Introduction
In the 19th century, Rudolf Virchow observed that
there were numerous leukocytes present within tumors, thus
providing the first indication of an association between
inflammation and cancer (1). More
recently, inflammation has been shown to be a critical component of
tumor progression (2). Furthermore,
numerous cancer types have been observed to arise from sites of
infection and inflammation (3). The
development of cancer from inflammation may be a process driven by
inflammatory cells, as well as a variety of chemical mediators
(2–5). Inflammatory cells and cytokines
establish a tumor inflammatory microenvironment, which is an
essential component of all tumors and is involved in tumor
progression by promoting proliferation, survival, immune evasion
and migration (2,6,7). The
immune cells and molecules secreted by these cells within the tumor
microenvironment have decisive dual roles in anti-tumor immunity
and immune evasion (8–10). Although this inflammatory response
may suppress tumors, it may also facilitate cancer development and
evasion via multiple signaling pathways (10,11).
The association between inflammation and cancer has provided a new
target for tumor biotherapy (12–14).
Programmed cell death-1 ligand 1 (PD-L1), also known
as B7-H1, is a cell surface protein of the B7 family (15). Upregulation of PD-L1 in cancer cells
has been observed in a variety of solid tumors, but not in normal
tissue (16–19). The interaction between PD-L1 on
cancer cells and programmed cell death-1 (PD-1) on immune cells has
been shown to suppress activation and proliferation and induce
apoptosis in the immune cells. Blocking the interaction of PD-L1
with PD-1 or the downregulation of PD-L1 surface expression in
cancer cells promotes host antitumor immunity and inhibits the
growth of tumor cells (20–23).
The mechanism by which PD-L1 expression is
stimulated on the surface of tumor cells is not well understood. A
previous study showed that interferon-γ (IFN-γ) is a potent
stimulator of PD-L1 surface expression in various types of tumor
cells in a time- and dose-dependent manner (24). In the present study, it was observed
that the surface expression of PD-L1 on Tca8113 oral squamous
carcinoma cells was increased by co-culture with activated immune
cells, as well as by the major inflammatory cytokines that are
secreted by immune cells following activation by antigens. These
results provide evidence that inflammation promotes tumor evasion
from the immune, suggesting that anti-inflammatory agents may offer
new possibilities for cancer therapy.
Material and methods
Animals
Nude mice (body weight, 23–25 g) were purchased from
the Animal Center of the Chinese Academy of Sciences (Shanghai,
China) and housed in a specific pathogen-free (SPF) laminar flow
room with a constant temperature (25–27°C) and humidity (40–50%).
All experiments and animal care procedures were approved by the
Animal Center of Sichuan University. The study was approved by the
Ethics Committee of the State Key Laboratory of Oral Diseases,
Sichuan University, Chengdu, Sichuan, China.
Reagents
Phycoerythrin (PE)-labeled anti-human PD-L1,
allophycocyanin (APC)-labeled anti-human CD8 and mouse IgG isotype
control antibodies were obtained from eBioscience (San Diego, CA,
USA). Recombinant human IFN-γ, interleukins (IL)-1, -2 and -6 and
tumor necrosis factor-α (TNF-α) were purchased from R&D Systems
(Minneapolis, MN, USA). The human tongue squamous carcinoma cell
line, Tca8113, was obtained from the State Key Laboratory of Oral
Diseases (Chengdu, China). The Annexin-V/FITC/Propidium Iodide (PI)
Apoptosis Assay kit was purchased from Invitrogen (Carlsbad, CA,
USA). Phytohemagglutinin (PHA) was purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Cell culture
The Tca8113 cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), 100 IU/ml
penicillin, 100 μg/ml streptomycin, 3% L-glutamine and 7.5%
sodium bicarbonate (Gibco Life Technologies, Carlsbad, CA, USA).
The cells were maintained as monolayers in 25-cm2
plastic tissue culture flasks at 37°C in a humidified atmosphere
with 5% CO2. Exponentially growing cells were used in
all experiments.
Proliferation assay
The Tca8113 cells were seeded at a density of
4×104 cells per well in 96-well plates. The cells were
grown overnight and the medium was replaced with maintenance medium
containing the desired concentrations of cytokines or medium. Cell
viability was assessed subsequent to 72 h using the
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) colorimetric assay.
Flow cytometry (FCM)
The pretreated Tca8113 cells were harvested and
washed twice with FCM buffer (PBS with 5% FBS and 0.1%
NaN3). Following incubation with PE-anti-human PD-L1 or
isotype control antibodies for 30 min at 4°C, the cells were
analyzed using a Beckman Coulter FC500 with Submit 5.2 software
(Beckman Coulter, Miami, FL, USA).
Cell cycle and apoptosis analysis
The analysis of the cell cycle distributions and the
measurements of the percentage of apoptotic cells were performed by
FCM. The Tca8113 cells were treated with the desired concentrations
of cytokines or medium. Following treatment, the floating cells in
the medium were combined with the attached cells collected by
trypsinization. The cell cycle distribution and levels of apoptosis
were analyzed using the Annexin V-FITC/PI Apoptosis kit
(Invitrogen) and a Beckman Coulter FC500 within 1 h of staining.
Cell cycle histograms were analyzed using Submit 5.2 and MultiCycle
software. The percentages of apoptotic cells were analyzed using
Submit 5.2 software.
Preparation of activated T cells and
supernatant
Briefly, peripheral blood mononuclear cells (PBMCs)
from healthy human donors were isolated using Lymphoprep density
gradient centrifugation. The PBMCs were plated at a density of
1×107 cells per well in 6-well plates and stimulated
with Tca8113 cell lysate or PHA. The tumor antigen-activated
lymphoblasts were isolated by gradient centrifugation following
stimulation with Tca8113 cell lysate for 7 days in culture medium
(CM; RPMI-1640 medium containing 10% FBS, 100 IU/ml penicillin, 100
μg/ml streptomycin and 1,000 IU rhIL-2). Following PHA
stimulation for three days, the supernatants were collected and
stored at −80°C as PHA-supernatant (PHA-sup).
