Introduction
The Wnt signaling pathway is important in embryonic
development as well as the initiation and progression of a number
of types of human cancer (1–3). The
Wnt/β-catenin signaling pathway, also known as the canonical Wnt
signaling pathway, involves the translocation of unphosphorylated
β-catenin into the nucleus. In the nucleus, β-catenin interacts
with transcription factors (including TCF or LEF) to initiate the
transcription of downstream target genes, including C-MYC, CCND1
and BIRC5, to regulate cell proliferation, differentiation and
survival (1).
As a Drosophila segment polarity gene,
porcupine (PPN) encodes a transmembrane protein located in the
endoplasmic reticulum which is necessary for the normal processing
of Wingless protein (4). Although
Hofmann predicted that PPN belongs to a superfamily of
membrane-bound O-acyltransferases (5), the active form of Wnt proteins were
not identified to be palmitoylated on a conserved cysteine until
2003 (6). Several studies suggest
that PPN-dependent palmitoylation is required for the activity and
distribution of Wnt proteins (6–9).
Given that PPN acts as an essential
palmitoyltransferase during the post-translational modification of
Wnt proteins, a diverse library of synthetic small molecules have
been screened to identify those that target Wnt-mediated cellular
responses (10). One class of small
molecules that inhibited the activity of PPN were named the
inhibitors of Wnt production (IWP) (10). The IWP compounds share an identical
core chemical structure and target the palmitoyltransferase PPN
(10,11). As the majority of currently used Wnt
pathway-targeting strategies have focused on Wnt-receiving cells
(12,13), the potential use of IWP may provide
new insights into the abrogation of aberrant Wnt signaling pathway
activity in Wnt-producing cells and cancer therapy.
Gastric cancer is one of the most common causes of
cancer-related mortality worldwide (14). In China, the incidence rate of
gastric cancer ranked as the third highest amongst the most common
cancers in 2005 (15). The
initiation and progression of gastric cancer have been linked to
the aberrantly activated Wnt/β-catenin signaling pathway (16–18).
However, little is known on the role of PPN in gastric cancer. In
this study, we first examined the expression profile of PPN in
paired human gastric cancer tissue samples. We then investigated
the effects of IWP on the cell growth and activity of the
Wnt/β-catenin signaling pathway in the gastric cancer cell line
MKN28.
Materials and methods
Tissue samples
Tissue samples of sixteen gastric cancer patients
from local hospitals were collected following receipt of written
informed consent and approval by the Tsinghua University School of
Medicine Ethical Review Committee, Beijing, China. Cancerous and
adjacent normal tissues of the same patient were obtained during
resection and immediately snap-frozen in liquid nitrogen. Normal
tissues were purchased from Biochain (Newark, CA, USA). Tissue
samples were stored at −80°C prior to analysis.
RNA extraction, reverse transcription and
real-time PCR
Total RNA was isolated from tissue or culture cell
samples using the RNeasy Plus kit (Qiagen Inc., Valencia, CA, USA).
The concentration of RNA was examined by Nanodrop 1000 (Thermo
Fisher Scientific, Wilmington, DE, USA). Reverse transcription and
real-time PCR were performed as previously reported (19). The sequences of primers and probes
used were: for PPN, forward: 5′-CATCCTCATCTACCTACTCAT-3′, reverse:
5′-CGCATCTTGTGCCATGTC-3′, probe: 5′-CGGTGTCTACCATGTGCATCTC-3′; for
internal control (ACTB), forward: 5′-GATCATTGCTCCTCCTGAGC-3′,
reverse: 5′-ACTCCTGCTTGCTGATCCAC-3′, probe:
5′-CTCGCTGTCCACCTTCCAGCAGAT-3′; for AXIN2, forward:
5′-ACATAGGTTCTGGCTATGTCTT-3′, reverse: 5′-GTCAGCGCATCACTGGATAT-3′,
probe: 5′-CCACCAGCGCCAACGACAGTG-3′; for C-MYC, forward:
5′-CCACGTCTCCACACATCAG-3′, reverse: 5′-TTGGCAGCAGGATAGTCCTT-3′,
probe: 5′-AACTACGCAGCGCCTCCCTCCAC-3′; for CCND1, forward:
5′-CGTCCATGCGGAAGATCGT-3′, reverse: 5′-TCCTCCTCGCACTTCTGTT-3′,
probe: 5′-CTCGCAGACCTCCAGCATCCAG-3′; for BIRC5 (encoding Survivin),
forward: 5′-TGGAGTCTGGGAAGGGTTGT-3′, reverse:
5′-GCTCTAACCTGCCATTGGAAC-3′, probe: 5′-TCACCCATAGCCCAGAAGCCTCA-3′.
