Introduction
Nasopharyngeal carcinoma (NPC) is particularly
common in areas of China and South-East Asia, and is highly
associated with Epstein-Barr virus (EBV) infection (1). EBV infection is present in all poorly
and undifferentiated non-keratinizing NPC regardless of geographic
origin, and the viral antigens expressed by NPC provide potential
targets for immunotherapy.
HLEAFab is a human monoclonal antibody directed
against latent membrane protein 1 (LMP1), which is considered to be
the most important oncoprotein encoded by EBV. We have previously
investigated the effects of an immunoconjugate (HLEAFab-MMC) on
proliferation and apoptosis in the NPC HNE2/LMP1 cell lines, as
well as the inhibition of the growth of NPC xenografts in nude mice
(1). The expression of LMP1 varies
in various types of NPC. LMP1 is expressed and detectable in only
20–65% of NPC patients, using western blotting,
immunohistochemistry (IHC) or other assays (2,3).
Therefore, the biological efficacy of the Fab-based immunoconjugate
varies in EBV-related carcinomas with various levels of LMP1
expression. Patients with LMP1-positive cancers were shown to
benefit from the effect of the immunoconjugate on standard
chemotherapy.
Prior to initiation of the targeted therapy with
HLEAFab-MMC, the expression of LMP1 was evaluated with IHC and
in situ hybridization (ISH) in samples from 36 NPC patients.
The present study shows that the HLEAFab antibody fragment has an
excellent correlation with S12 in IHC-positive cases and ISH also
yields identical results. S12 is a purified mouse anti-human latent
membrane protein 1 monoclonal antibody from, which is used as the
standard control as it has in other studies (4). HLEAFab may be an ideal candidate for
NPC treatment using the novel immunoconjugate of HLEAFab-MMC.
Patients and methods
Patients
A total of 36 cases of NPC were selected from the
Department of Otolaryngology Head and Neck Surgery, Second
Affiliated Hospital of Nanjing Medical University (Nanjing, China)
between 2002 and 2010. The diagnosis of NPC was determined
according to the latest WHO criteria (5). The study was approved by the Ethics
Committee of The Second Affiliated Hospital of Nanjing Medical
University, Nanjing, China. Written informed consent was obtained
from the patient for publication of this study and any accompanying
images.
IHC for LMP1 expression
The EnVision method was used for immunohistochemical
analysis (6–8). NPC tissues were excised and sent for
paraffin wax-embedding and processing. Sections (5-μm thick)
were deparaffinized with xylene, then dehydrated in decreasing
concentrations of alcohol. Endogenous peroxidase activity was
blocked by hydrogen peroxidase (3%) in Tris-buffered saline (TBS)
for 30 min. The sections were then boiled for 10 min under pressure
in citrate buffer for antigen retrieval. Nonspecific binding was
blocked with 5% goat serum in TBS for 15 min and the tissues were
incubated with HLEAFab and S12 antibodies (cat. 559898; Becton
Dickinson Medical Devices Co. Ltd, Franklin Lakes, NJ, USA) in TBS
containing 1% bovine serum albumin for 60 min. The sections were
then washed with TBS and incubated with goat Anti-Human IgG (Fab
specific)-peroxidase (Sigma, St. Louis, MO, USA; Cat. No. A0293;
1:100) and EnVision goat anti-mouse IgG peroxidase antibody
(EB-2305, ZhongShan, Godbridge, China; 1:200) for 60 min. The color
was developed in diaminobenzidine solution and counterstained with
Mayer’s hematoxylin.
