Introduction
The detection of polypoid lesions of the gallbladder
(PLGs) has increased significantly due to the widespread use of
ultrasonography (US) imaging techniques (1–3). US is
the first choice in the diagnosis of this disease and enhanced
computed tomography (CT) and magnetic resonance imaging (MRI)
confirm its nature (4–8). Many studies have indicated that the
size of tumor is vital for diagnosis. A diameter >10 mm is
regarded as the threshold for malignancy (3,9–11). In
this study, a large tumor size did not necessarily mean that the
lesion was malignant; the configuration of the tumor and the
gallbladder wall and the existence of space between the two were
the main indicators of the nature of the lesion. This knowledge
will enable optimal management of this type of patient. In this
article, a retrospective analysis of clinical data of PLG patients
over a 5-year time period was performed.
Materials and methods
Patients
The study was approved by the Ethics Committee of
Zhejiang Provincial People’s Hospital, Hangzhou, China. The study
was retrospective so consent was not required. Two qualified
radiologists reviewed images from 130 patients from 2005 to 2011
with PLGs larger than 10 mm who consented to the removal of the
gallbladder or biopsy. All patients were pathologically diagnosed
by routine postoperative examination; the age of patients ranged
from 28 to 82 years old.
CT, MR and US protocol
Patients fasted for at least 8 h before examination;
no oral contrast medium or water was administered. CT examinations