The 2−ΔCt value demonstrates the relative PPN expression
(relative to internal control) in cancer cell lines and normal
tissues. The 2−ΔΔCt value demonstrates the fold change
of the relative PPN expression (relative to internal control) in
cancer tissues normalized to adjacent normal tissues (20) and 2−ΔΔCt >1.5 was
regarded as overexpression.
Cell culture and Wnt palmitoyltransferase
inhibitor
Human gastric cancer cell lines MKN28 were obtained
from the China Center for Type Culture Collection (Wuhan, China).
The cells were cultured in Dulbecco’s modified Eagle’s medium
supplemented with 10% fetal bovine serum. Cells were cultured at
37°C in a humid incubator with 5% CO2. Wnt
palmitoyltransferase inhibitor (IWP-2) was purchased from Sigma
Aldrich (St. Louis, MO, USA) and 30 μM was used to treat the
cells.
Western blot analysis
The detailed western blot procedures were performed
as previously reported (21). The
primary antibodies included anti-β-actin (1:5,000; Sigma Aldrich),
anti-β-catenin (1:2,000; BD Biosciences, San Jose, CA, USA),
anti-Axin 2 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-Cyclin D1 (1:2,000; Cell Signaling Technology, Inc.),
anti-c-myc (1:1,000; Cell Signaling Technology, Inc.),
anti-Survivin (1:1,000; Cell Signaling Technology, Inc.). β-catenin
was detected in cytosolic proteins. All other targets were detected
in total proteins.
Cell proliferation assay
CellTiter 96 Aqueous Non-Radioactive Cell
Proliferation Assay kit (Promega Corporation, Madison, WI, USA) was
used as previously reported (21).
Colony formation and soft agar
assays
The detailed procedures for colony formation and
soft agar assays were performed as previously reported (21).
Luciferase assay
Dual-Glo Luciferase Assay System (Promega
Corporation) was used as previously reported (21). The ratio between firefly luciferase
activity and renilla luciferase activity (FL/RL) was calculated to
examine the TCF/LEF transcriptional activity.
Caspase-3/7 activity assay
Caspase-Glo 3/7 Assay (Promega Corporation) was used
for measuring caspase-3/7 activity according to the manufacturer’s
instructions. Treated cells (100 μl of 5×103)
were incubated with an equal volume of the assay reagent at room
temperature. After 1.5 h, luminescence was measured to calculate
caspase-3/7 activity.
Transwell migration and invasion
assay
Haptotaxis chambers (8-μm pore size, Corning
Costar, Cambridge, MA, USA) without or with 5 mg/ml matrigel (Sigma
Aldrich) were used for migration and invasion assays, respectively.
The experiments were repeated three times independently.
Statistical analysis
Experimental data were analyzed using GraphPad Prism
5.0 for Windows (GraphPad Software, Inc., La Jolla, CA, USA). The
differences in the relative expression levels of genes between two
unpaired and paired groups were analyzed by the Student’s t-test
and the Wilcoxon matched pairs test, respectively. For
independently repeated experiments, mean values ± SD (error bars)
are represented in Figs. 1–4. For all statistical tests, a two-tailed
P<0.05 was considered to indicate a statistically significant
result. P<0.05, P<0.01 and P<0.001 are represented as *,
** and ***, respectively.
Results
Overexpression of PPN in gastric cancer
tissue samples and MKN28 cell line
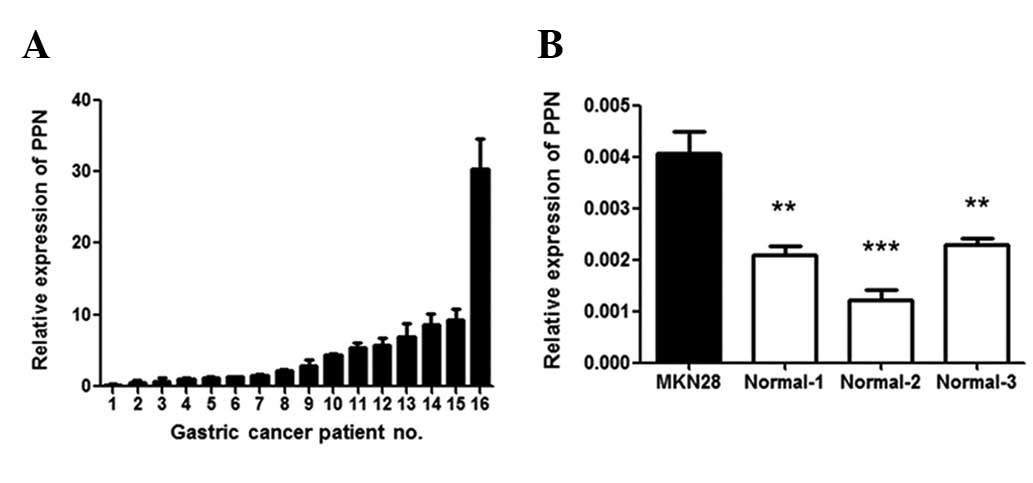
PPN was overexpressed in 62.5% (10/16) of gastric
cancer tissue samples compared with adjacent normal tissue samples
(paired test, P<0.05; Fig. 1A).
