Introduction
Currently, the acceptable predictors of cancers
include the tumor type and malignant potential of the respective
tumor, including its proliferation, invasion and angiogenesis
properties. An increasing number of studies indicate that matrix
metalloproteinases (MMPs) contribute to the malignant phenotype,
and that the evaluation of MMP expression may be helpful for the
assessment of patients’ prognosis (1–5). MMPs
are a family of zinc-dependent endopeptidases with multiple
functions, including proteolytic activity. Studies have
demonstrated that MMPs play essential roles in numerous
pathological processes such as tissue remodeling, wound healing,
angiogenesis, apoptosis and tumor progression. MMPs not only
degrade almost all the extracellular matrix (ECM) components,
thereby promoting cancer invasion and metastasis (6), but also regulate cellular adhesion
(7) and promote cancer angiogenesis
(8).
MMP-26, a novel member of the MMP family, was first
cloned in 2000 (9). MMP-26 has a
variety of properties that distinguish it from other MMPs. It lacks
a hinge region. The conservative PRCGXXD cysteine switch is
replaced by PHCGVPD in MMP-26, which is the basis of its unorthodox
activation and distinct functions (10). In order to investigate the roles of
MMP-26 in the growth, invasion and angiogenesis of breast cancer,
we prepared the recombinant plasmid pcDNA3.1(+)-neo carrying the
proMMP-26 coding sequence, and transfected it into breast cancer
MCF-7 cells. MMP-26 expression was measured by RT-PCR,
immunofluorescence assay and flow cytometry. The observations of
in vitro and in vivo growth and the invasive
potential of MMP-26-transfected cells indicated that MMP-26
overexpression was closely correlated with the malignant phenotype,
increased invasion ability and enhanced angiogenesis in the
cancers. Thus, we speculated that the evaluation of MMP-26
expression may have clinical implications in predicting the
prognosis of breast cancer.
Materials and methods
Cell line and cell culture
The human breast carcinoma cell line (MCF-7 cells)
was purchased from ATCC and grown in H-Dulbecco’s modified Eagle’s
medium (DMEM; Gibco, Invitrogen Life Technologies, Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS; Sigma, St. Louis, MO,
USA) at 37°C. The ethics approval was given by the medical ethics
committee of Basic Medical College, Jilin University, Jilin,
China.
DNA transfection and clonal
selection
pcDNA3.1(+)-neo expression plasmid carrying the
proMMP-26 coding sequence (provided by Dr Alex Strongin; Burnham
Institute, La Jolla, CA, USA) (8),
was used to transfect MCF-7 cells using SuperFect (Qiagen, Hilden,
Germany) following the manufacturer’s instructions. An
800-μg/ml concentration of G418 (Sigma) was used to select
the MMP-26 stable transfectants. Single clones with G418 resistance
were selected and expanded for further analysis. MMP-26 expression
was determined in MCF-7 cells transfected with the pcDNA3.1 plasmid
and in non-transfected MCF-7 cells, used as controls, by the
methods described below.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. cDNA was synthesized using AMV reverse transcriptase
(Takara Bio Inc., Tokyo, Japan), oligo dT and 2 μg total
RNA. The reaction was carried out at 48°C for 30 min, 99°C for 5
min and 5°C for 5 min. In the PCR assay, cDNA was amplified by 30
cycles of reactions (denaturing at 94°C for 30 sec, annealing at
55°C for 30 sec and extension at 72°C for 1 min) using primers for
MMP-26 (forward, 5′-TGACATGCAGATGCATGCTCTGC-3′; and reverse,
5′-CTAGGGTCGTGATACCAGTAAGTG-3′) according to a method previously
described (9). The anticipated size
of the PCR products was 500 bp. β-actin was amplified (forward,
5′-TGGAATCCTGTGGCATCCATGAAAC-3′; and reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′) and the expected size of β-actin
was 360 bp.