were performed using Phlilips Brilliance 16 (Philips Medical
Systems, Best, Netherlands). Each patient received 100 ml of a
nonionic contrast material (iopromide; Ultravist 370, Bayer
HealthCare, Berlin, Germany) through an 18-gauge angiographic
catheter inserted into a forearm vein. CT scans were routinely
obtained with the patient in a supine position. The contrast
material was injected at a rate of 3 ml/sec with an automatic power
injector. Magnetic resonance cholangiopancreatography was taken
using a Siemens 3.0 Tesla MR magnet (Megnetom Trio; Siemens AG,
Erlangen, Germany); sonography was performed using Esaote MyLab 70
(Esaote SPA, Genova, Italy) with 1–8 MHz linear array probes or a
Toshiba (Applio XG 790; Toshiba, Tokyo, Japan) with 3–6 MHz 6C1
probes. Specific attention was given to the size and the base of
the lesion, the space between the lesion and the wall, and the
configuration of the wall to elucidate the differences between
benign and malignant lesions.
Results
Patient characteristics
Twenty-two patients were diagnosed as malignant by
CT and 20 of them were confirmed by biopsy or postoperative
pathological examination. Two patients with lesions larger than 50
mm were suspected to be malignant preoperatively but were benign on
histological examination. One patient had two polypoid lesions. On
pathological examination one was benign and the other was malignant
(Table I).
 | Table IPatients’ clinical data. |
Table I
Patients’ clinical data.
| Characteristics | No. | Percentage |
|---|
| Total (n=130) | | |