We also noted that PPN expression in the gastric cancer cell line
MKN28 was significantly higher than that in three normal gastric
tissue samples that we examined (P<0.05; Fig. 1B). These results indicate that PPN
may be important in gastric cancer.
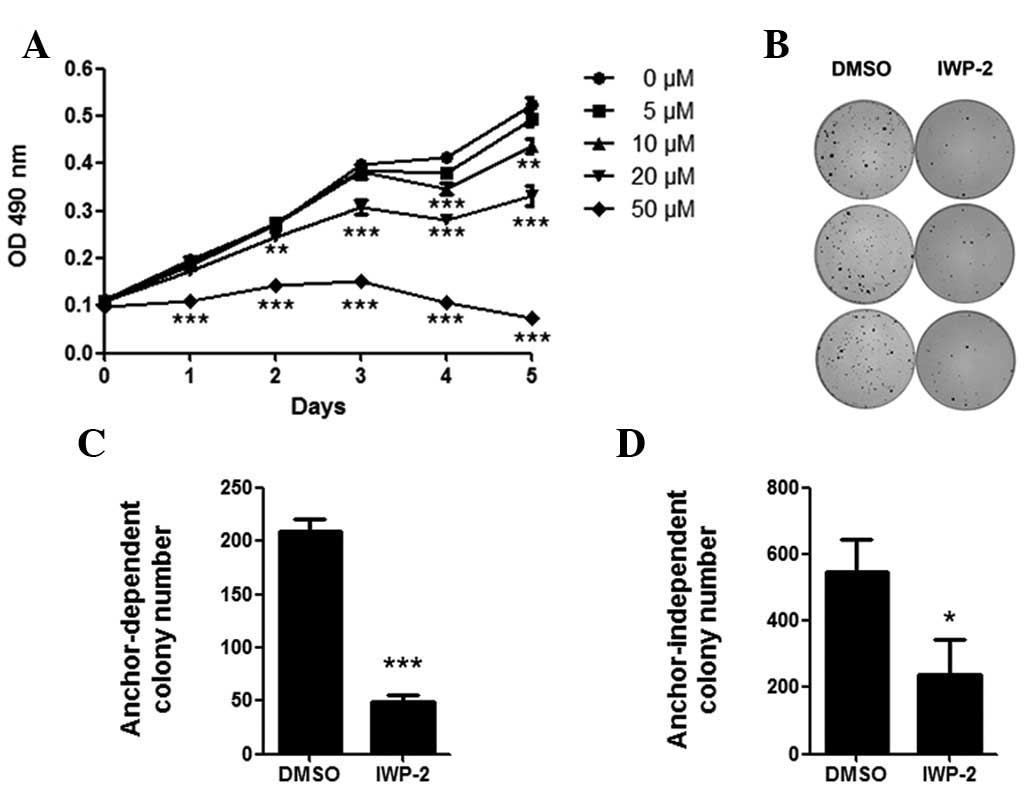
PPN inhibitor suppresses MKN28 cell
growth
To investigate the role of PPN as a Wnt
palmitoyltransferase in gastric cancer, we used a
palmitoyltransferase inhibitor (IWP-2) specific for PPN activity
(10,11). Following treatment in the MKN28 cell
line for four days, 10–50 μM IWP-2 significantly suppressed
the proliferation of MKN28 cells (P<0.05; Fig. 2A). In addition, anchor-dependent and
anchor-independent colony numbers were significantly decreased
following IWP-2 treatment (P<0.05; Fig. 2B–D).