Preparation of DIG-labeled probes and
ISH
LMP1 probes were prepared under the following
conditions: i) DNA was extracted from a 3-mm thick section based on
published protocols for formalin-fixed, paraffin-embedded tissues
(9). Sections were digested with
proteinase K (Roche Molecular Biochemicals, Mannheim, Germany). The
samples were then centrifuged to pellet the cell debris and the
supernatant was used for PCR amplification. The PCR template was
performed with primers 1 and 2 (primer 1, 5′-AGAAACACGCGTTACT
CTGACG-3′; primer 2, 5′-ACAATGCCTGTCCGTGCAAAT TCC-3′) using a PCR
instrument (MJ Research Inc., St. Bruno, QC, Canada). Amplification
was performed with 30 cycles at 90°C for 1 min, 55°C for 1 min and
72°C for 1 min and the final extension was at 72°C for 15 min. The
PCR product, which was the first exon of the LMP1 gene (BNLF1), was
analyzed on an ethidium bromide-stained 1.5% agarose gel. The
positive control for the PCR reactions contained the fragment of
the plasmid DNA BNLF1 in double distilled water for disinfection
(10). ii) Digoxin-labeled probe:
The PCR-positive product was used as a template for PCR DIG
Labeling Mix (Roche Molecular Biochemicals; Cat. No. 11585550910)
and primers 1 and 2 were synthesized by PCR probes according to
product instructions, followed by ultrafiltration unit (Millipore)
centrifugation to remove free dNTP and the purified probe. iii) ISH
was performed with the LMP1 gene in all cases. Sections
(5-μm) were deparaffinized prior to the RNA enzyme treatment
to remove RNA and then placed in pretreatment solution at 95°C for
10 min, followed by 15 min cooling at room temperature. Sections
were then washed twice with wash buffer for 3 min. After being
blocked with 3% H2O2 and methanol for 30 min
and digested by proteinase K for 20 min with thorough rinsing, the
sections were post-fixed in 4% cold PFA-PBS for 15 min. The
sections were then rinsed with PBS and distilled water,
pre-hybridized for 4 h and hybridized with 100 μl of
digoxigenin-labelled LMP1 probe for 18 h at 42°C. Following
thorough rinsing with SSC and incubation in blocking solution, the
slides were processed with mouse anti-digoxin-biotin antibody,