Analysis of the surface expression of
PD-L1 on the Tca8113 cells
The Tca8113 cells (4×105) were
co-cultured with 1×106 PBMCs per well in 6-well plates.
The cells were grown overnight and the PHA, the PHA-supernatant,
the desired concentrations of cytokines and 20 μg/ml
anti-cytokine antibodies were added to the corresponding cells. All
cells were collected by trypsinization, stained with PE-anti-PD-L1
antibody and analyzed with FCM at day 3.
The Tca8113 cells were pre-labeled with
carboxyfluorescein diacetate succinimidyl ester (CFSE;
Invitrogen-Molecular Probes, Eugene, OR, USA). Briefly, the cells
were washed and resuspended at a density of 5×106
cells/ml in serum-free RPMI-1640, then incubated with a final
concentration of 10 μM CFSE at 37°C for 10 min with gentle
agitation, washed twice with RPMI-1640 supplemented with 10% FBS
and resuspended in RPMI-1640 supplemented with 10% FBS. The Tca8113
cells (4×105) were co-cultured or separated by transwell
inserts with 1×106 PBMCs per well in 24-well plates,
with or without 100 μg/ml PHA, for 72 h. Following
treatment, the attached cells were collected by trypsinization,
stained with PE-anti-PD-L1 antibody and analyzed with FCM.
CD8+ T cell in vitro
apoptosis assay
The activated T cells were then harvested and
co-cultured with the Tca8113 cells that had been pretreated with
inflammatory cytokines or PHA-supernatant for 18 h. Anti-PD-L1
blocking antibody (10 μg/ml) was added into the indicated
wells to block PD-1/PD-L1 interactions. All cells were then
harvested and stained with APC-anti-CD8 antibodies and annexin
V-FITC/PI. The apoptosis of the CD8+ T cells was
calculated as the percentage of annexin
V+/PI+ cells that were first gated on the
CD8+ T cell population.
In vivo immune evasion of Tca8113 cells
pretreated with PHA
The Tca8113 cells were pretreated with
PHA-supernatant for 72 h. Tumor cells (2×107) were
injected subcutaneously into nude mice to establish a subcutaneous
xenotransplantation tumor model. Untreated control Tca8113 cells
were injected around the neck and the PHA-supernatant pretreated
Tca8113 cells were injected posteriorly. Tca8113 antigen-specific T
cells (5×107) or PBS as a control were injected via the
tail vein at 0, 3 and 7 days subsequent to the tumor cells being
injected. All the mice were sacrificed on the 21st day
post-transplantation. Images of the tumors were captured, prior to
the tumors being removed and weighed
Statistical analysis
All values were expressed as the mean ± SEM. The
data were analyzed by a one-way analysis of variance (ANOVA)
followed by the Bonferroni test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PHA-activated immune cells promote
expression of PD-L1 on Tca8113 tumor cells
PHA is a plant lectin, which acts as a mitogen to
trigger the activation and cell division of T lymphocytes. The
supernatant of the PHA-stimulated PBMCs contains a number of
inflammatory cytokines, which are present in the inflammatory
microenvironment in the body (25).
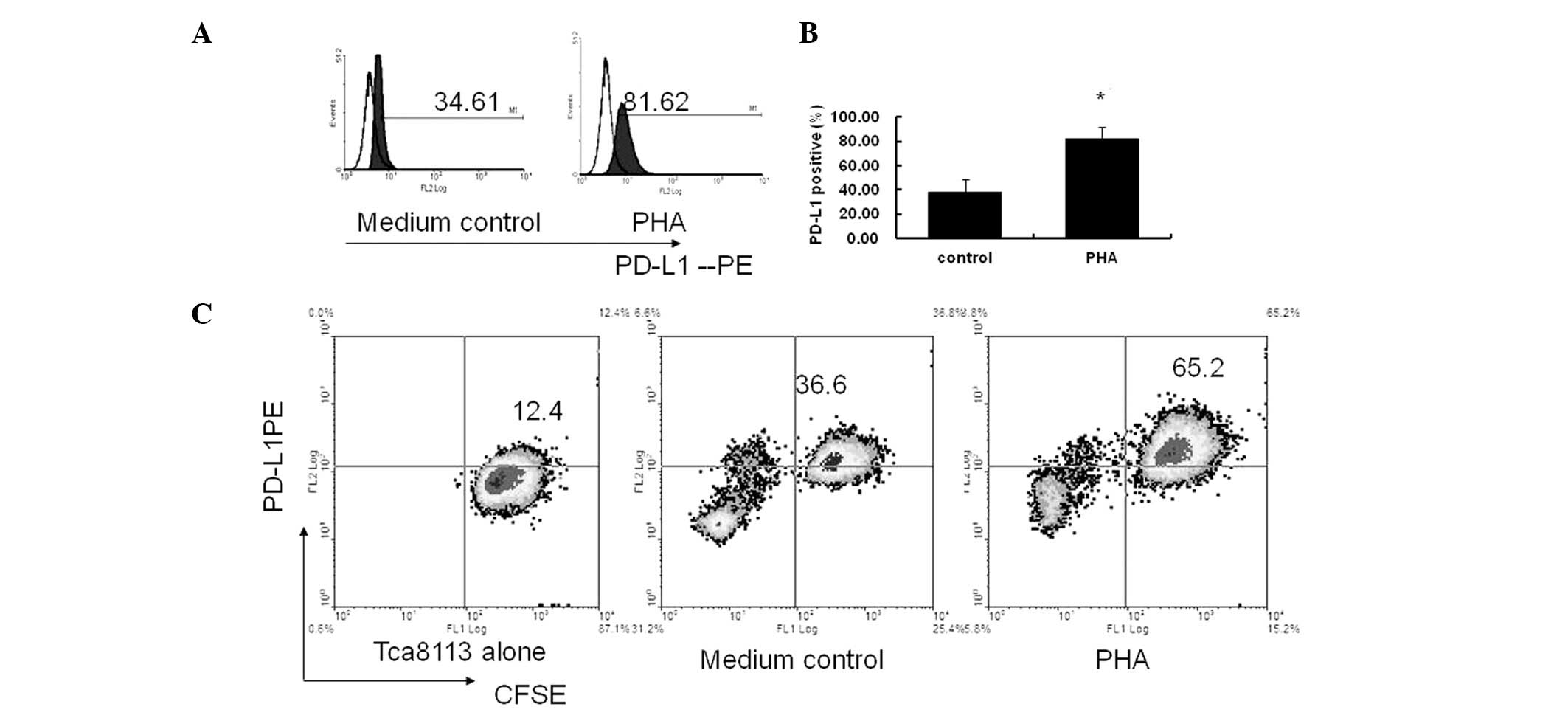
In the present study, when the PBMCs were co-cultured with the
Tca8113 cells and stimulated with PHA, the expression of PD-L1 by
the Tca8113 cells was significantly increased compared with the
cells cultured in medium alone (P= 0.006) (Fig. 1A and B).