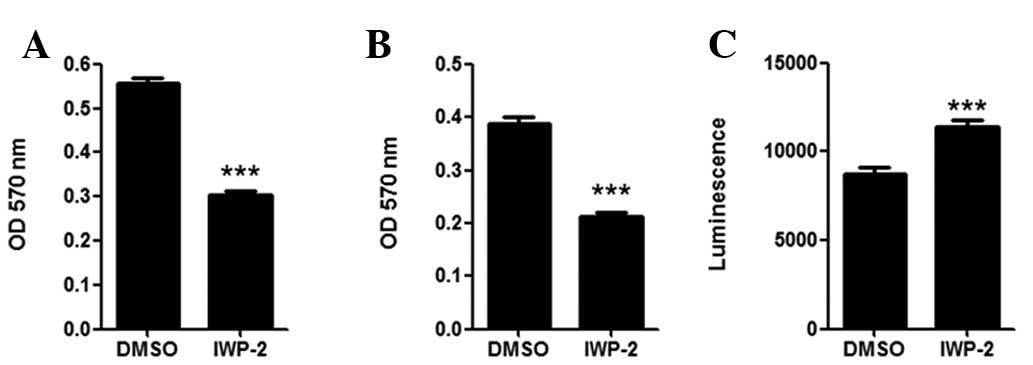
PPN inhibitor downregulates MKN28 cell
migration and invasion, and elevates caspase 3/7 activity
In cell migration and invasion assays, MKN28 cells
demonstrated almost 50% reduction in migration (Fig. 3A) and invasion (Fig. 3B) following IWP-2 treatment
(P<0.05). IWP-2 treatment also significantly elevated the
cellular caspase 3/7 activity in MKN28 cells (P<0.05),
indicating that IWP-2 may induce cell apoptosis.
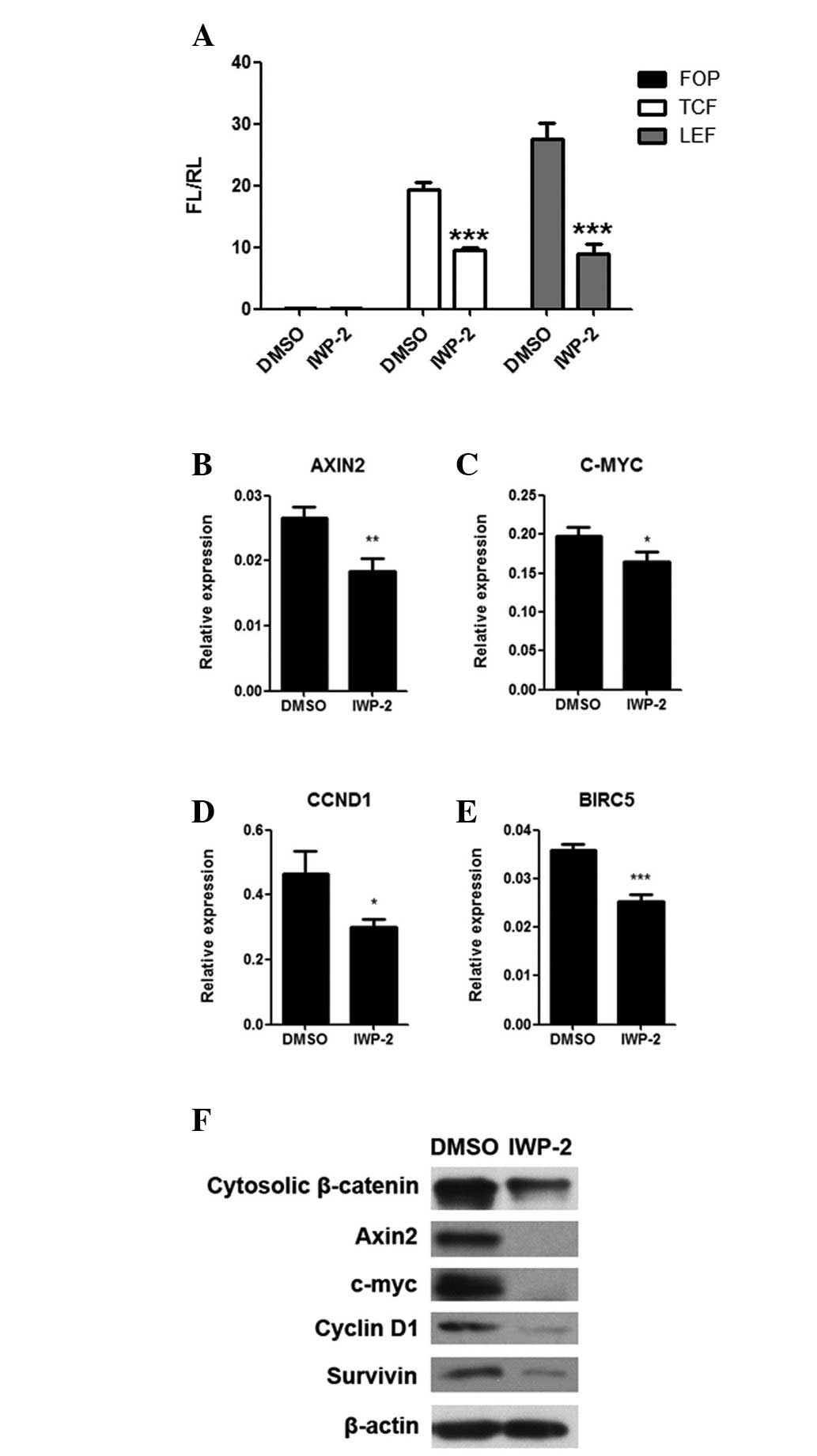
PPN inhibitor downregulates activity of
the Wnt/β-catenin signaling pathway
Due to the correlation of proliferation, migration
and invasion of MKN28 cells with the activity of the Wnt/β-catenin
signaling pathway (21), we
examined the effects of IWP-2 on the Wnt/β-catenin signaling
pathway in gastric cancer cells. We observed that the
transcriptional activity of two transcription factors (TCF and LEF)
were significantly decreased in MKN28 cells following IWP-2
treatment (P<0.05; Fig. 4A). The
mRNA and protein expression levels of four downstream Wnt/β-catenin
signaling pathway target genes (AXIN2, C-MYC, CCND1 and BIRC5) were
also significantly downregulated in MKN28 cells following IWP-2
treatment (P<0.05; Fig. 3B–F).
Furthermore, the expression levels of cytosolic β-catenin protein,
a hallmark of Wnt/β-catenin signaling pathway activation, was also
decreased (Fig. 3F). Taken
together, these data suggest that the inhibition of PPN abrogates
activity of the Wnt/β-catenin signaling pathway, providing more
evidence of the importance of PPN activity in the aberrant
activation of the Wnt/β-catenin signaling pathway in gastric
cancer.
Discussion
Although PPN has been demonstrated to be important
in the processing and distribution of Wnt proteins (6–9),
little is known with regard to its characteristics in human cancer.
We previously identified that PPN was overexpressed in lung cancer