SABC-POD and streptavidin-HRP at 37°C, in sequence. At each step,
the slides were rinsed three times with PBS. Finally, the slides
were colored with DAB and counterstained with hematoxylin (11).
Statistical analysis
SPSS 18.0 (SPSS, Inc.) was used for all statistical
analyses. The Pearson χ2 test was used to compare
discrete variables. P≤0.05 was considered to indicate statistically
significant differences for all comparisons.
Results
Tumor characteristics
The 36 NPC samples were subjected to IHC and ISH
analyses. Among the cases, there were 16 females and 20 males. The
mean age was 61.58 years (range, 42–78 years). Of the 36 cases, 2
were squamous cell carcinoma (5.5%), 11 were non-keratinizing
tumors (30.6%) and the remaining 23 cases (64%) were
undifferentiated.
IHC
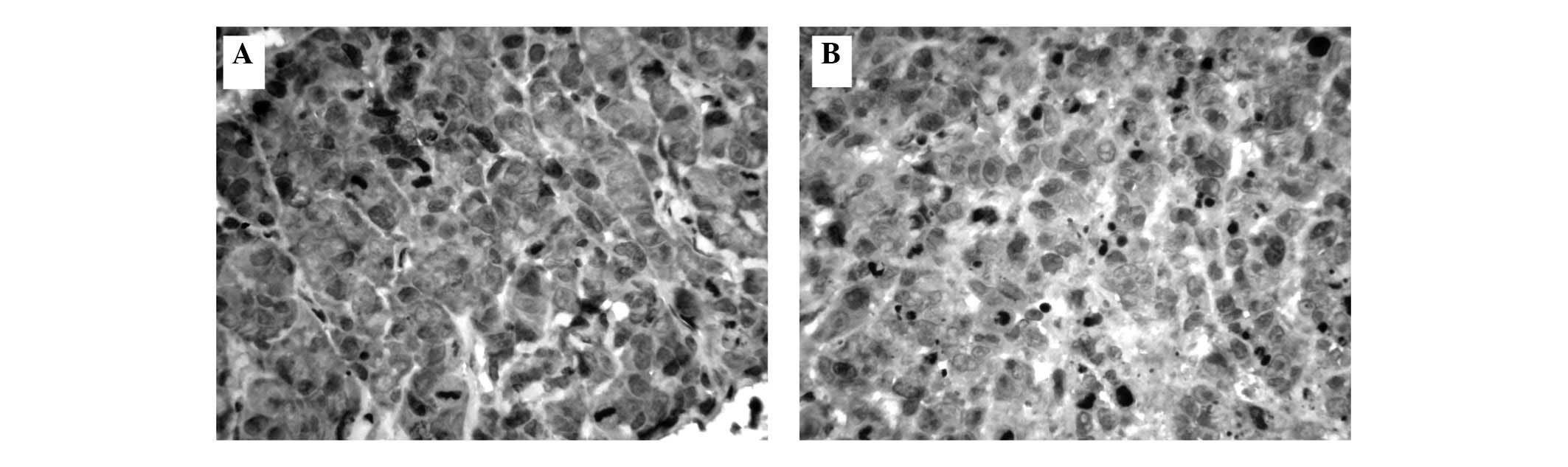
Positive staining with HLEAFab or S12 was observed
in the NPC cell membranes and cytoplasm (Fig. 1). LMP1 expression was observed in
20/36 (55.6%) cases in the HLEAFab group and 22/36 (61.1%) cases in
the S12 group. Similarly, LMP1 expression was detected in 2
squamous cell carcinoma cases, 7/11 cases of non-keratinizing
tumors and 13/23 cases of undifferentiated tumors in the HLEAFab
group, compared with the same 2 squamous cell carcinoma cases, 6/11
cases of non-keratinizing tumors and 16/23 cases of
undifferentiated tumors in the S12 group (Table I). Positive staining for LMP1 was
not significantly different between the HLEAFab and S12 groups
(P>0.05). Staining was entirely absent with the unrelated
antibody Fab fragment as the negative control antibody.
 | Table IComparison of LMP1 expression by IHC
with HLEAFab and S12. |
Table I
Comparison of LMP1 expression by IHC
with HLEAFab and S12.
| HLEAFab
| S12
|
|---|
| Negative | Positive | Total | Negative | Positive | Total |
|---|
| Squamous cell
carcinoma | 2 | 0 | 2 | 2 | 0 | 2 |
| Non-keratinizing | 4 | 7 | 11 | 5 | 6 | 11 |
| Undifferentiated | 10 | 13 | 23 | 7 | 16 | 23 |
| Total | 16 | 20 | 36 | 14 | 22 | 36 |
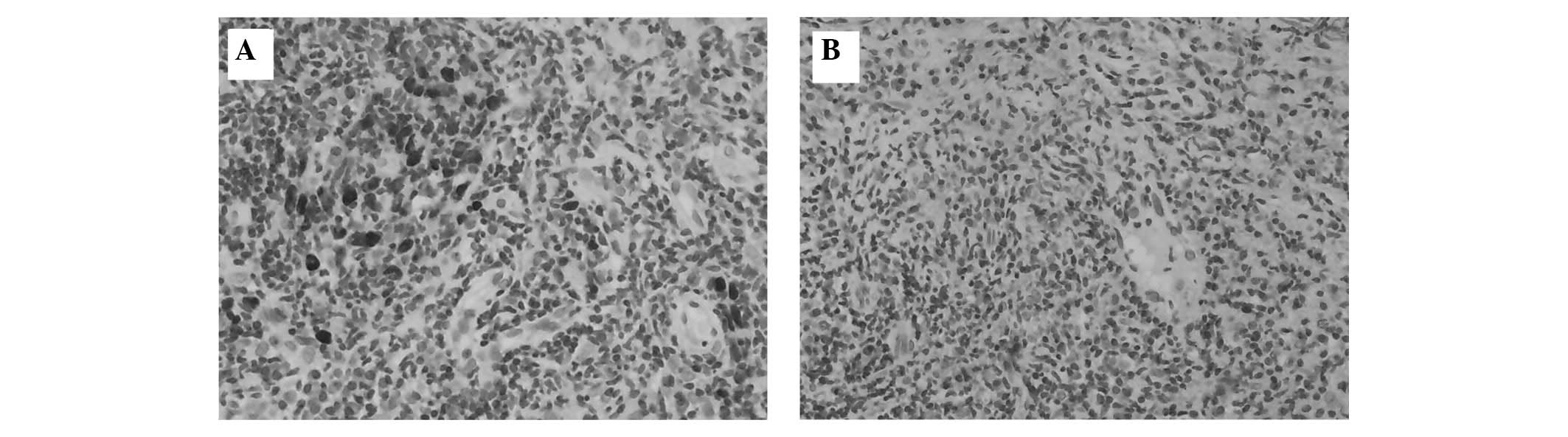
ISH
The in situ detection of BNLF1 using an LMP1
probe in NPC sections is shown in Fig.