Immunofluorescence assay
The cultured cells were fixed for 30 min in 4%
paraformaldehyde, and permeabilized in 0.1% Triton X-100 (Sigma)
for 10 min. The endogenous peroxidase was inactivated with hydrogen
peroxide (0.3% in methanol) followed by washing in
phosphate-buffered saline (PBS). The cells were incubated with
blocking serum for 30 min at room temperature and then with
anti-human MMP-26 polyclonal antibody (a gift from Burnham
Institute) (8) for 1 h at 37°C
followed by washing in PBS. FITC-conjugated anti-rabbit antibody
(Sigma) was used to treat the cells for 30 min followed by washing
for 30 min at room temperature. The primary antibody was replaced
with PBS serving as a negative control.
Flow cytometry
The MMP-26-transfected MCF-7 cells were harvested
and fixed in 4% paraformaldehyde for 60 min at 4°C. After washing
in PBS, the cells were treated with 0.1% Triton X-100 for 10 min
and washed in PBS. Anti-human MMP-26 polyclonal antibody was used
to treat the cells for 40 min at 4°C followed by washing in PBS.
FITC-conjugated anti-rabbit antibody was applied to treat these
cells for 40 min at 4°C followed by washing. The cells were then
re-suspended in 500 μl PBS and subjected to flow
cytometry.
Spreading of tumor cells on Matrigel
A single cell suspension in serum-free medium was
firstly prepared. Cells (1x104) were seeded into 96-well
plates precoated with Matrigel™ (BD Biosciences, Franklin Lakes,
NJ, USA). The cells were grown for 1.5 h and washed with PBS. The
morphology of spreading cells was observed under the
microscope.
Boyden chamber assay
Boyden chambers (Falcon; BD Biosciences) were
precoated with Matrigel (60 μl/well) and incubated for 30
min at 37°C. The Matrigel precoated chamber was supplemented with
anti-MMP-26 polyclonal antibody (100 μg/ml) in the MMP-26
transfected group. Cells in 200 μl serum-free H-DMEM were
stained with rhodamine (Invitrogen Life Technologies) and seeded
into the upper chambers. The lower chambers were filled with NIH3T3
culture supernatant to create a chemotactic gradient. Incubation
was performed at 37°C with 5% CO2. In the migration
assay, the distances that cells migrated on the Matrigel were
measured under a laser confocal microscope after 2 h. In the
invasion assay, the cells on the upper surface with the Matrigel
were removed by wiping the surface firmly with a cotton swab after
4 h of incubation. The filters were photographed under a laser
confocal microscope (Olympus, Tokyo, Japan). The number of cells
penetrating the filter was counted under the microscope at a
magnification of x100. Ten visual fields were counted on each
filter. The results are expressed as the mean ± SD.
In vivo nude mice bearing breast cancer
model
Cells (1x105) were harvested and
inoculated into the groin region of 6–8-week-old female nude mice
(nu/nu; Lianhelihua Company, Beijing, China). The mice were bred in
the Experimental Animal Center of Jilin University (Changchun,
Jilin, China). Twenty days later, the mice were sacrificed and the
tumors were collected. The volumes of solid tumors were estimated
by measuring the long and short diameter of the tumor. Tumor
tissues were fixed in 10% formalin, paraffin-embedded and cut into
5-μm sections. Hematoxylin and eosin staining was then
performed. The morphologic characteristics of the tumors were
observed under a light microscope.
Tumor cell-induced angiogenesis
model
Cells (1x105) were harvested and
inoculated subcutaneously into the backs of nude mice. The mice
were sacrificed after 4 days. The over-lying skin was collected.
The injection site was photographed under the dissecting microscope
(magnification, x7). Ten visual fields of each nude mouse were
employed and the number of blood vessels was counted. The results
are expressed as the mean ± SD of vessel numbers per visual
field.
Results
Expression of MMP-26 mRNA in
MMP-26-transfected cells
MCF-7 cells were transfected with pcDNA3.1(+)-neo
plasmid carrying the proMMP-26 coding sequence. Following G418
resistance screening, neomycin-resistant clones were selected and
the expression of MMP-26 mRNA was determined by RT-PCR. Results
showed that the expression level of MMP-26 mRNA was significantly
increased in MMP-26 transfected cells compared to non-transfected
MCF-7 cells and pcDNA3.1(+) vector-transfected MCF-7 cells.