| Benign | 110/130 | 85 |
| Malignant | 20/130 | 15 |
| Malignant (n=20) | | |
| Platform | 13/20 | 65 |
| Protruding
lesions | 7/20 | 35 |
| Female | 16/20 | 80 |
| Male | 4/20 | 20 |
| Benign (n=110) | | |
| Lesion >20
mm | 8/110 | 7 |
| Lesion >50
mm | 2/110 | 1.8 |
| Laparoscopic
cholecystectomy | 102/110 | 93 |
| Open
cholecystectomy | 8/110 | 7 |
Differences in morphology between large
adenoma and protruding type cancer
Before the operation, 2 patients were diagnosed with
gallbladder cancer due to the large size of their tumors but these
were revealed to be adenoma. One was tubular adenoma and the other
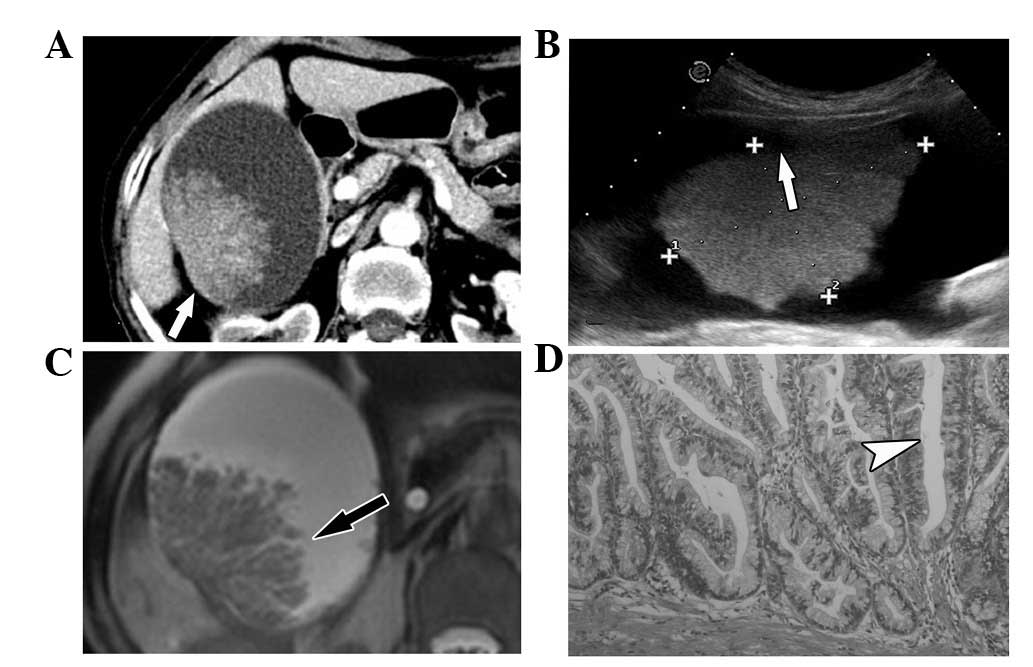
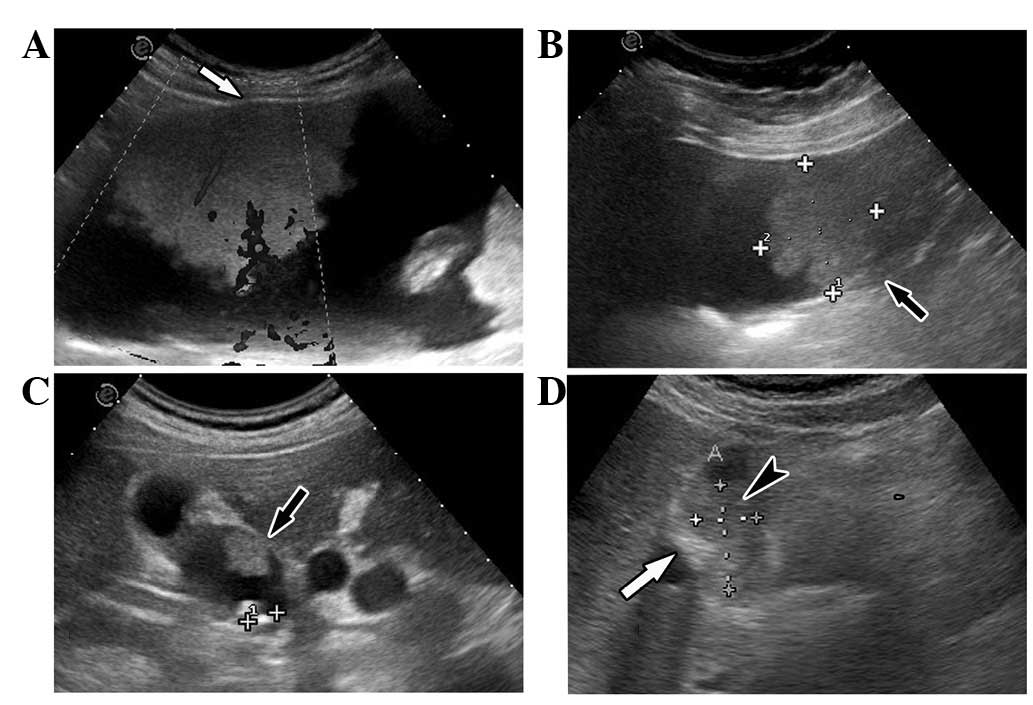
was rare villious adenoma. Following CT scans, the villious adenoma
turned out to be akin to dendritic processes; there were spaces
within the mass, the surface had no contraction and the whole shape
appeared to be natural like dendritic processes in the MRI scan
(Fig. 1). The CT and MRI image of
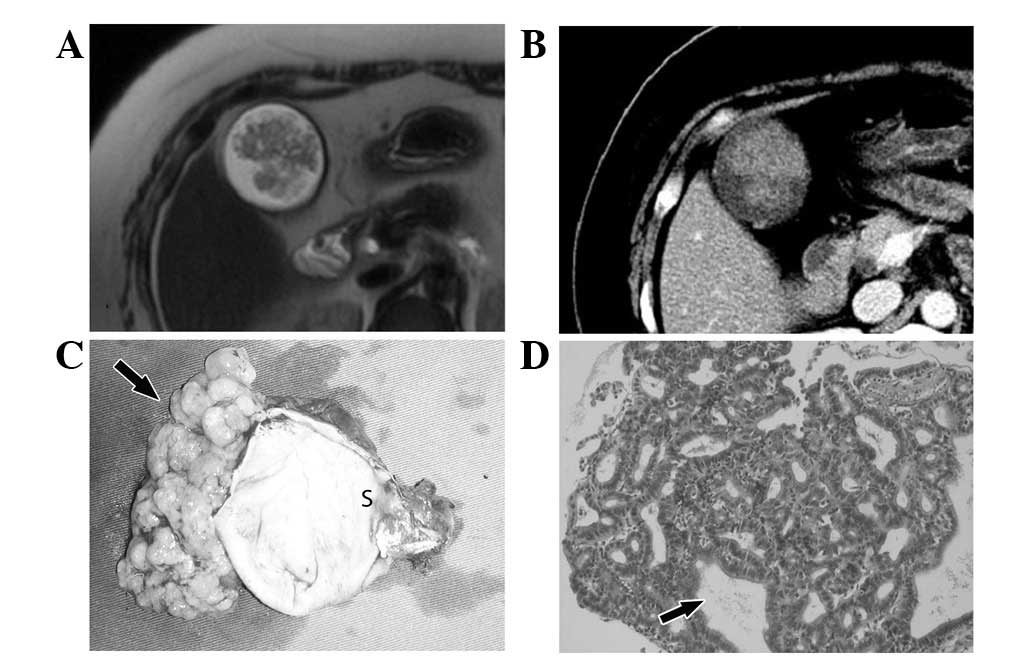
the tubular adenoma was different from that of villious adenoma,
the appearance was similar to a congregation of mulberries
(Fig. 2). In contrast, the surface
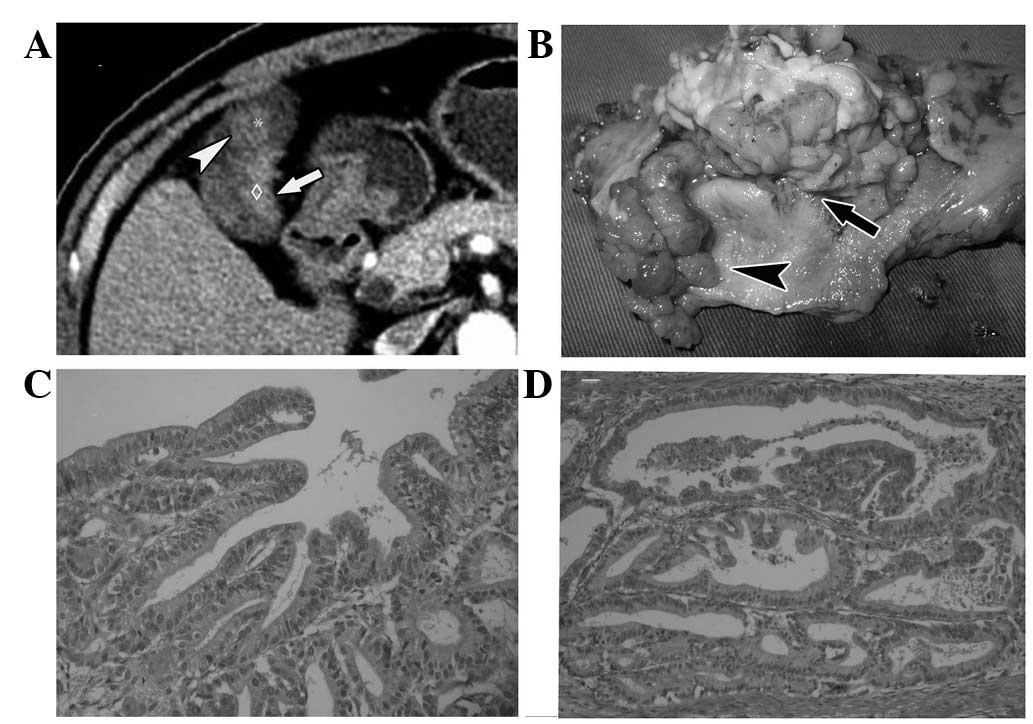
of the malignant tumor was uneven, the enhancement of the solid
entity of the tumor seemed to be inhomogenous and grew in a solid
mass (Fig. 3). In Fig. 4, where a benign and malignant tumor
grew together in the same gallbladder, the malignant tumor became
more enhanced than the benign one (Fig.