In order to demonstrate that the observed PD-L1 was
expressed primarily on the Tca8113 cells in the co-cultured system,
the Tca8113 cells were pre-labeled with CFSE and co-cultured with
PBMC again. Fig. 1 shows that there
was no decrease in CFSE following 72 h in culture (Fig. 1C, Tca8113 alone). The percentage of
the CFSE+PD-L1+ cells was significantly
increased following stimulation with PHA compared with the control
cells cultured in medium only (P= 0.006) (Fig. 1C, medium control and PHA). These
results indicate that the PD-L1 was primarily expressed on the
Tca8113 cells and not on the PBMCs. Only PHA-activated, but not
resting, immune cells promoted the significant expression of PD-L1
on the Tca8113 cells.
PHA-activated immune cells promote
expression of PD-L1 on tumor cells via secreted inflammatory
cytokines, but not by cell-cell contact
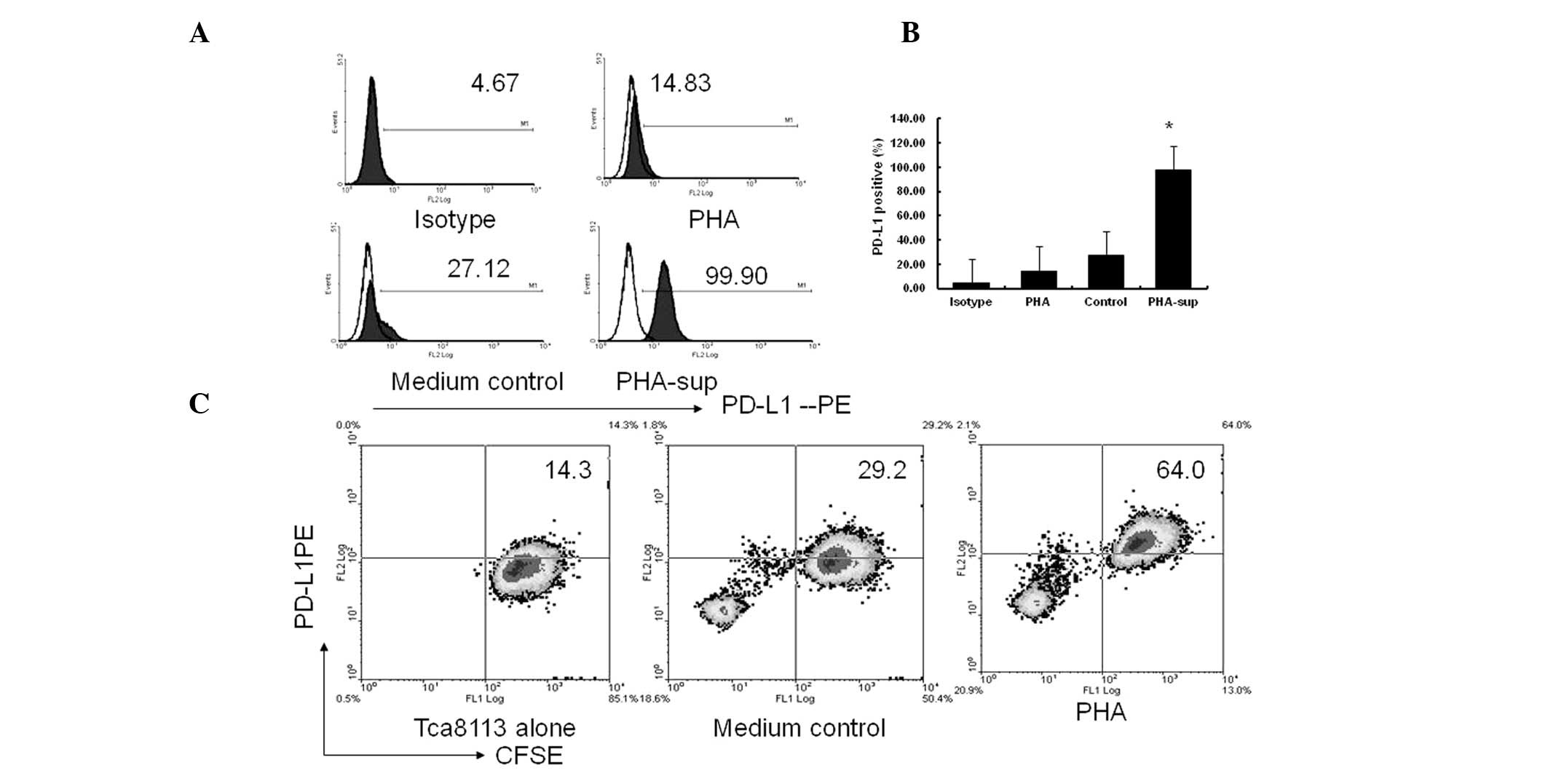
PHA had no direct effect on the surface expression
of PD-L1 when using the Tca8113 cells alone. However, the surface
expression of PD-L1 on the Tca8113 cells was significantly
increased following exposure to PHA-supernatant for 72 h (P= 0.001)
(Fig. 2A and 2B). Similar to the PHA-supernatant, the
CFSE+PD-L1+ cell (PD-L1+Tca8113)
subset was significantly increased in the transwell culture system.