Immunofluorescence assay of MMP-26
expression
MMP-26 polyclonal antibody was used to determine
MMP-26 expression in MMP-26-transfected cells and cells in
controls. Results showed that the protein expression of MMP-26 was
significantly increased in the MMP-26-transfected cells compared
with that in control groups. The immunofluorescence assay
demonstrated high expression of MMP-26 in the cytoplasm of
MMP-26-transfected cells compared with weak expression in MCF-7
cells and pcDNA3.1(+) vector-transfected cells.
Protein expression of MMP-26 by flow
cytometry
MMP-26 protein expression was also determined by
flow cytometry. The peak of MMP-26 in the MMP-26-transfected cells
demonstrated a rightward shift and the average intensity was
doubled compared to the non-transfected MCF-7 cells and
pcDNA3.1-transfected cell clones.
Morphologic changes of MMP-26-transfected
MCF-7 cells
Following transfection with the pcDNA3.1(+)-neo
expression plasmid carrying the proMMP-26 coding sequence, the
MCF-7 cells showed evident morphologic changes compared to the
control groups. The cells appeared larger and more pleomorphic with
abnormal nuclei. The ultrastructure of transfected cells were as
follows: the number of mitotic cells increased, pathological
karyokinesis was noted and myelin-like bodies appeared in the
cytoplasm of several cells. These features indicate high
proliferation and a high degree of malignancy.
Spreading of MMP-26-transfected
cells
The MMP-26-transfected cells and those in the
control group were seeded into 96-well plates precoated with
Matrigel and were grown in serum-free H-DMEM at 37°C for 1.5 h. The
spreading ability of MMP-26 transfected cells increased
considerably compared to the control groups. The shape of cells
became polygonal and more pseudopods were observed (Fig. 1).
Migration and invasion of
MMP-26-transfected cells
To detect the migration of the transfected cells in
the Boyden chambers, a laser confocal microscope was employed to
observe cells 2 h after incubation in the Boyden chamber precoated
with Matrigel. Results showed the migration ability of
MMP-26-transfected cells was markedly higher compared with the
control group. The migration ability of MMP-26-transfected cells
was dramatically reduced in the presence of MMP-26 antibody. Four
hours after incubation, the number of cells that invaded the filter
was counted. The number of invasive cells in the MMP-26-transfected
cells was significantly higher than in the control group
(P<0.01). The number of invasive cells in the presence of MMP-26
antibody was significantly reduced (P<0.01).
Growth of MCF-7 cell-induced breast
cancer in nude mice
MMP-26-transfected, pcDNA3.1-transfected and
non-transfected MCF-7 cells were inoculated subcutaneously into
nude mice and the tumor growth was detected in vivo. Solid
tumors formed 7 days after inoculation. Twenty days later, the
tumors were collected, and tumor size and weight were measured.
Results showed that there was no significant difference in the
tumor size among the three groups (P>0.05; data not shown).