4).
Difference in the base size of the tumor,
and the space between the tumor and the gallbladder wall
The base of the benign polypus was smaller than the
malignant one and there was space between the tumor and the
gallbladder wall. In the case of tubular adenoma, the CT and MR
images showed that the mass had almost no contact with the
gallbladder wall and the whole mass appeared to be floating in the
water. Macroscopically, the mass was connected to the wall of the
gallbladder with small and slender fibers and could easily be
removed from the wall of the gallbladder, while the gallbladder
wall remained smooth with integrity (Fig. 2). In the case where benign and
malignant tumors coexisted, there was a space between the wall and
the tumor, while the space disappeared between the malignant tumors
and the wall (Fig. 4). In the case
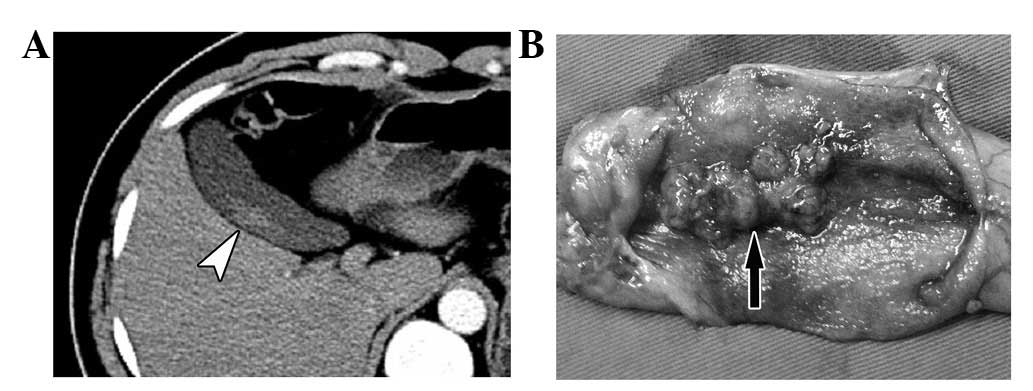
where the lesion was a polypoid with a slender pedicel, the CT scan
showed a strip parallel with the wall resembling a trailing plant,
and was separated from the wall by a narrow space (Fig. 5). In contrast, the base of the
protruding carcinoma was wide, the space between the polypus and
the gallbladder wall disappeared and the enhancement became more
evident close to the base of the tumor (Figs. 4 and 6).
Shape of the gallbladder wall
The gallbladder wall with the benign tumor was
smooth and soft, had the normal thickness and normal extent of
enhancement, with the normal space between the wall and the liver.
The wall of malignant lesions, however, became thicker and stiff
and the space became obscure. In some cases it disappeared. In
certain cases, the wall had broken and the lesion had infiltrated
the liver parenchyma, which was a sign of a malignant lesion.
Discussion
Polypoid lesions of the gallbladder are an imaging
feature, which indicate a wide variety of pathology. They affect
∼5% of the adult population (3,12,13).
Although most of these lesions are benign, some early carcinomas of
the gallbladder present as polypoid lesions; malignant
transformation is always a concern. The differentiation of benign
and malignant lesions can be challenging. Several features,
including patient age, tumor size and the speed of growth of the
lesions, are important discriminating features between benign and
malignant polyps. Many studies have shown that age >50 years and
a polyp size >1 cm are the two most important factors predicting
malignancy in polypoid lesions of the gallbladder (3,9–11).
Microscopically, malignant tumors no longer possess
the normal structure of the original tissue. There is frequent
necrosis and subsequent repair within the tumor; the surface of a
malignant tumor is often uneven and the whole configuration is
unnatural and becomes stiff (14).
