Introduction
According to the World Health Organization (WHO)
statistics, breast cancer is the most common type of cancer in
females worldwide in developed and developing countries (1). It is estimated that 226,870 females
will be diagnosed with breast cancer and 39,510 will have succumbed
to the disease in the United States in 2012. According to the
National Institute of Health (NIH), breast cancer is the second
leading cause of cancer-related mortality, following lung cancer
(2). Data from the Ministry of
Health of The People’s Republic of China (MOHC) show that in China
the incidence of breast cancer has been rising steadily in the last
few years and that it is the most commonly diagnosed cancer in
females. Elemene has been approved by the State Food and Drug
Administration (SFDA) of China for the treatment of advanced
cancers, including breast, gynecological and lung cancer. The
essential oil extracted from Curcuma wenyujin, Y.H. Chen et
C. Ling, a traditional Chinese herbal medicine, is a mixture
containing β-, γ- and δ-elemene. β-elemene is the major active
component (1).
Although β-elemene has been shown to inhibit tumor
cell growth and an elemene emulsion has been approved for cancer
treatment (3–7), the objective response rate with
elemene has been modest in the majority of tumor types. Moreover,
β-elemene is an essential oil with poor water-solubility and
stability; therefore its use in cancer treatment is limited. To
improve the activity and solubility of β-elemene, we synthesized a
series of derivatives which contained piperazine, morpholine,
tetrahydropyrrole, thiophenylethylamine or cyclohexamine groups in
a previous study. Among these derivatives,
13,14-bis(cis-3,5-dimethyl-1-piperazinyl)-β-elemene (IIi) was found
to be one of the most potent agents for inhibiting the
proliferation of human cancer cells and it also demonstrated an
inhibitory effect on mTOR (8) The
structures of β-elemene and IIi are shown in Fig. 1A.
In the present study, we aimed to investigate the
antitumor activities of IIi in vitro and in vivo, and
to elucidate the possible associated mechanisms of action. The
results show that IIi inhibited the proliferation of a wide
spectrum of human cancer cells in vitro, including breast,
ovarian and lung cancer cells. IIi also inhibited the growth of
S-180 sarcoma in mice. The present study also showed that IIi
inhibits mTOR activity and induces autophagy in breast cancer
cells. These findings may aid in the verification of the antitumor
activity of IIi and enable the rational design of a novel elemene
analog.
Materials and methods
Cell culture and reagents
MCF-7, BT-474, MDA-MB-231, MDA-MB-468, HeLa, SKOV-3,
A549, HepG-2, BEL-7402, HL-60, K562, HCT-116 and HCT-15 cells were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and maintained in appropriate medium, as suggested by the
ATCC. MKN-45 and SGC-7901 human gastric cancer cells were obtained
from the Cancer Research Foundation of Japan and were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS).
The cells were incubated in a humidified atmosphere of 95% air with
5% CO2 at 37°C. β-elemene was obtained from Yuanda
Pharmaceuticals (Dalian, China). IIi was obtained from the Key
Laboratory of Structure-Based Drug Design and Discovery of Shenyang
Pharmaceutical University, China. The IIi compund was synthesized
using similar methods to those described in our previous study and
characterized with infrared (IR) spectroscopy, proton nuclear
magnetic resonance (1HNMR) spectroscopy, mass
spectrometry and elemental analyses (6).
This study was approved by the Ethics Committee of
Taizhou College, Taizhou, China.
Immunoblotting and
immunofluorescence
Immunoblotting and immunofluorescence analysis were
conducted with standard procedures, using antibodies against S6K1,
phosphorylated S6K1, 4EBP1, phosphorylated 4EBP1, LC3 (Cell
Signaling Technology, Beverly, MA, USA) and GAPDH (Ding Guo
Biotechnology, Beijing, China).
Animals and antitumor activity in
vivo
Seven-week-old specific pathogen-free male Kunming
mice (weight, 18–22 g) were supplied by the Laboratory Animal
Center of Shenyang Pharmaceutical University, Liaoning, China. The
mice were inoculated subcutaneously into the right armpit with
S-180 sarcoma cells. After 24 h, normal saline, cyclophosphamide
(CTX), β-elemene and IIi were administered by intraperitoneal
injection for 7 days. Following treatment, the animals were
sacrificed by cervical dissociation and the solid tumors were
removed and weighed. The inhibition rate was calculated as:
[(Average tumor weight of normal saline group − average tumor
weight of test group) / average tumor weight of normal saline
group] ×100.