However, no significant differences were observed when comparing
the co-culture system with the transwell system (P=0.125) (Fig. 1C vs. Fig. 2C). These results indicate that
activated immune cells significantly promote PD-L1 expression on
Tca8113 cells via the use of secreted inflammatory cytokines,
instead of cell-cell contact.
IL-1α, IL-6, TNF-α and IFN-γ are the
major inflammatory cytokines that promote PD-L1 expression on tumor
cells
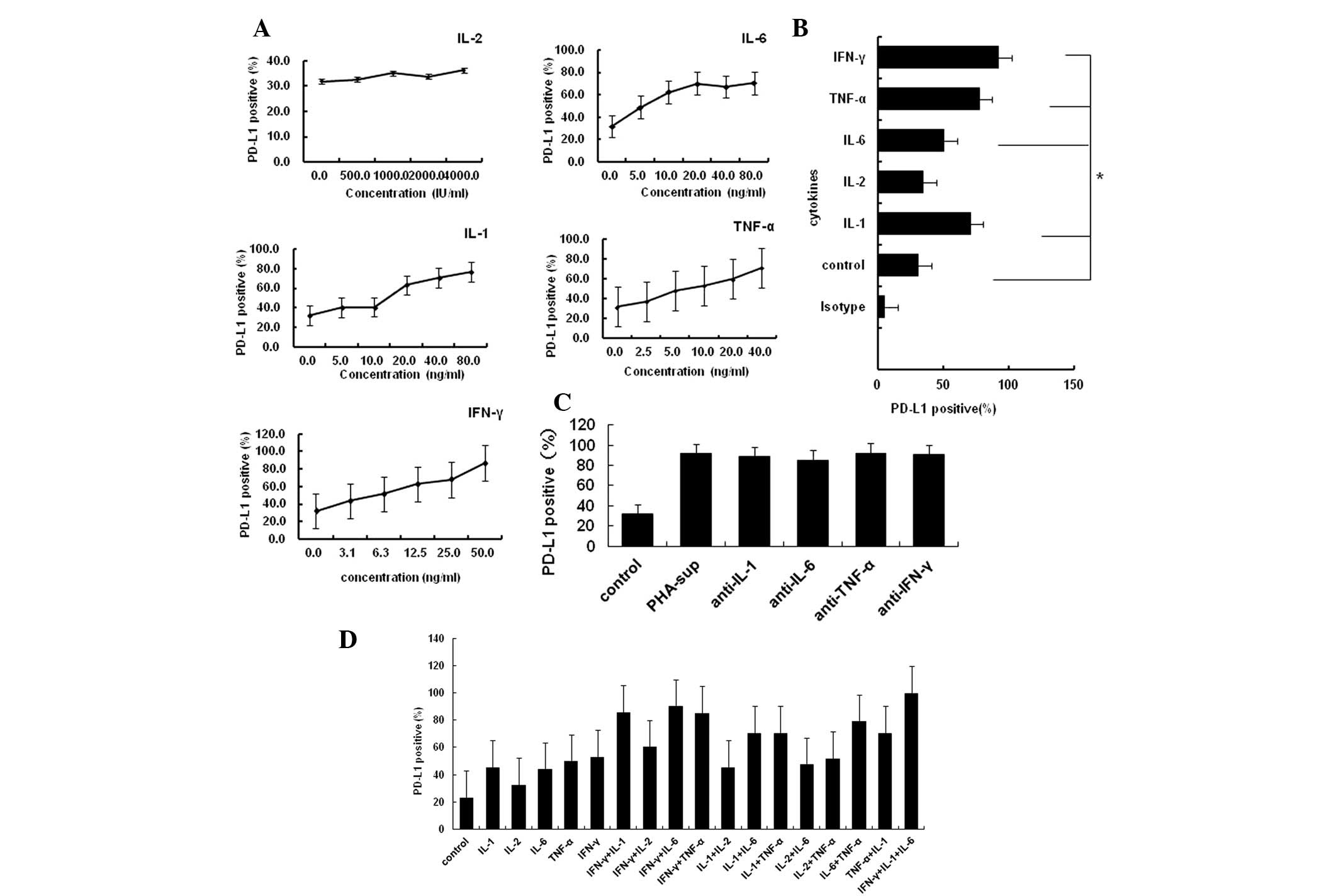
As IL-1α, IL-2, IL-6, TNF-α and IFN-γ are the major
inflammatory cytokines secreted by immune cells activated by PHA,
they are also located in the tumor microenvironment (25). Next, the present study examined
whether these cytokines induced the expression of PD-L1 on tumor
cells. The Tca8113 cells were exposed specifically to each of these
cytokines at a desired concentration. The majority of these
inflammatory cytokines induced PD-L1 expression on the Tca8113
cells in a dose-dependent manner, with the exception of IL-2
(Fig. 3A and B). However, 20
μg/ml anti-cytokine antibodies against these cytokines did
not block the expression of PD-L1 induced by PHA-supernatant (all
P<0.05) (Fig. 3C). The majority
of these cytokines had an additive effect with each other to
promote the expression of PD-L1 (Fig.
3D). These results suggest that the inflammatory
cytokine-induced surface expression of PD-L1 on Tca8113 cells is a
result of multiple stimulatory factors.