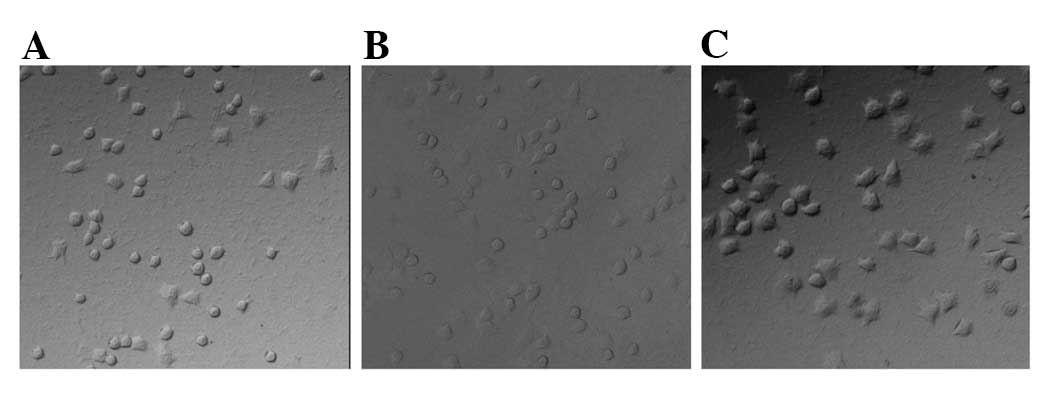
Under a microscope (magnification, x200), the cancer nests in the
mice inoculated with MMP-26-transfected cells were smaller than
those in the controls, with more surrounding stroma (Fig. 2A, B and C). The angiogenesis in the
tumors of nude mice treated with MMP-26-transfected cells (Fig. 2D) was dramatically increased
compared with that in the controls.
At high magnification (x400), the parenchymal cells
in the tumors of mice treated with MMP-26-transfected cells had
larger size and were more pleomorphic than those in controls. There
were more tumor giant cells (Fig.
2E) and more mitotic figures, including more atypical mitotic
figures (Fig. 2F) than the controls
(Fig. 2G). These findings indicate
that the tumors of mice treated with MMP-26-transfected cells were
highly malignant and had higher proliferation when compared with
tumors of mice treated with cells in the control group.
MMP-26-transfected cells induced
angiogenesis
MMP-26-transfected, pcDNA3.1-transfected and
non-transfected MCF-7 cells were inoculated subcutaneously into
nude mice and the angiogenesis of formed tumors was evaluated. The
skin was removed 4 days later and the blood vessels were measured.
Results showed that MMP-26-transfected cell-induced tumors had
significantly increased angiogenesis as compared to the control
group. The newly generated blood vessels were large and abundant in
branches forming the vascular networks. Quantitative analysis of
the newly generated blood vessels revealed that the number of
vascular branches and the total length of these vessels in
MMP-26-transfected cell induced tumors were significantly different
from those in control cell-induced tumors (P<0.01; Table I).
 | Table I.Angiogenesis in tumors following
allogeneic cancer cell inoculation. |
Table I.
Angiogenesis in tumors following
allogeneic cancer cell inoculation.
| Groups of
cells | No. of
branches | Total length of
vascular branches (cm) | P-value |
|---|
| Non-transfected
MCF-7 | 15.3±2.50 | 17.02±10.07 | <0.05a |
|
pcDNA3.1-transfected | 24.4±10.0 | 31.68±3.78 | |
|
MMP-26-transfected | 47.0±13.8 | 47.70±8.75 | |
Discussion
The invasion and metastasis of malignant tumors is a
complex and multi-stage process associated with the enhancement of
proteolytic activity and the degradation of the ECM. There are four
main types of proteolytic enzymes taking part in the degradation of
ECM, among which MMPs are the largest family with the most
complicated functions and high proteolytic activity. MMPs are
involved in the metastasis and invasion of cancers by degrading the
ECM, regulating cell adherence and promoting angiogenesis (1–5).
MMP-26 is a novel member of the MMP family. Numerous
studies have been conducted to investigate the prokaryotic
expression, spatial structure, in vitro activation and the
substrate cleavage specificity of MMP-26 (9–13).
However, the functions of MMP-26 in cancer progression and their
clinical significance are still poorly understood. Herein, pcDNA3.1
vector carrying the full-length gene of MMP-26 was transfected into
MCF-7 cells, a human breast cancer cell line, and the roles of
MMP-26 in the malignant phenotype of these cells were
evaluated.
In the present study, the cellular atypia of MCF-7
cells increased significantly once they were transfected with the
MMP-26 gene in vivo and in vitro. In vitro, the
MMP-26-transfected cells were larger and more pleomorphic with more
tumor giant cells compared with the non-transfected cells and
pcDNA3.1-transfected cells. In vivo, MMP-26-transfected
cells had similar morphologic features to those in vitro.