Statistical analysis
Data are presented as the mean ± SD, and
significance was assessed with the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
IIi inhibits cancer cell proliferation in
vitro
As we had previously shown that IIi was able to
inhibit the proliferation of HeLa and SGC-7901 cells during an
experiment for drug screening (8),
in the present study, we first verified the effects of IIi on the
growth of human cancer cells in a wide spectrum of cell lines. The
IC50 of IIi on the K562 and HL-60 cells was determined
using a cell counting kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Haimen, China), while a sulforhodamine B (SRB) assay
was used for the other cells. The cells treated with
dimethylsulphoxide (DMSO) were used as the vehicle control. IIi
displayed potent cytotoxicity in a dose-dependent manner in
diversified cancer cell lines, including those of breast, ovarian,
lung, gastric, hepatocellular and colon cancer, as well as leukemia
cells (Fig. 1B). The average
IC50 values of IIi against the 15 human tumor cell lines
that were tested was 3.44 μmol/l, and each IC50
was below 10 μmol/l. The breast and ovarian cancer cells and
the leukemia cells appeared to be more sensitive to IIi, with
average IC50 values of 1.98, 2.40 and 1.90
μmol/l, respectively.
IIi overcomes drug resistance in
vitro
To determine whether IIi was able to overcome drug
resistance, the effect of IIi was examined on two
multidrug-resistant (MDR) sublines, K562/A02 and MCF-7/Adriamycin.
The drug-sensitive parental K562 and MCF-7 cell lines and
conventional anticancer drugs Adriamycin and β-elemene were used as
references. IIi and β-elemene displayed significant cytotoxicity in
the MDR sublines examined, with average IC50 values of
2.7 and 318.2 μmol/l, respectively, which were close to
those values observed in the corresponding parental cell lines
(average IC50, 1.7 and 281.8 μmol/l). The average
resistance factor (RF) of IIi on the MDR cells was 1.66 vs. 107.28
for Adriamycin (Table I).
 | Table ICytotoxicity of IIi in MDR and
drug-sensitive parental cell lines. |
Table I
Cytotoxicity of IIi in MDR and
drug-sensitive parental cell lines.
| IC50 (mean
± SD, n=3) (μM)
| | IC50 (mean
± SD, n=3) (μM)
| |
|---|
| Compounds | K562 | K562/A02 | RF | MCF-7 | MCF-7/ADR | RF |
|---|
| IIi | 2.2±0.5 | 3.1±0.4 | 1.41 | 1.2±0.2 | 2.3±0.3 | 1.92 |
| β-elemene | 256.7±49.5 | 309.8±43.2 | 1.21 | 306.9±60.7 | 326.7±69.2 | 1.06 |
| ADR | 0.4±0.2 | 50.2±7.8 | 125.50 | 1.8±0.4 | 160.3±38.2 | 89.06 |
IIi arrests tumor growth in mice
implanted with S-180 sarcoma
Kunming male mice inoculated with S-180 sarcoma
cells were used to determine the effect of IIi on tumor growth
in vivo. IIi showed dose-dependent effects on the tumor
growth in mouse S-180 sarcoma models (Table II). Intraperitoneal administration
of IIi at a dose of 40 mg/kg for 7 days reduced tumor growth by
50.4% and, at 20 mg/kg for 7 days, reduced tumor growth by 26.0%.
CTX and β-elemene at doses of 30 and 50 mg/kg with the same
schedule reduced tumor growth by 73 and 42.8%, respectively.
 | Table IIEffects of IIi on the growth of S-180
sarcoma in mice. |
Table II
Effects of IIi on the growth of S-180
sarcoma in mice.
| Treatment group | Dosage
(i.p.)/(mg/kg/day) x no. of days | Mouse number
(start/end) | Body weight (g)
| Tumor weight (g) | Inhibition rate
(%) |
|---|
| Start | End |
|---|
| NS | - | 20/20 | 20.02±2.13 | 23.20±4.01 | 1.19±0.12 | - |
| CTX | 30×7 | 10/9 | 20.03±1.95 | 17.59±2.34b | 0.31±0.09a | 73.9 |
| β-elemene | 50×7 | 10/10 | 20.24±1.87 | 23.14±3.56 | 0.68±0.13a | 42.8 |
| IIi | 40×7 | 10/9 | 20.64±1.94 | 19.62±3.47 | 0.59±0.14a | 50.4 |
| 20×7 | 10/9 | 20.00±1.83 | 19.64±2.43 | 0.88±0.23b | 26.0 |
| 10×7 | 10/10 | 20.11±1.96 | 22.83±1.64 | 1.06±0.14 | 10.9 |
IIi inhibits the mTOR target
In our previously published paper, we demonstrated
that IIi could inhibit the mTOR pathway in K562 cells (6). In the present study, we explored
whether IIi demonstrated the same function in breast cancer cells.