Effect of inflammatory cytokines on
proliferation or apoptosis of tumor cells
Previous studies have shown that the majority of
inflammatory cytokines promote the proliferation of tumor cells
(26). However, the results of the
present study showed that 10 ng/ml of IL-1α, IL-2, IL-6, TNF-α and
IFN-γ, the same concentration which was used to induce PD-L1
expression, had no significant effect on the proliferation of the
Tca8113 cells (Fig. 4A) or the
progression of the cell cycle (Fig.
4B). With the exception of TNF-α, all the cytokines
investigated were also unable to induce apoptosis of the tumor
cells (all P<0.05) (Fig.
4C).
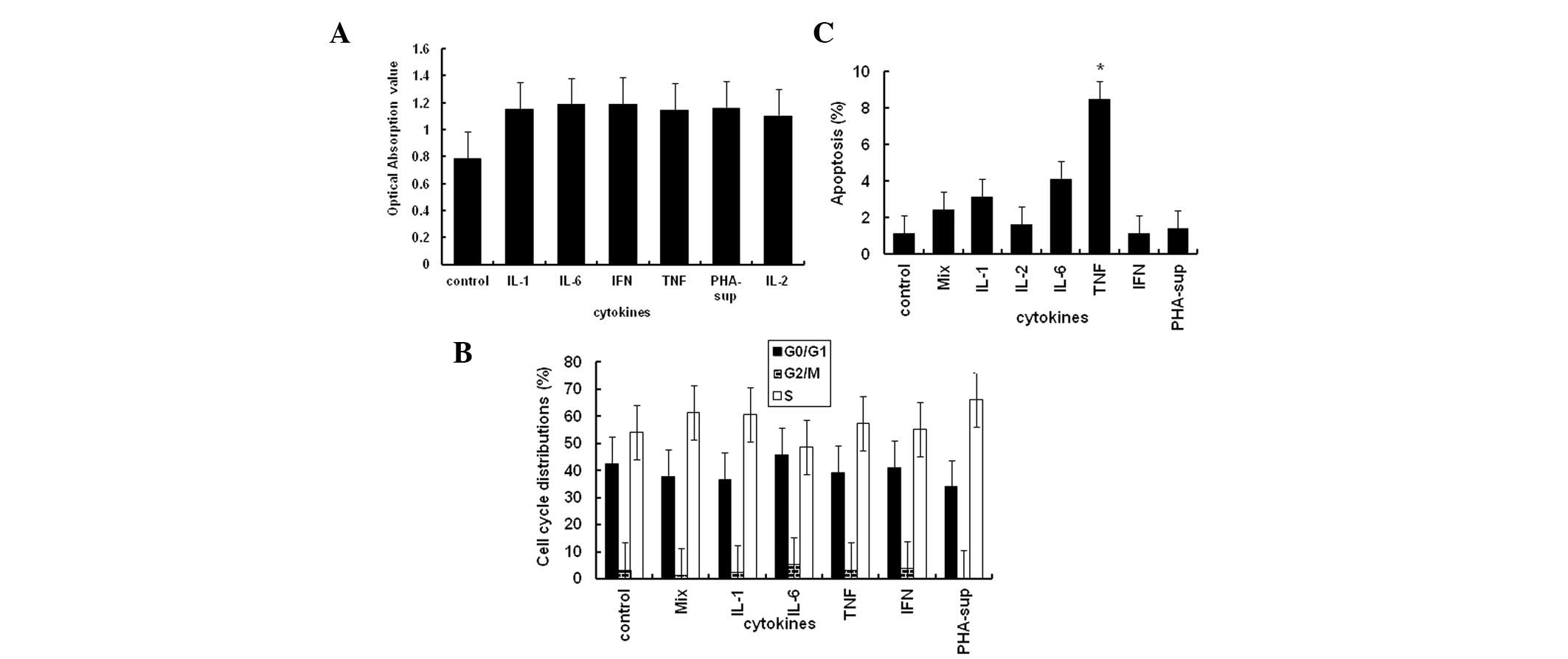 | Figure 4Effect of inflammatory cytokines on
Tca8113 cell proliferation, apoptosis and cell cycle progression.
(A) Cell viability was assessed using the MTT colorimetric assay at
72 h. (B) Tca8113 cells were collected by trypsinization and
stained with PI. The percentages of cells undergoing apoptosis were
analyzed on a Beckman Coulter FC500 with Submit 5.2 software. (C)
The Tca8113 cells were collected by trypsinization and stained
using an apoptosis assay kit. Cell cycle progression was analyzed
on a Beckman Coulter FC500 with MultiCycle software. The results
are representative of three independent experiments. *
indicates values significantly increased compared with untreated
control. PD-L1, programmed cell death-1 ligand 1; PHA,
phytohemagglutinin; MTT,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide;
PI, propidium iodide; IFN-γ, interferon-γ; TNF-α, tumor necrosis
factor-α; IL, interleukin; sup, supernatant. |
Inflammatory cytokines promote expression
of PD-L1 on Tca8113 cells and induce the apoptosis of tumor
antigen-specific T cells
As the PD-L1/PD-1 pathway is known to inhibit
antitumor T cell-mediated immune responses, tumor antigen-specific
T cells were co-cultured with the Tca8113 cells pretreated with
inflammatory cytokines or PHA-supernatant. Anti-PD-L1 blocking
antibody was added to demonstrate that the observed apoptosis was
occurring via the PD-1/PD-L1 pathway. The percentage of
CD8+ T cells undergoing apoptosis significantly
increased subsequent to the co-culture with the Tca8113 cells
pretreated with inflammatory cytokines (all P<0.05) (Fig. 5A and B). The apoptosis of the
CD8+ T cells was significantly decreased following the
addition of anti-PD-L1 to block the PD-1/PD-L1 pathway (all
P<0.05) (Fig. 5B). These results
suggest that inflammatory cytokines induce tumor cells to express
PD-L1 to evade immune attraction.