2. BNLF1 was observed in 19/36 NPC cases (52.8%). Among all the
positive cases, 16/20 (80%) were amplified with positive IHC
staining in the HLEAFab group, while 3/16 (19%) were amplified with
negative IHC staining. By comparison, 17/22 (77%) cases were
amplified with positive IHC staining in the S12 group, while 2/14
(14%) were amplified with negative IHC staining. There was only 1
case with negative IHC staining in the HLEAFab group but positive
IHC staining in the S12 group (Table
II). The sensitivity of the amplification was 84.2% for the
IHC-positive cases in the HLEAFab group and 89.5% in the S12 group.
The specificity was 76.5% for the IHC-positive cases in the HLEAFab
group and 70.6% in the S12 group. The ISH results of the 36 samples
were consistent with the IHC data (P>0.05).
 | Table IIComparison of LMP1 expression by ISH
with HLEAFab and S12. |
Table II
Comparison of LMP1 expression by ISH
with HLEAFab and S12.
| Negative | Positive | Total |
|---|
| HLEAFab (−) | 13 (81%) | 3 (19%) | 16 |
| HLEAFab (+) | 4 (20%) | 16 (80%) | 20 |
| S12 (−) | 12 (86%) | 2 (14%) | 14 |
| S12 (+) | 5 (23%) | 17 (77%) | 22 |
| Total | 17 | 19 | 36 |
Discussion
LMP1, which is encoded by EBV, is significantly
expressed in several EBV-associated malignancies, particularly NPC.
LMP1 is an oncoprotein which acts by constitutive activation of
various signaling pathways and induces the promotion of cell growth
and inhibition of apoptosis in a variety of cell types in
vitro(12). In addition, LMP1
has been demonstrated to contribute to B cell and epithelial cell
tumourigenesis in vivo in 4 transgenic mice (13–15).
These unique characteristics of LMP1 lead to the suggestion that
LMP1 may be a good therapeutic target in the treatment of
EBV-associated carcinomas. Numerous studies have shown that certain
antibodies are able to target LMP1 successfully by binding to its
intracellular activating regions (16–18).
In our previous study, HLEAFab was a humanized antibody fragment
Fab directed against LMP1, targeting the extramembrane domains
(EMD) of the polypeptide.
In the present study, IHC was used for detection of
LMP1 with HLEAFab and S12 antibodies. Positive staining of LMP1 was
observed in the membrane and cytoplasm of NPC cells. LMP1 staining
was detected in 55.6% cases in the HLEAFab group compared with
61.1% cases in the S12 group. LMP1 staining was not significantly
different between the HLEAFab and S12 groups (P>0.05). BNLF1 was
observed in 19/36 NPC cases (52.8%). The sensitivity for
amplification was 84.2% for the HLEAFab group and 89.5% for the S12
group with positive IHC cases. The specificity was 76.5% for
HLEAFab-positive IHC and 70.6% for S12-positive IHC. The results
showed that the antibody fragment HLEAFab had an excellent
correlation with S12 in IHC-positive cases and ISH analysis yielded
identical results in 36 samples.