(22). As gastric cancer is one of
the most common causes of cancer-related mortality worldwide and in
China (14,15), we aimed to study the role of PPN
activity in gastric cancer. We identified that PPN was
overexpressed in gastric cancer tissues and the MKN28 cell line.
Notably, the Wnt/β-catenin signaling pathway was aberrantly
activated in gastric cancer tissues (18) and in the MKN28 cell line (21). Therefore, our results indicate that
PPN overexpression correlates with the activation status of the
Wnt/β-catenin signaling pathway in gastric cancer.
We previously demonstrated that the proliferation of
MKN28 cells correlated with the activity of the Wnt/β-catenin
signaling pathway in a Wnt ligand-dependent manner (21), suggesting the potential influence
that PPN inhibition may exert on MKN28 cell proliferation. In the
present study, we demonstrated that IWP-2, a newly developed small
molecule PPN inhibitor (10),
inhibited anchor-dependent and anchor-independent proliferation of
MKN28 cells, providing evidence that inhibiting PPN activity may be
a novel strategy for suppressing gastric cancer cell
proliferation.
As downstream target genes of the Wnt/β-catenin
signaling pathway, CCND1 and C-MYC encode regulators of cancer cell
proliferation, migration and invasion (21,23,24).
Therefore, downregulation of these genes following IWP-2 treatment
correlated with downregulated activity of the Wnt/β-catenin
signaling pathway and inhibition of MKN28 cell proliferation,
migration and invasion induced by IWP-2. In addition,
downregulation of BIRC5, encoding Survivin which inhibits caspase
activity and cell apoptosis (25),
correlated with elevated activity of caspase 3/7 in MKN28 cells
following IWP-2 treatment. These results provide further evidence
for the use of PPN inhibitors to abrogate the aberrantly activated
Wnt/β-catenin signaling pathway.