Growing in an infiltrative way, the malignant tumors invade
neighboring tissue; thus, the demarcation between the tumor and the
normal tissue is obscure. Benign tumors do not grow uncontrollably;
most benign tumors have a similar structure to the tissue they
originate from and do not invade neighboring tissues and spread
throughout the body. Since adenoma has the structure of gland tube,
which is characterized by short fibrovascular stalks that are
covered by dysplastic or neoplastic cells, the CT and MR images of
large adenoma have the similar shapes, akin to float grass. In
contrast, gallbladder cancer infiltrates the base and there is
necrosis on the inside of the tumor and the image appears to be
inhomogenous without a natural texture. Macroscopically, the
gallbladder tumors are classified into the protruding, flat or
infiltrative type (15). US, CT and
MR images are used to classify these types (8,16–20).
In this study, pathological differences in the shape
and texture of the mass, the base of the mass, the space between
the tumor and gallbladder wall, and the changes of the gallbladder
wall were used to determine whether the lesion was benign or
malignant. The results support the differences between large
adenoma and protruding type carcinoma. In gallbladder adenoma, even
though the lesions were very large there were still spaces within
the tumor, indicating that the tumor was not a solid mass; in the
villous adenoma, the spaces were narrow and resembled dendritic
processes; in the tubular adenoma, the spaces were irregular.
Microscopically, this kind of spaces exist between the
fibrovascular stalks that are covered with gland. Since the
malignant tumor grows in an infiltrative way and always has a wide
base, there is no space between the tumor and the gallbladder wall
and the gallbladder walls are always thickened and contracted; the
spaces between the gall-bladder wall and the liver disappear when
the tumors break the gallbladder wall and invade the liver
parenchyma.
Many studies have demonstrated that lesion size and
patient age are strong indicators of a malignant tumor. In this
study, the large size of polypoid lesions of the gallbladder did
not necessarily mean the tumor was malignant. A possible reason for
this may be that the cancerous change needs time and the speed of
this change is relatively slow. Conversely, small adenoma does not
always mean benign. According to a study conducted by Roa et
al(21), adenomas associated
with cancer may measure <5 mm. Using polyp size to decide
surgical behavior may, therefore, be misleading. Adenoma is a
precancerous disease which could transform to adenocarcinoma in an
adenoma-dysplasia-cancer manner (22,23). A
protruding type of gallbladder was likely the result of adenoma
under continuing stimuli such as gallstones or chronic
inflammation. In this study, the co-existence of a benign and
malignant tumor in one patient was evidence of this theory.
Although it is difficult to discriminate between
large adenoma and cancer, removing the gallbladder by open
laparotomy is usually the first choice of treatment for these two
diseases. However, in an era of minimally invasive surgery, both
doctors and patients prefer to choose laparoscopic cholecystectomy.
Size may not be the key indicator to discriminate benign from
malignant lesions, or the indication for a laparoscopic or open
procedure. Preoperative images indicate that the feasibility of
laparoscopic cholecystectomy mainly depends on the lesion’s
configuration; they should be confined to the lumen of the
gallbladder, the wall of the gallbladder should be smooth and thin
and the space between the wall and liver should be clear; this
indicates that the separation of the gallbladder from the liver
should be relatively simple. Many articles have reported that even
PLGs with a diameter >1.5 mm, a potential early-stage cancer,
could still be resected by laparoscopic cholecystectomy with
full-thickness dissection (24–26).
The key issue with such surgery is to maintain the integrity of the
gallbladder and protect the Trocar port in order to avoid the
dissemination of the cancer in case the lesion is malignant
(27,28).
References
|
1
|
Ozdemir A, Ozenc A, Bozoklu S and Cosķun
T: Ultrasonography in the diagnosis of gallbladder polyps. Br J
Surg. 80:3451993. View Article : Google Scholar
|
|
2
|
Kratzer W, Haenle MM, Voegtle A, et al:
Ultrasonographically detected gallbladder polyps: a reason for
concern? A seven-year follow-up study. BMC Gastroenterol.
8:412008.PubMed/NCBI
|
|
3
|
Myers RP, Shaffer EA and Beck PL:
Gallbladder polyps: epidemiology, natural history and management.
Can J Gastroenterol. 16:187–194. 2002.PubMed/NCBI
|
|
4
|
Park KW, Kim SH, Choi SH and Lee WJ:
Differentiation of nonneoplastic and neoplastic gallbladder polyps
1 cm or bigger with multi-detector row computed tomography. J
Comput Assist Tomogr. 34:135–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SJ, Lee JM, Lee JY, et al: Accuracy of
preoperative T-staging of gallbladder carcinoma using MDCT. AJR Am
J Roentgenol. 190:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalra N, Suri S, Gupta R, et al: MDCT in
the staging of gall-bladder carcinoma. AJR Am J Roentgenol.