This poor immunogenicity, particularly due to the
short extracellular structure of LMP1, may explain why IHC
exhibited different results for EBV-LMP1 with regard to the
reactivity of HLEAFab and S12. HLEAFab, a humanized anti-LMP1 EMD
Fab fragment, is able to bind to the extra-cellular domains of
LMP1. LMP-specific mAb S12 and other monoclonal antibodies are
known to recognize large intracellular regions of LMP1 for ligand
binding. Consequently, LMP1 expression in the present study was
shown to be identical when using HLEAFab or S12 in the IHC
method.
The lack of detectable BNLF amplification in 4 cases
of IHC-positive staining in the HLEAFab group and 5 cases in the
S12 group, as well as BNLF amplification in 3 cases of negative
staining in the HLEAFab group and 3 cases in the S12 group,
indicates that LMP1 expression is not required for the maintenance
or regulation of latent EBV infection in epithelial cells and thus
BNLF1 fragments may be lost following EBV-induced cell
transformation in the cell differentiation and proliferation
process. Modulation of LMP1 expression is affected by a number of
regulatory gene factors, as well as the heterogeneity of host cells
(19,20). These lead to the positive expression
of BNLF1 fragment without LMP1 expression in certain cases, as
observed in the present study.
The search for targeted therapies for NPC has been
continuing. As the number of studies is growing, standardization of
all steps of the testing process from tissue fixation to final
interpretation is likely to ensure accurate identification of
patients who may benefit from specific therapies. Preclinical
trials with the antibody Fab fragments are ongoing to broaden the
spectrum of possibilities of targeted approaches to the
personalized treatment of NPC.
Acknowledgements
The present study was supported by the
grants from the Youth Funds of the Second Affiliated Hospital of
Nanjing Medical University (No. QN201004) and Technology Support
Program of Jiangsu (No. BE2009152).
References
|
1
|
Chen R, Zhang D, Mao Y, Zhu J, Ming H, Wen
J, Ma J, Cao Q, Lin H, Tang Q, Liang J and Feng Z: A human
Fab-based immunoconjugate specific for the LMP1 extracellular
domain inhibits nasopharyngeal carcinoma growth in vitro and
in vivo. Mol Cancer Ther. 11:594–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young LS, Dawson CW, Clark D, Rupani H,
Busson P, Tursz T, Johnson A and Rickinson AB: Epstein-Barr virus
gene expression in nasopharyngeal carcinoma. J Gen Virol.
69:1051–1065. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brooks L, Yao QY, Rickinson AB and Young
LS: Epstein-Barr virus latent gene transcription in nasopharyngeal
carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts.
J Virol. 66:2689–2697. 1992.PubMed/NCBI
|
|
4
|
Jiwa NM, Oudejans JJ, Dukers DF, Vos W,
Horstman A, van der Valk P, Middledorp JM, Walboomers JM and Meijer
CJ: Immunohistochemical demonstration of different latent membrane
protein-1 epitopes of Epstein-Barr virus in lymphoproliferative
diseases. J Clin Pathol. 48:438–442. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnes L, Eveson JW, Reichart P and
Sidransky D; World Health Organization Classification of Tumours:
Pathology and Genetics of Head and Neck Tumours. IARC Press; Lyon:
pp. 81–105. 2005
|
|
6
|
Mao Y, Zhang DW, Wen J, Cao Q, Chen RJ,
Zhu J and Feng ZQ: A novel LMP1 antibody synergizes with mitomycin
C to inhibit nasopharyngeal carcinoma growth in vivo through
inducing apoptosis and downregulating vascular endothelial growth
factor. Int J Mol Sci. 13:2208–2218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao Y, Zhang DW, Zhu H, Lin H, Xiong L,
Cao Q, Liu Y, Li QD, Xu JR, Xu LF and Chen RJ: LMP1 and LMP2A are
potential prognostic markers of extranodal NK/T-cell lymphoma,
nasal type (ENKTL). Diagn Pathol. 7:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao Y, Zhang DW, Lin H, Xiong L, Liu Y, Li
QD, Ma J, Cao Q, Chen RJ, Zhu J and Feng ZQ: Alpha B-crystallin is
a new prognostic marker for laryngeal squamous cell carcinoma. J
Exp Clin Cancer Res. 31:1012012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan LX, Diss TC, Peng HZ and Isaacson PG:
Clonality analysis of defined B-cell populations in archival tissue
sections using microdissection and the polymerase chain reaction.