The downstream effectors of the target protein were examined in
MCF-7 and MDA-MB-468 cells upon IIi treatment. β-elemene and
rapamycin were used as references. As shown in Fig. 2, IIi functioned as the mTOR
inhibitor in a dose-dependent manner. IIi treatment at 0.25
μmol/l for 2 h slightly decreased the phosphorylated p70S6K1
and 4EBP1 levels in the MCF-7 cells, but appeared to have no
function in the MDA-MB-468 cells. However, the exposure of the
MCF-7 and MDA-MB-468 cells to IIi at a dose of 1 μmol/l
significantly decreased the phosphorylated p70S6K1 and 4EBP1 in the
two cells within 2 h, indicating that the mTOR activity was
inhibited significantly by IIi. Rapamycin and β-elemene, at doses
of 0.1 and 250 μmol/l with the same schedule, showed a
similar inhibitory effect.
IIi induces autophagy in MCF-7 cells
It has been reported that the mTOR inhibitors
produce antitumor activities and induce autophagy (9), and that β-elemene also induces
autophagy in certain cancer cells (10). As IIi has been confirmed as a novel
β-elemene derivative with mTOR inhibiting activity, further
examination of whether IIi was able to induce autophagy was
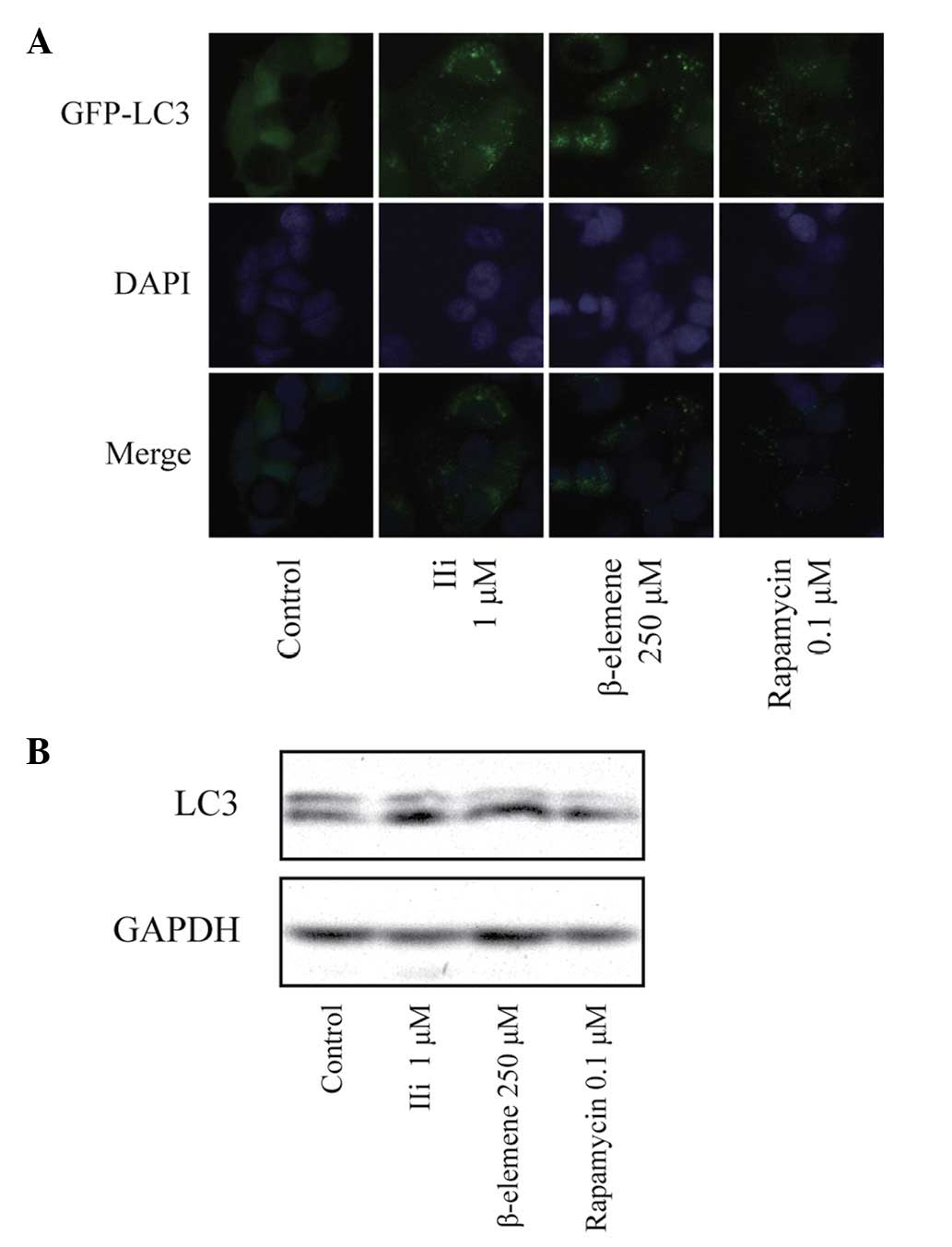
performed in MCF-7 cells expressing LC3 fused to EGFP. As shown in
Fig. 3A, a diffused distribution of
LC3 was observed in the control cells, whereas a punctate pattern
of LC3 was observed in the IIi-treated cells. To further confirm
whether IIi was able to induce autophagy in the MCF-7 cells, an
immunoblotting analysis was used to detect the cleavage of the LC3
protein. As shown in Fig. 3B,
western blot analysis showed a significantly increased expression
of cleaved LC3 in the IIi-treated MCF-7 cells. These results
suggested that IIi induced autophagy in the MCF-7 cells.
Discussion
In the present study, we showed that IIi, a novel
β-elemene derivative, inhibits the proliferation of human cancer
cells in a dose-dependent manner in numerous cancer cells,
including breast, ovarian and lung cancer cells, and also inhibits
the growth of S-180 sarcoma in vivo. IIi was also found to
inhibit mTOR activity and induce autophagy in breast cancer cells.
The results indicate that IIi may be a potent new anticancer drug
candidate or leading compound.
The synthesis and evaluation of promising novel
anticancer compounds remains an important challenge for new