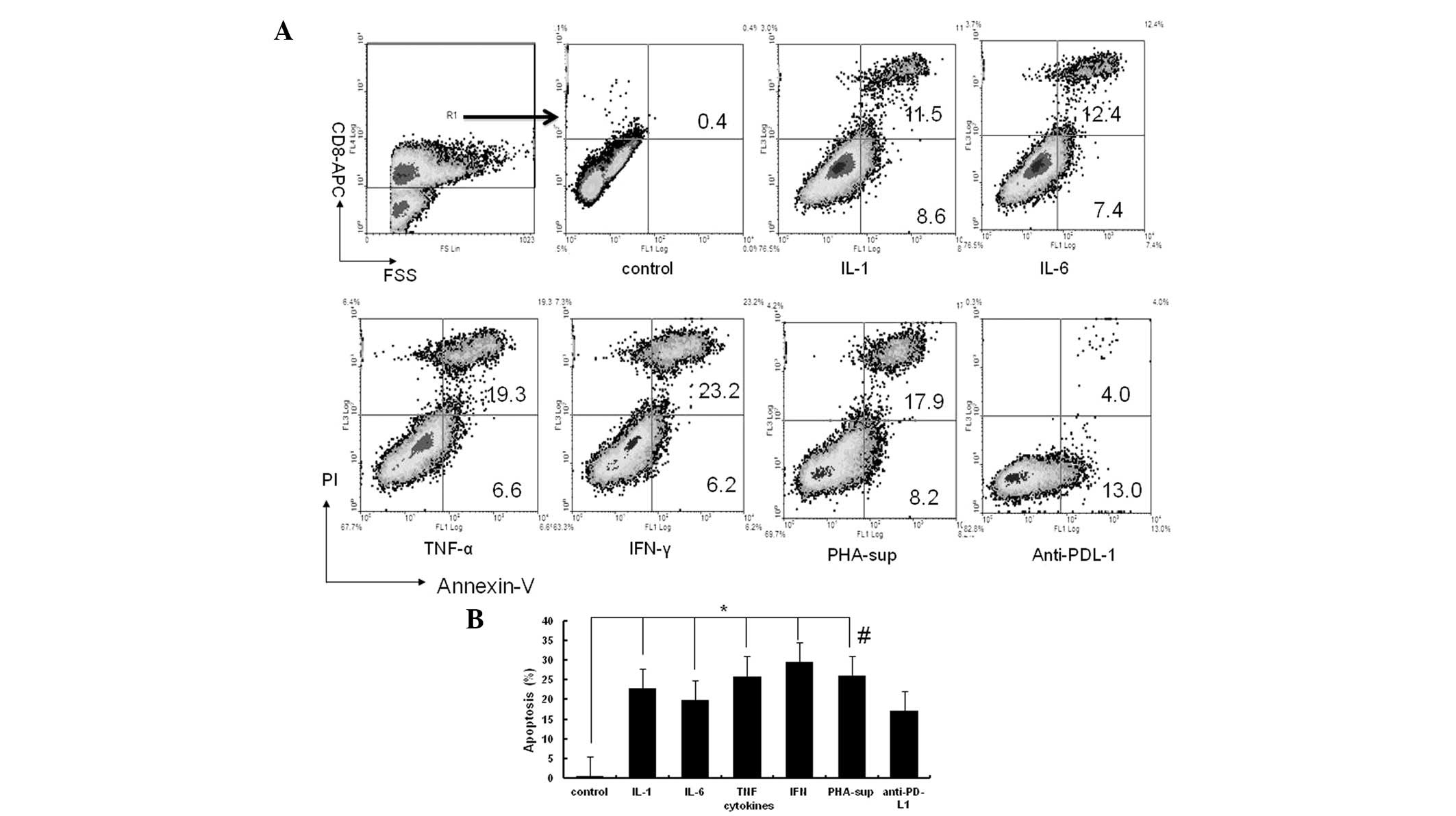 | Figure 5Inflammatory cytokines induce
apoptosis of tumor antigen-specific T cells. Tca8113
antigen-activated human PBMCs were co-cultured at a ratio of 50:1
with the Tca8113 cells that had been pretreated with inflammatory
cytokines. In the PHA-supernatant-pretreated sample, anti-PD-L1 mAb
was added in an additional well for 16 h. The cells were then
harvested and stained with anti-annexin V-FITC/PI and APC-anti-CD8
antibodies. (A) The apoptosis of the CD8+ T cells was
analyzed on a Beckman Coulter FC500 with Submit 5.2 software gated
first for the CD8-APC-positive group. The results are
representative of three independent experiments. (B) Error bars
represent the mean ± standard deviation of three independent
experiments. *P<0.05 vs. untreated Tca8113 cells,
#P<0.05 vs. anti-PD-L1 antibody block. PD-L1,
programmed cell death-1 ligand 1; PHA, phytohemagglutinin; PBMC,
peripheral blood mononuclear cell; IFN-γ, interferon-γ; TNF-α,
tumor necrosis factor-α; IL, interleukin; PI, propidium iodide;
sup, supernatant; FSC, forward angle light scatter. |
Tca8113 cells pretreated with
inflammatory cytokines promote tumor immune evasion in vivo
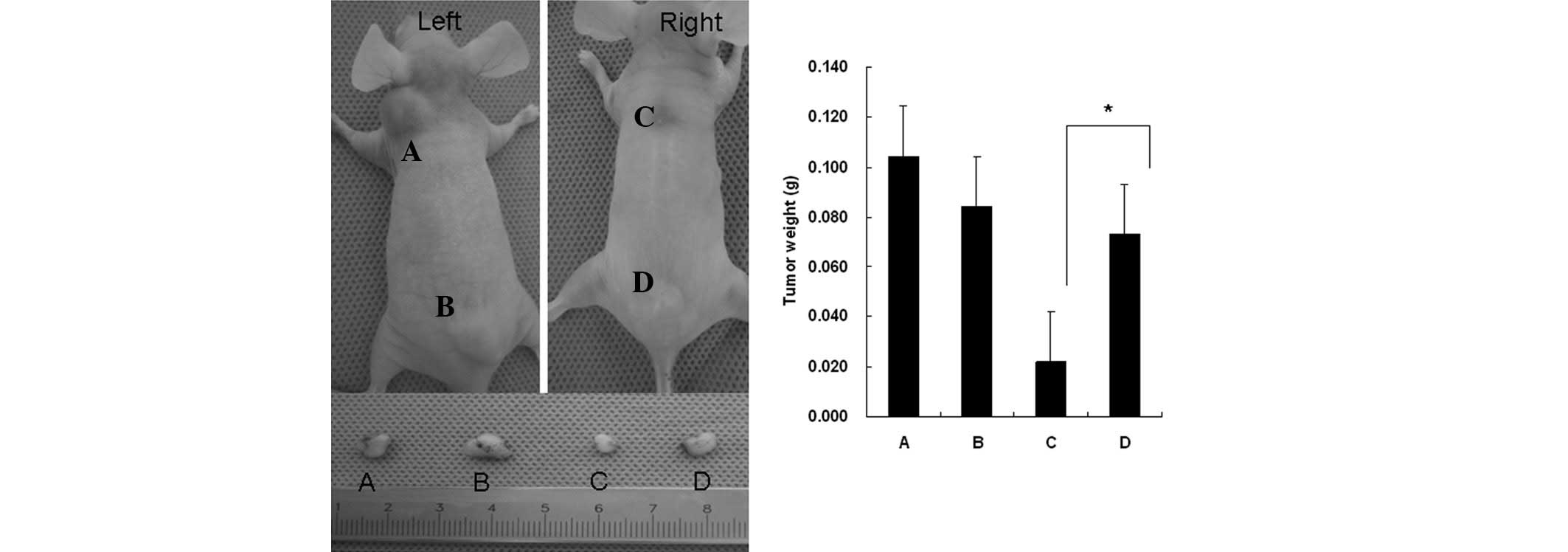
The untreated Tca8113 cells (Fig. 6A and C), or those pretreated with
PHA-supernatant (Fig. 6B and D),
were subcutaneously inoculated into nude mice. Certain mice also
received Tca8113 antigen-specific T cells (Fig. 6, right mice). Pretreatment of the
Tca8113 cells with PHA-supernatant did not affect the growth of the
tumors in vivo (Fig. 6A vs.
B). However, PHA-supernatant pretreatment of the Tca8113 cells
(Fig. 6D) significantly promoted
Tca8113 evasion from the Tca8113 antigen-specific T cell
reaction.
Discussion
The growth and reappearance of spontaneous tumors
are considered to be the result of the resistance to therapy and
evasion from the host immune reaction (27). A number of mechanisms for tumor
immune evasion in the tumor microenvironment have been proposed
(8,27,28).
Although immunotherapy has shown great promise in
the treatment of human cancer, the resistance of cancer cells to
this treatment remains a challenge. Clinical data have shown that
PD-L1 is important for immune evasion by tumor cells. The
expression of PD-L1 on tumors is markedly correlated with the
survival of cancer patients. Targeting PD-L1/PD-1 interactions may
improve the efficacy of adoptive cell therapies for chronic
infections, as well as cancer (21,22).
However, the mechanism by which PD-L1 is expressed
on tumor cells is not well understood. Studies have shown that
IFN-γ is a potent stimulator of PD-L1 expression in various types