The parenchymal cells in the MMP-26-transfected cell-induced tumors
appeared to be more pleomorphic with more tumor giant cells and
more mitotic figures, including atypical mitotic figures. All of
these findings suggest that the overexpression of MMP-26 turned the
less malignant phenotype of MCF-7 cells into a highly malignant
phenotype. The increased number of mitotic figures also suggests
enhanced cell proliferation. The mechanism of MMP-26-induced
promotion of cell proliferation has not been elucidated to date.
Golubkov et al (14)
hypothesized that MMPs acted as oncogenes promoting the malignant
transformation of normal cells rather than just as enzymes
supporting the growth of pre-existing cancers. To validate this
hypothesis, normal 184B5 human mammary epithelial cells were
transfected with MT1-MMP (184B5-MT1 cells). Results showed that
184B5-MT1 cells exhibited aneuploidy and were efficient in
generating cancers in an orthotopic xenograft model in
immunodeficient mice. They also found that the oncogenic functions
of MT1-MMP were related to its proteolysis of pericentrin, one of
the most notable scaffolding proteins of pericentriolar material
surrounding the centrosome (15).
These observations may be useful for further studies on the
oncogenic mechanism of MMP-26.
Penetration of the basement membrane by breast
cancer cells is a key step in which in situ cancer becomes
infiltrating cancer. Thus, the migration and invasion of cancer
cells are important indicators in evaluating the degree of
malignancy. In the present study, MMP-26-transfected cells adhered
to the Matrigel more rapidly and had increased pseudopodia or
altered shapes (from round to polygonal) when compared with cells
in the control group. In the migration and invasion assay,
MMP-26-transfected cells had significantly increased migration and
invasion through the filter as compared to the cells in the control
group. However, the migration and invasion of MMP-26-transfected
cells were effectively inhibited in the presence of MMP-26
antibody. These results suggest that MMP-26 promotes the adherence,
migration and invasion of MCF-7 cells. Matrigel is an analog of the
basement membrane and its components and structure are similar to
to those of the basement membrane in vivo. Therefore, we
speculate that MMP-26 may play a key role in the early invasion of
breast cancer by promoting adhesion to the basement membrane and
migration or invasion through it. By immunofluorescence microscopy,
Zhao et al (16) detected
MMP-26 expression in human breast ductal carcinoma in situ
(DCIS), infiltrating ductal carcinoma (IDC), atypical intraductal
hyperplasia and normal breast epithelia adjacent to ductal DISC and
IDC. Their results revealed that MMP-26 expression in DCIS was
significantly higher than in the other tissues. We postulate that
the increased MMP-26 expression in DCIS may promote the
infiltrating ability of the cancer cells, allowing them to
eventually penetrate the basement membrane.
Cancer growth is also dependent on the angiogenesis
within it. The blood vessels in cancers not only supply nutrition,
but also provide potential pathways for hematogenous metastasis. A
variety of factors produced by the cancer cells induce angiogenesis
in cancers, including MMPs (3). In
the present study, angiogenesis in MMP-26-transfected cell-induced
cancers was significantly promoted and the blood vessels were
larger in diameter and longer in total length than those in the
MCF-7 and pcDNA3.1-transfected cell-induced cancers. In short,
there were more newly generated capillaries in the stroma of
MMP-26-transfected cell-induced cancers than in the control group.
These findings demonstrate that MMP-26 is a potent inducer of
angiogenesis in cancers.
As a novel member of MMPs, the function of MMP-26 is
of great interest. Herein, our results demonstrate that MMP-26
elevates the malignant phenotypes of MCF-7 breast cancer cells,
including atypia, mitosis, spreading, migration and angiogenesis.
Although the mechanisms underlying these effects have not been
identified, these effects of MMP-26 have significant clinical
implications. Since the high expression of MMP-26 is accompanied by
increased malignant phenotypes, MMP-26 may be used as an important
predictor in determining the degree of malignancy of breast cancer.