anti-cancer drug idenitifications. The rational optimization of
lead compounds from natural products is one of the practical
strategies for new drug development (11). Structural modifications have been
demonstrated to successfully increase water solubility and/or
antitumor activity of several natural compounds, including
salvicine (12), camptothecin
(13) and taxol (14). β-elemene as a novel anticancer
herbal medicine has shown a range of antitumor effects in
vitro and in vivo(4–7). It
has been approved by the State Food and Drug Administration of
China for the treatment of malignant effusions and certain solid
tumors, following the positive results of phase III trials
(7). However, β-elemene inhibits
cell growth only at high concentrations and is an essential oil
with poor water-solubility and stability. Previously, we
synthesized and characterized a series of β-elemene derivatives and
demonstrated that IIi is the most potent agent for inhibiting the
proliferation of the human cancer cells HeLa and SGC-7901 (8). In the present study, we first used a
variety of breast cancer (MCF-7, BT-474, MDA-MB-231 and
MDA-MB-468), ovarian cancer (HeLa and SKOV-3), lung cancer (A549),
gastric cancer (MKN-45 and SGC-7901), hepatocellular cancer (HepG-2
and BEL-7402), leukemia (K562 and HL-60) and colon cancer cell
lines (HCT-15 and HCT-116) to evaluate the potential of IIi to
induce cytotoxicity. The results show that IIi treatment
significantly affected cell viability at lower concentrations, with
IC50 <10 μmol/l. Furthermore, the results show
that the IIi treatment led to a significant reduction in the tumor
size in mice implanted with S-180 sarcoma, when compared with
untreated animals with tumors. Thus, the effectiveness of IIi in
human cell lines and in mice makes it a potential cancer
therapeutic agent. However, this requires further investigation in
human cancer xenografts.
mTOR, a serine/threonine kinase, sits in the center
of the PI3K pathway and has been validated as an important target
for chemotherapy (15,16). The rapalogs, temsirolimus (Torisel
and Wyeth) and everolimus (Afinitor and Novartis), were approved by
the FDA for the treatment of cancer. However, the objective
response rates with the rapalogs were modest in the majority of
tumor types and highly variable (17,18).