of tumor cells (24). The
association between inflammation and cancer was observed 150 years
ago. It is now becoming clear that the tumor microenvironment,
which is largely orchestrated by inflammatory cells and molecules
secreted by immune cells, is essential for neoplastic
proliferation, survival and migration (8,29–31).
In the present study, the surface expression of PD-L1 on the
Tca8113 cells was observed to be significantly increased following
co-culture with PBMCs exposed to PHA (P=0.022), although PHA alone
was unable to induce the expression of PD-L1. In addition, without
the stimulation of PHA in the Tca8113/PBMC co-culture system, there
was no significant increase in the expression of PD-L1. When the
Tca8113 cells and PBMCs were separated in a transwell system, there
was no significant change in the expression of PD-L1 on the Tca8113
cells compared with the increase observed when the Tca8113 cells
were co-cultured with the PBMCs. The supernatant of the PBMCs
exposed to PHA had the same effect on the surface expression of
PD-L1 on the Tca8113 cells. These results demonstrate that
activated immune cells, not resting immune cells, are able to
promote the expression of PD-L1 via the use of secreted
inflammatory cytokines, but not by cell-cell contact (Figs. 1 and 2).
PHA is a multiclonal T cell activator. When PBMCs
are exposed to PHA, the CD4+ and CD8+ T cells
are activated and secrete a number of inflammatory cytokines,
including IL-2, IL-1α, IL-6, TNF-α and IFN-γ. The response of the T
cells to PHA is a typical inflammatory response, similar to the
reaction to a specific antigen (25). In the present study, the Tca8113
cells were treated with the supernatant of PHA-stimulated PBMCs, as
well as recombinant human inflammatory cytokines, to simulate the
inflammatory tumor microenvironment. The results indicated that PHA
supernatant induced the positive expression of PD-1 on 99% of the
Tca8113 cells (Fig. 2). With the
exception of IL-2, all the inflammatory cytokines tested, including
IL-1α, IL-6, TNF-α and IFN-γ, promoted the expression of PD-L1 on
the Tca8113 cells in a dose-dependent and additive manner (Fig. 3A and D). However, anti-cytokine
antibodies were unable to block the effect of the cytokines
(Fig. 3C), suggesting that the
cytokine-stimulated induction of the expression of PD-L1 on the
Tca8113 cells is multifactorial.
It has been well-established that the inflammatory
response in the tumor microenvironment is part of the normal host
defense for the elimination of pathogens. As a result, the tumor
microenvironment is composed of numerous innate and adaptive immune
cells in addition to the cancer cells and their surrounding stroma.