In summary, we demonstrated that PPN expression
levels are upregulated in gastric cancer tissues. We also revealed
that IWP-2, a palmitoyltransferase inhibitor specific for PPN,
inhibited cell proliferation, migration and invasion, as well as
inducing apoptosis in gastric cancer cells. Further analysis showed
that activity of the Wnt/β-catenin signaling pathway was also
downregulated by IWP-2 in these gastric cancer cells. Our data
indicate that PPN activity may be critical for cell proliferation
and activation of the Wnt/β-catenin signaling pathway in gastric
cancer. These data also provide evidence for the viability of
targeting the Wnt/β-catenin signaling pathway in Wnt-producing
cells of gastric cancer and shed light on the potential use of PPN
inhibitors as a therapeutic strategy for the treatment of gastric
cancer.
Acknowledgements
This study was partially supported by
the National Key Basic Research and Development (973) Program of
China (No. 2011CB910800 and No. 2012CB917304), the China Natural
Science Foundation (No. 31170732 and No. 31270854), the Natural
Science Foundation of Zhejiang Province (No. Y204499) and a
research fund from Beijing ACCB Biotech Ltd.
References
|
1
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
2
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
4
|
Kadowaki T, Wilder E, Klingensmith J,
Zachary K and Perrimon N: The segment polarity gene porcupine
encodes a putative multitransmembrane protein involved in Wingless
processing. Genes Dev. 10:3116–3128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofmann K: A superfamily of membrane-bound
O-acyltransferases with implications for wnt signaling. Trends
Biochem Sci. 25:111–112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willert K, Brown JD, Danenberg E, et al:
Wnt proteins are lipid-modified and can act as stem cell growth
factors. Nature. 423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nusse R: Wnts and Hedgehogs:
lipid-modified proteins and similarities in signaling mechanisms at
the cell surface. Development. 130:5297–5305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galli LM, Barnes TL, Secrest SS, Kadowaki
T and Burrus LW: Porcupine-mediated lipid-modification regulates
the activity and distribution of Wnt proteins in the chick neural
tube. Development. 134:3339–3348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coudreuse D and Korswagen HC: The making
of Wnt: new insights into Wnt maturation, sorting and secretion.
Development. 134:3–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen B, Dodge ME, Tang W, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dodge ME, Moon J, Tuladhar R, et al:
Diverse chemical scaffolds support direct inhibition of the
membrane-bound O-acyltransferase porcupine. J Biol Chem.
287:23246–23254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi-Yanaga F and Kahn M: Targeting
Wnt signaling: can we safely eradicate cancer stem cells? Clin
Cancer Res. 16:3153–3162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen W, Chen M and Barak LS: Development
of small molecules targeting the Wnt pathway for the treatment of
colon cancer: a high-throughput screening approach. Am J Physiol
Gastrointest Liver Physiol. 299:G293–G300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
15
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
16
|
Du R, Xia L, Sun S, et al: URG11 promotes
gastric cancer growth and invasion by activation of beta-catenin
signalling pathway. J Cell Mol Med. 14:621–635. 2010.PubMed/NCBI
|
|
17
|
Ganesan K, Ivanova T, Wu Y, et al:
Inhibition of gastric cancer invasion and metastasis by PLA2G2A, a
novel beta-catenin/TCF target gene. Cancer Res. 68:4277–4286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H and Xue Y: Wnt pathway is involved
in advanced gastric carcinoma. Hepatogastroenterology.
55:1126–1130. 2008.PubMed/NCBI
|
|
19
|
Mo ML, Chen Z, Li J, et al: Use of serum
circulating CCNB2 in cancer surveillance. Int J Biol Markers.
25:236–242. 2010.PubMed/NCBI
|
|
20
|
Chen Z, Fan JQ, Li J, et al: Promoter
hypermethylation correlates with the Hsulf-1 silencing in human
breast and gastric cancer. Int J Cancer. 124:739–744. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Mo ML, Chen Z, et al: HSulf-1
inhibits cell proliferation and invasion in human gastric cancer.
Cancer Sci. 102:1815–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Li J, Li QS, et al: Suppression of
PPN/MG61 attenuates Wnt/beta-catenin signaling pathway and induces
apoptosis in human lung cancer. Oncogene. 27:3483–3488. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Hou Y, Ashktorab H, et al: The
impact of C-MYC gene expression on gastric cancer cell. Mol Cell
Biochem. 344:125–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolfer A and Ramaswamy S: MYC and
metastasis. Cancer Res. 71:2034–2037. 2011. View Article : Google Scholar
|
|
25
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|