One important strategy to improve the efficacy of mTOR inhibitors
is to identify new drugs over rapalogs (19). The present study showed that IIi
decreases phosphorylated p70S6K1 and 4EBP1 in human breast cancer
MCF-7 and MDA-MB-468 cells in a dose-dependent manner. It appears
that IIi is a new mTOR inhibitor. Additional experiments are
required to determine the possible mechanisms involved, including
the interaction with FKBP-12 and the effects on the mTOR kinase
domain. Among the signaling pathways implicated in the control of
autophagy, the most well-characterized autophagy regulator to date
is mTOR. Increased autophagic activity has been frequently observed
in malignant cells in response to treatment with therapeutic mTOR
inhibitors (9). In the present
study, we also showed that IIi induces human cancer cell autophagy,
in an identical manner to the mTOR inhibitor, rapamycin.
In summary, we have shown that IIi may be a
potential cancer therapeutic agent candidate with mTOR inhibitory
activity. However, additional animal models and clinical research
are required to evaluate the effectiveness and safety of IIi.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 81201530) and
Zhejiang Provincial Natural Science Foundation of China (No.
Y2110474 and LY12H31001).
References
|
1
|
World Health Organization: Programmes and
projects: Cancer: Breast Cancer Awareness Month in October.
http://www.who.int/cancer/events/breast_cancer_month/en/index.html.
Accessed October 31, 2011.
|
|
2
|
National Cancer Institute: Cancer
statistics: SEER stat fact sheets, breast. http://seer.cancer.gov/statfacts/html/breast.html.
Accessed April 5, 2011.
|
|
3
|
Guo YT: Isolation and identification of
elemene from the essential oil of Curcuma wenyujin. Zhong Yao Tong
Bao. 8(31)1983.(In Chinese).
|
|
4
|
Tan P, Zhong W and Cai W: Clinical study
on treatment of 40 cases of malignant brain tumor by elemene
emulsion injection. Zhongguo Zhong Xi Yi Jie He Za Zhi. 20:645–648.
2000.(In Chinese).
|
|
5
|
Peng X, Zhao Y, Liang X, et al: Assessing
the quality of RCTs on the effect of beta-elemene, one ingredient
of a Chinese herb, against malignant tumors. Contemp Clin Trials.
27:70–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao L, Zhou L, Zheng L and Yao M: Elemene
displays anti-cancer ability on laryngeal cancer cells in vitro and
in vivo. Cancer Chemother Pharmacol. 58:24–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Zhang H and Sun Y: Phase III
clinical trial of elemenum emulsion in the management of malignant
pleural and peritoneal effusions. Zhonghua Zhong Liu Za Zhi.
18:464–467. 1996.(In Chinese).
|
|
8
|
Xu L, Tao S, Wang X, et al: The synthesis
and anti-proliferative effects of beta-elemene derivatives with
mTOR inhibition activity. Bioorg Med Chem. 14:5351–5356. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carew JS, Kelly KR and Nawrocki ST:
Autophagy as a target for cancer therapy: new developments. Cancer
Manag Res. 4:357–365. 2012.
|
|
10
|
Liu J, Hu XJ, Jin B, Qu XJ, Hou KZ and Liu
YP: β-Elemene induces apoptosis as well as protective autophagy in
human non-small-cell lung cancer A549 cells. J Pharm Pharmacol.
64:146–153. 2012.
|
|
11
|
Butler MS: The role of natural product
chemistry in drug discovery. J Nat Prod. 67:2141–2153. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qing C, Zhang JS and Ding J: In vitro
cytotoxicity of salvicine, a novel diterpenoid quinone. Zhongguo
Yao Li Xue Bao. 20:297–302. 1999.PubMed/NCBI
|
|
13
|
Kollmannsberger C, Mross K, Jakob A, Kanz
L and Bokemeyer C: Topotecan - A novel topoisomerase I inhibitor:
pharmacology and clinical experience. Oncology. 56:1–12. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI. The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
17
|
Hudes G, Carducci M, Tomczak P, et al:
Global ARCC Trial: Temsirolimus, interferon alfa, or both for
advanced renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007.
View Article : Google Scholar
|
|
18
|
Motzer RJ, Escudier B, Oudard S, et al
RECORD-1 Study Group: Efficacy of everolimus in advanced renal cell
carcinoma: a double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan X and Helman LJ: The biology behind
mTOR inhibition in sarcoma. Oncologist. 12:1007–1018. 2007.
View Article : Google Scholar : PubMed/NCBI
|