During the inflammatory response to the tumor, there is a balance
between antitumor immunity and the promotion of tumor growth
(32). In the tumor
micro-environment, mature T cells exert tumor suppressive and tumor
promoting effects that are determined by their effector functions.
The inflammatory cytokines in the tumor microenvironment may be
more relevant than the specific immune cell content. Various
cytokines may either inhibit or promote tumor development and
progression, regardless of their source (29).
The present study demonstrated that IL-1α, IL-2,
IL-6, TNF-α, IFN-γ and the supernatant from the PHA-stimulated
PBMCs had no effect on the proliferation of the Tca8113 tumor
cells. With the exception of TNF-α, the cytokines also did not
affect the apoptosis or cell cycle progression of the Tca8113 cells
(Fig. 4). However, the Tca8113
cells pretreated with inflammatory cytokines significantly induced
the apoptosis of the tumor antigen-specific CD8+ T cells
in vitro and this effect was reversed by the anti-PD-L1
antibody (Fig. 5). Pretreatment of
the Tca8113 cells with inflammatory cytokines did not affect their
growth in vivo (Fig. 6A vs.
B), but significantly decreased the antitumor effect of the
tumor antigen-specific T cells (Fig.
6D). These results indicate a new mechanism for the promotion
of tumor immune evasion by the tumor inflammatory
microenvironment
Acknowledgements
The present study was supported by the
National Natural Science Foundation of China (30972764, 30901689
and 81172579).
References
|
1
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aggarwal BB, Shishodia S, Sandur SK, et
al: Inflammation and cancer: how hot is the link? Biochem
Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grange JM, Krone B and Mastrangelo G:
Infection, inflammation and cancer. Int J Cancer. 128:2240–2241.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A and Pierotti MA: Cancer and
inflammation: a complex relationship. Cancer Lett. 267:180–181.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eiró N and Vizoso FJ: Inflammation and
cancer. World J Gastrointest Surg. 4:62–72. 2012.
|
|
6
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan TT and Coussens LM: Humoral immunity,
inflammation and cancer. Curr Opin Immunol. 19:209–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arias JI, Aller MA and Arias J: Cancer
cell: using inflammation to invade the host. Mol Cancer. 6:292007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal BB and Gehlot P: Inflammation and
cancer: how friendly is the relationship for cancer patients? Curr
Opin Pharmacol. 9:351–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore MM, Chua W, Charles KA and Clarke
SJ: Inflammation and cancer: causes and consequences. Clin
Pharmacol Ther. 87:504–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexandrescu DT, Riordan NH, Ichim ET, et
al: On the missing link between inflammation and cancer. Dermatol
Online J. 17:102011.PubMed/NCBI
|
|
12
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Babbar N and Gerner EW: Targeting
polyamines and inflammation for cancer prevention. Recent Results
Cancer Res. 188:49–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demaria S, Pikarsky E, Karin M, Coussens
LM, et al: Cancer and inflammation: promise for biologic therapy. J
Immunother. 33:335–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakanishi J, Wada Y, Matsumoto K, et al:
Overexpression of B7-H1 (PD-L1) significantly associates with tumor
grade and postoperative prognosis in human urothelial cancers.
Cancer Immunol Immunother. 56:1173–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J, Xu K, Wu C, et al: PD-L1 expression
analysis in gastric carcinoma tissue and blocking of
tumor-associated PD-L1 signaling by two functional monoclonal
antibodies. Tissue Antigens. 69:19–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghebeh H, Mohammed S, Al-Omair A, et al:
The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in
breast cancer patients with infiltrating ductal carcinoma:
correlation with important high-risk prognostic factors. Neoplasia.
8:190–198. 2006. View Article : Google Scholar
|
|
19
|
Fife BT, Pauken KE, Eagar TN, et al:
Interactions between PD-1 and PD-L1 promote tolerance by blocking
the TCR-induced stop signal. Nat Immunol. 10:1185–1192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blank C, Gajewski TF and Mackensen A:
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T
cells as a mechanism of immune evasion: implications for tumor
immunotherapy. Cancer Immunol Immunother. 54:307–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blank C, Kuball J, Voelkl S, Wiendl H, et
al: Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell
responses in vitro. Int J Cancer. 119:317–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blank C and Mackensen A: Contribution of
the PD-L1/PD-1 pathway to T-cell exhaustion: an update on
implications for chronic infections and tumor evasion. Cancer
Immunol Immunother. 56:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karwacz K, Arce F, Bricogne C, et al:
PD-L1 co-stimulation, ligand-induced TCR down-modulation and
anti-tumor immunotherapy. Oncoimmunology. 1:86–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Feng Y, Lu L, et al:
Interferon-γ-induced PD-L1 surface expression on human oral
squamous carcinoma via PKD2 signal pathway. Immunobiology.
217:385–393. 2012.
|
|
25
|
Fan J, Nishanian P, Breen EC, McDonald M
and Fahey JL: Cytokine gene expression in normal human lymphocytes
in response to stimulation. Clin Diagn Lab Immunol. 5:335–340.
1998.PubMed/NCBI
|
|
26
|
Germano G, Allavena P and Mantovani A:
Cytokines as a key component of cancer-related inflammation.
Cytokine. 43:374–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dranoff G: Immune recognition and tumor
protection. Curr Opin Immunol. 14:161–164. 2002. View Article : Google Scholar
|
|
28
|
Chow MT, Möller A and Smyth MJ:
Inflammation and immune surveillance in cancer. Semin Cancer Biol.
22:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keibel A, Singh V and Sharma MC:
Inflammation, microenvironment, and the immune system in cancer
progression. Curr Pharm Des. 15:1949–1955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rauch D, Gross S, Harding J, Bokhari S,
Niewiesk S, et al: T-cell activation promotes tumorigenesis in
inflammation-associated cancer. Retrovirology. 6:1162009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9:2122007. View Article : Google Scholar : PubMed/NCBI
|