186:758–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimitsu K, Honda H, Shinozaki K, et al:
Helical CT of the local spread of carcinoma of the gallbladder:
evaluation according to the TNM system in patients who underwent
surgical resection. AJR Am J Roentgenol. 179:423–428. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar A and Aggarwal S: Carcinoma of the
gallbladder: CT findings in 50 cases. Abdom Imaging. 19:304–308.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee KF, Wong J, Li JC and Lai PB: Polypoid
lesions of the gall-bladder. Am J Surg. 188:186–190. 2004.
View Article : Google Scholar
|
|
10
|
Terzi C, Sökmen S, Seçkin S, Albayrak L
and Uğurlu M: Polypoid lesions of the gallbladder: report of 100
cases with special reference to operative indications. Surgery.
127:622–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Csendes A, Burgos AM, Csendes P, Smok G
and Rojas J: Late follow-up of polypoid lesions of the gallbladder
smaller than 10 mm. Ann Surg. 234:657–660. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CY, Lu CL, Chang FY and Lee SD: Risk
factors for gall-bladder polyps in the Chinese population. Am J
Gastroenterol. 92:2066–2068. 1997.
|
|
13
|
Ozmen MM, Patankar RV, Hengirmen S and
Terzi MC: Epidemiology of gallbladder polyps. Scand J
Gastroenterol. 29:4801994. View Article : Google Scholar
|
|
14
|
Hamilton SR: Pathology and genetics of
tumours of the digestive system. IARC; Lyon: pp. 201–213. 2000
|
|
15
|
Sumiyoshi K, Nagai E, Chijiiwa K and
Nakayama F: Pathology of carcinoma of the gallbladder. World J
Surg. 15:315–321. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim BS, Ha HK, Lee IJ, et al: Accuracy of
CT in local staging of gallbladder carcinoma. Acta Radiol.
43:71–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuchiya Y: Early carcinoma of the
gallbladder: macroscopic features and US findings. Radiology.
179:171–175. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sadamoto Y, Kubo H, Harada N, Tanaka M,
Eguchi T and Nawata H: Preoperative diagnosis and staging of
gallbladder carcinoma by EUS. Gastrointest Endosc. 58:536–541.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz LH, Black J, Fong Y, et al:
Gallbladder carcinoma: findings at MR imaging with MR
cholangiopancreatography. J Comput Assist Tomogr. 26:405–410. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng JH, Wan YL, Hung CF, et al:
Diagnosis and staging of gallbladder carcinoma. Evaluation with
dynamic MR imaging. Clin Imaging. 26:177–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roa I, de Aretxabala X, Morgan R, et al:
Clinicopathological features of gallbladder polyps and adenomas.
Rev Med Chil. 132:673–679. 2004.(In Spanish).
|
|
22
|
Chang HJ, Jee CD and Kim WH: Mutation and
altered expression of beta-catenin during gallbladder
carcinogenesis. Am J Surg Pathol. 26:758–766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanagisawa N, Mikami T, Saegusa M and
Okayasu I: More frequent beta-catenin exon 3 mutations in
gallbladder adenomas than in carcinomas indicate different
lineages. Cancer Res. 61:19–22. 2001.PubMed/NCBI
|
|
24
|
Kubota K, Bandai Y, Noie T, Ishizaki Y,
Teruya M and Makuuchi M: How should polypoid lesions of the
gallbladder be treated in the era of laparoscopic cholecystectomy?
Surgery. 117:481–487. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf P, Bhargava P, Pellegrini C and Peter
C: Management of unsuspected gallbladder cancerin the era of
minimally invasive surgery. J Cancer Ther. 1:152–159. 2010.
View Article : Google Scholar
|
|
26
|
de Aretxabala X, Leon J, Hepp J, Maluenda
F and Roa I: Gallbladder cancer: role of laparoscopy in the
management of potentially resectable tumors. Surg Endosc.
24:2192–2196. 2010.PubMed/NCBI
|
|
27
|
Paolucci V, Schaeff B, Schneider M and
Gutt C: Tumor seeding following laparoscopy: international survey.
World J Surg. 23:989–995; discussion 996–997. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paolucci V: Port site recurrences after
laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg.
8:535–543. 2001. View Article : Google Scholar : PubMed/NCBI
|