Histopathology. 24:323–327. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng W, Zhou M and Lin H: The significance
of detecting Epstein-Barr virus BNLF1 fragment and its expression
in Hodgkin’s disease in the Guangdong area. Zhonghua Bing Li Xue Za
Zhi. 26:27–30. 1997.In Chinese.
|
|
11
|
QingLing Z, LiNa Y, Li L, Shuang W, YuFang
Y, Yi D, Divakaran J, Xin L and YanQing D: LMP1 antagonizes
WNT/β-catenin signalling through inhibition of WTX and promotes
nasopharyngeal dysplasia but not tumourigenesis in LMP1 (B95-8)
transgenic mice. J Pathol. 223:574–583. 2011.PubMed/NCBI
|
|
12
|
Kieff E and Rickinson AB: Epstein-Barr
virus and its replication. Field’s Virology. Knipe M and Howley PM:
4th edition. Lippincott Williams & Wilkins; Philadelphia, PA:
pp. 2511–2627. 2001
|
|
13
|
Wilson JB, Weinberg W, Johnson R, Yuspa S
and Levine AJ: Expression of the BNLF-1 oncogene of Epstein-Barr
virus in the skin of transgenic mice induces hyperplasia and
aberrant expression of keratin 6. Cell. 61:1315–1327. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kulwichit W, Edwards RH, Davenport EM,
Baskar JF, Godfrey V and Raab-Traub N: Expression of the
Epstein-Barr virus latent membrane protein 1 induces B cell
lymphoma in transgenic mice. Proc Natl Acad Sci USA.
95:11963–11968. 1998. View Article : Google Scholar
|
|
15
|
Stevenson D, Charalambous C and Wilson JB:
Epstein-Barr virus latent membrane protein 1 (CAO) up-regulates
VEGF and TGF alpha concomitant with hyperlasia, with subsequent
up-regulation of p16 and MMP9. Cancer Res. 65:8826–8835. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gennari F, Mehta S, Wang Y, St Clair
Tallarico A, Palu G and Marasco WA: Direct phage to intrabody
screening (DPIS): demonstration by isolation of cytosolic
intrabodies against the TES1 site of Epstein Barr virus latent
membrane protein 1 (LMP1) that block NF-kappaB transactivation. J
Mol Biol. 335:193–207. 2004. View Article : Google Scholar
|
|
17
|
Fang CY, Chang YS, Chow KP, Yu JS and
Chang HY: Construction and characterization of monoclonal
antibodies specific to Epstein-Barr virus latent membrane protein
1. J Immunol Methods. 287:21–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piché A, Kasono K, Johanning F, Curiel TJ
and Curiel DT: Phenotypic knock-out of the latent membrane protein
1 of Epstein-Barr virus by an intracellular single-chain antibody.
Gene Ther. 5:1171–1179. 1998.PubMed/NCBI
|
|
19
|
Salamon D, Adori M, Ujvari D, Wu L, Kis
LL, Madapura HS, Nagy N, Klein G and Klein E: Latency
type-dependent modulation of Epstein-Barr virus-encoded latent
membrane protein 1 expression by type I interferons in B cells. J
Virol. 86:4701–4707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ning S, Hahn AM, Huye LE and Pagano JS:
Interferon regulatory factor 7 regulates expression of Epstein-Barr
virus latent membrane protein 1: a regulatory circuit. J Virol.
77:9359–9368. 2003. View Article : Google Scholar : PubMed/NCBI
|