sestamibi brain SPECT after chemoradiotherapy is
prognostic of survival in patients with high-grade gliomaJ Nucl
Med45409413200415001680
|
|
18.
|
P BeauchesneC SolerCorrelation of
99mTc-MIBI brain spect (functional index ratios) and survival after
treatment failure in malignant glioma patientsAnticancer
Res22308130852002
|
|
19.
|
S NagamachiS JinnouchiK NabeshimaR NishiiL
Flores IIT KodamaThe correlation between 99mTc-MIBI uptake and
MIB-1 as a nuclear proliferation marker in glioma - a comparative
study with
201TlNeuroradiology4310231030200110.1007/s00234010062911792039
|
|
20.
|
VP DeltuvaN JurkieneI KulakieneA
BuneviciusA MatukeviciusA TamasauskasIntroduction of novel
semi-quantitative evaluation of 99mTc-MIBI SPECT before and after
treatment for gliomaMedicina (Kaunas)4811521201222481370
|
|
21.
|
L FilippiR SantoniC ManniR DanieliR
FlorisO SchillaciImaging primary bain tumors by single-photon
emission computerized tomography (SPECT) with technetium-99m
sestabimi (MIBI) and tetrofosminCurrent Medical Imaging
Reviews16166200510.2174/1573405052953047
|
|
22.
|
RE HenkinD BovaGL DillehayJR HalamaSM
KareshRH WagnerNuclear Medicine2nd editionMosby
ElsevierPhiladelphia1371532006
|
|
23.
|
T SembaY SugawaraT OchiT FujiiT MochizukiT
OhnishiThallium-201 SPECT in prognostic assessment of malignant
gliomas treated with postoperative radiotherapyAnn Nucl
Med20287294200610.1007/BF0298464516856572
|
|
24.
|
MV PadmaS SaidM JacobsDR HwangK DuniganM
SatterPrediction of pathology and survival by FDG PET in gliomasJ
Neurooncol64227237200310.1023/A:102566582000114558598
|
|
25.
|
NJ PatronasG Di ChiroC KuftaD BairamianPL
KornblithR SimonPrediction of survival in glioma patients by means
of positron emission tomographyJ
Neurosurg62816822198510.3171/jns.1985.62.6.08162987440
|
|
26.
|
WS ChenKE LukerJL DahlheimerCM PicaGD
LukerD Piwnica-WormsEffects of MDR1 and MDR3 P-glycoproteins, MRP1,
and BCRP/MXR/ABCP on the transport of (99m) Tc-tetrofosminBiochem
Pharmacol60413426200010.1016/S0006-2952(00)00341-510856437
|
|
27.
|
LI Delmon-MoingeonD Piwnica-WormsAD Van
den AbbeeleBL HolmanA DavisonAG JonesUptake of the cation
hexakis(2-methoxyisobutylisonitrile)-technetium-99m by human
carcinoma cell lines in vitroCancer Res50219820219902317808
|
|
28.
|
U PichlmeierA BinkG SchackertW StummerALA
Glioma Study GroupResection and survival in glioblastoma
multiforme: an RTOG recursive partitioning analysis of ALA study
patientsNeuro
Oncol1010251034200810.1215/15228517-2008-05218667747
|
|
29.
|
N SanaiMY PolleyMW McDermottAT ParsaMS
BergerAn extent of resection threshold for newly diagnosed
glioblastomasJ
Neurosurg11538201110.3171/2011.2.JNS1099821417701
|