Further studies are warranted to evaluate the role of MMP-26 in the
prognosis of breast cancer.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant nos. 30470662
and 30870970) and Jilin Provincial Science and Technology Projects
(grant nos. 20050118, 200705358 and 20090513). We also acknowledge
Dr F. William Orr for his critical suggestions on the organization
of the manuscript.
References
|
1.
|
Z WerbECM and cell surface proteopysis:
regulating cellular
ecologyCell91439442199710.1016/S0092-8674(00)80429-89390552
|
|
2.
|
YJ ShinJH KimThe role of EZH2 in the
regulation of the activity of matrix metalloproteinases in prostate
cancer cellsPLoS
One7e30393201210.1371/journal.pone.003039322272343
|
|
3.
|
WG Stetler-StevensonMatrix
metalloproteinases in angiogenesis: a moving target for therapeutic
interventionJ Clin Invest10312371241199910.1172/JCI687010225966
|
|
4.
|
L KnopfovaP BenesL Pekarcikovac-Myb
regulates matrix metalloproteinases 1/9, and cathepsin D:
implications for matrix-dependent breast cancer cell invasion and
metastasisMol Cancer1115201210.1186/1476-4598-11-1522439866
|
|
5.
|
S HeymansA LuttunD NuyensInhibition of
plasminogen activators or matrix metalloproteinases prevents
cardiac rupture but impairs therapeutic angiogenesis and causes
cardiac failureNat Med511351142199910.1038/13459
|
|
6.
|
K RyggvasonM HöyhtyäT SaloProteolytic
degradation of extracellular matrix in tumor invasionBiochimica et
Biophysica Acta90719121719872823896
|
|
7.
|
G GiannelliJ Falk-MarzillierO
SchiraldiInduction of cell migration by matrix metalloprotease-2
cleavage of
laminin-5Science277225228199710.1126/science.277.5323.2259211848
|
|
8.
|
RL BarnhillMW PiepkornAJ CochranTumor
vascularity, proliferation, and apoptosis in human melanoma
micrometastases and macrometastasesArch
Dermatol13499199419989722729
|
|
9.
|
HI ParkJ NiFE GerkemaIdentification and
characterization of human endometase (Matrix metalloproteinase-26)
from endometrial tumorJ Biol
Chem2752054020544200010.1074/jbc.M00234920010801841
|
|
10.
|
GN MarchenkoBI RatnikovDV
RozanovCharacterization of matrix metalloproteinase-26, a novel
metalloproteinase widely expressed in cancer cells of epithelial
originBiochem J356705718200110.1042/0264-6021:356070511389678
|
|
11.
|
ND MarchenkoGN MarchenkoRN
WeinrebBeta-catenin regulates the gene of MMP-26, a novel
metalloproteinase expressed both in carcinomas and normal
epithelial cellsInt J Biochem Cell
Biol36942956200410.1016/j.biocel.2003.12.00715006646
|
|
12.
|
W LiAY SavinovDV RozanovMatrix
metalloproteinase-26 is associated with estrogen-dependent
malignancies and targets alpha1-antitrypsin serpinCancer
Res6486578665200410.1158/0008-5472.CAN-04-301915574774
|
|
13.
|
Y ZhangH ZhaoY WangY LinY TanX FangL
ZhengNon-small cell lung cancer invasion and metastasis promoted by
MMP-26Mol Med Report412011209201121805034
|
|
14.
|
VS GolubkovAV ChekanovAY SavinovMembrane
type-1 matrix metalloproteinase confers aneuploidy and
tumorigenicity on mammary epithelial cellsCancer
Res661046010465200610.1158/0008-5472.CAN-06-299717079467
|
|
15.
|
VS GolubkovAY StronginProteolysis-driven
oncogenesisCell Cycle6147150200710.4161/cc.6.2.370617245132
|
|
16.
|
YG ZhaoAZ XiaoRG NewcomerActivation of
pro-gelatinase B by endometase/matrilysin-2 promotes invasion of
human prostate cancer cellsJ Biol
Chem2781505615064200310.1074/jbc.M21097520012586837
|